Coronavirus Disease 2019 (COVID-19) manifests differently in children than adults, as children usually have a milder course of disease, mild symptoms if any, and lower fatality rates are recorded among children. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission also seems to be different between children and adults. Many factors are proposed to explain the milder outcome in children, e.g., a more appropriate immune response (especially active innate response), trained immunity, a lack of immunosenescence, and the reduced prevalence of comorbidities. A better understanding of the differences in susceptibility and outcome in children compared with adults could lead to greater knowledge of risk factors for complicated COVID-19 cases and potential treatment targets.
- SARS-CoV-2
- COVID-19
- coronavirus
- children
- adolescents
- young people
- ACE2
- immune system
- transmission
- viral infection
1. Strong Innate Response
2. Activated Immunity Providing Cross-Protection
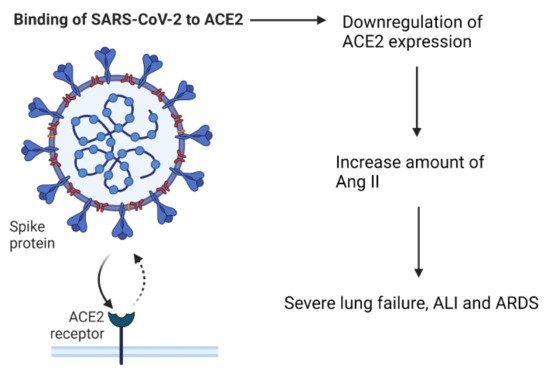
3. The Role of Angiotensin Converting Enzyme-2
4. Immunosenescence
5. Comorbidities
6. Melatonin Levels
7. Difference in Microbiota
References
- Neeland, M.R.; Bannister, S.; Clifford, V.; Dohle, K.; Mulholland, K.; Sutton, P.; Curtis, N.; Steer, A.C.; Burgner, D.P.; Crawford, N.W.; et al. Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children. Nat. Commun. 2021, 12, 1084.
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2021, 39, 1–6.
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020, 12, eabd5487.
- Dhochak, N.; Singhal, T.; Kabra, S.K.; Lodha, R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J. Pediatr. 2020, 87, 537–546.
- Snape, M.D.; Viner, R.M. COVID-19 in children and young people. Science 2020, 370, 286–288.
- Moorlag, S.; Arts, R.; Van Crevel, R.; Netea, M. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478.
- Messina, N.; Zimmermann, P.; Curtis, N. The impact of vaccines on heterologous adaptive immunity. Clin. Microbiol. Infect. 2019, 25, 1484–1493.
- Higgins, J.P.T.; Soares-Weiser, K.; López, J.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.; Reingold, A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 2016, 355, i5170.
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542.
- Mysore, V.; Cullere, X.; Settles, M.L.; Ji, X.; Kattan, M.W.; Desjardins, M.; Durbin-Johnson, B.; Gilboa, T.; Baden, L.R.; Walt, D.R.; et al. Protective heterologous T cell immunity in COVID-19 induced by the trivalent Measles-Mumps-Rubella and Tetanus-Diptheria-Pertussis vaccine antigens. Med 2021, 2, 1050–1071.e7.
- Moorlag, S.J.C.F.M.; van Deuren, R.C.; van Werkhoven, C.H.; Jaeger, M.; Debisarun, P.; Taks, E.; Mourits, V.P.; Koeken, V.A.; de Bree, L.C.J.; ten Doesschate, T.; et al. Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: A Retrospective Cohort Study. Cell Rep. Med. 2020, 1, 100073.
- Ingraham, N.E.; Barakat, A.G.; Reilkoff, R.; Bezdicek, T.; Schacker, T.; Chipman, J.G.; Tignanelli, C.; Puskarich, M. Understanding the renin—angiotensin—aldosterone—SARS-CoV axis: A comprehensive review. Eur. Respir. J. 2020, 56, 2000912.
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2021, 106, 429–439.
- Song, R.; Preston, G.; Yosypiv, I.V. Ontogeny of angiotensin-converting enzyme 2. Pediatr. Res. 2012, 71, 13–19.
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448.
- Liu, M.-Y.; Zheng, B.; Zhang, Y.; Li, J.-P. Role and mechanism of angiotensin-converting enzyme 2 in acute lung injury in coronavirus disease 2019. Chronic Dis. Transl. Med. 2020, 6, 98–105.
