Several processes involved in cancer development, such as cell growth, migration, and angiogenesis, are regulated by the arachidonic acid derivative thromboxane A2 (TXA2). Higher levels of circulating TXA2 are observed in patients with multiple cancers, and this is accompanied by overexpression of TXA2 synthase (TBXAS1, TXA2S) and/or TXA2 receptors (TBXA2R, TP). Overexpression of TXA2S or TP in tumor cells is generally associated with poor prognosis, reduced survival, and metastatic disease. However, the role of TXA2 signaling in the stroma during oncogenesis has been underappreciated. TXA2 signaling regulates the tumor microenvironment by modulating angiogenic potential, tumor ECM stiffness, and host immune response.
- thromboxane A2 synthase
- thromboxane A2 receptor
- isoforms
1. Thromboxane A2 Receptor Isoforms and Signaling
2. TXA2 Signaling in Cancer
2.1. TP Isoforms in Cancer