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116.
- Sodhi, C.P.; Nguyen, J.; Yamaguchi, Y.; Werts, A.D.; Lu, P.; Ladd, M.R.; Fulton, W.B.; Kovler, M.; Wang, S.; Prindle, T.; et al. A Dynamic Variation of Pulmonary ACE2 Is Required to Modulate Neutrophilic Inflammation in Response to Pseudomonas aeruginosa Lung Infection in Mice. J. Immunol. 2019, 203, 3000–3012.
- Sharif-Askari, N.S.; Sharif-Askari, F.S.; Alabed, M.; Temsah, M.-H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6.
- Muus, C.; Luecken, M.; Eraslan, G.; Waghray, A.; Heimberg, G.; Sikkema, L.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smilie, C.; et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. BioRxiv 2020.
- Wang, A.; Chiou, J.; Poirion, O.B.; Buchanan, J.; Valdez, M.J.; Verheyden, J.M.; Hou, X.; Kudtarkar, P.; Narendra, S.; Newsome, J.M.; et al. Single-cell multiomic profiling of human lungs reveals cell-type-specific and age-dynamic control of SARS-CoV2 host genes. eLife 2020, 9, 1–28.
- Xie, X.; Chen, J.; Wang, X.; Zhang, F.; Liu, Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006, 78, 2166–2171.
- Caruso, C.; Buffa, S.; Candore, G.; Colonna-Romano, G.; Dunn-Walters, D.; Kipling, D.; Pawelec, G. Mechanisms of immunosenescence. Immun. Ageing 2009, 6, 10.
- McElhaney, J.E.; Effros, R.B. Immunosenescence: What does it mean to health outcomes in older adults? Curr. Opin. Immunol. 2009, 21, 418–424.
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID -19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre- COVID -19 ARDS. Med. J. Aust. 2020, 213, 54–56.e1.
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343.
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768.
- Song, J.-W.; Zhang, C.; Fan, X.; Meng, F.-P.; Xu, Z.; Xia, P.; Cao, W.-J.; Yang, T.; Dai, X.-P.; Wang, S.-Y.; et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020, 11, 1–10.
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665.
- Bai, K.; Liu, W.; Liu, C.; Fu, Y.; Hu, J.; Qin, Y.; Zhang, Q.; Chen, H.; Xu, F.; Li, C. Clinical Analysis of 25 COVID-19 Infections in Children. Pediatr. Infect. Dis. J. 2020, 39, e100–e103.
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 103, 246–256.
- Williams, N.; Radia, T.; Harman, K.; Agrawal, P.; Cook, J.; Gupta, A. COVID-19 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review of critically unwell children and the association with underlying comorbidities. Eur. J. Nucl. Med. Mol. Imaging 2020, 180, 689–697.
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242.
- Posadzki, P.P.; Bajpai, R.; Kyaw, B.M.; Roberts, N.J.; Brzezinski, A.; Christopoulos, G.I.; Divakar, U.; Bajpai, S.; Soljak, M.; Dunleavy, G.; et al. Melatonin and health: An umbrella review of health outcomes and biological mechanisms of action. BMC Med. 2018, 16, 18.
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525.
- Wang, L.-F.; Shi, Z.; Zhang, S.; Field, H.; Daszak, P.; Eaton, B.T. Review of Bats and SARS. Emerg. Infect. Dis. 2006, 12, 1834–1840.
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162.
- Iguchi, H.; Kato, K.-I.; Ibayashi, H. Age-Dependent Reduction in Serum Melatonin Concentrations in Healthy Human Subjects. J. Clin. Endocrinol. Metab. 1982, 55, 27–29.
- Waldhauser, F.; Weiszenbacher, G.; Tatzer, E.; Gisinger, B.; Schemper, M.; Frisch, H. Alterations in Nocturnal Serum Melatonin Levels In Humans With Growth and Aging. J. Clin. Endocrinol. Metab. 1988, 66, 648–652.
- Miller, S.C.; Pandi, P.S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87.
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583.
- Camp, O.G.; Bai, D.; Gonullu, D.C.; Nayak, N.; Abu-Soud, H.M. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J. Inorg. Biochem. 2021, 223, 111546.
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706.
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010.
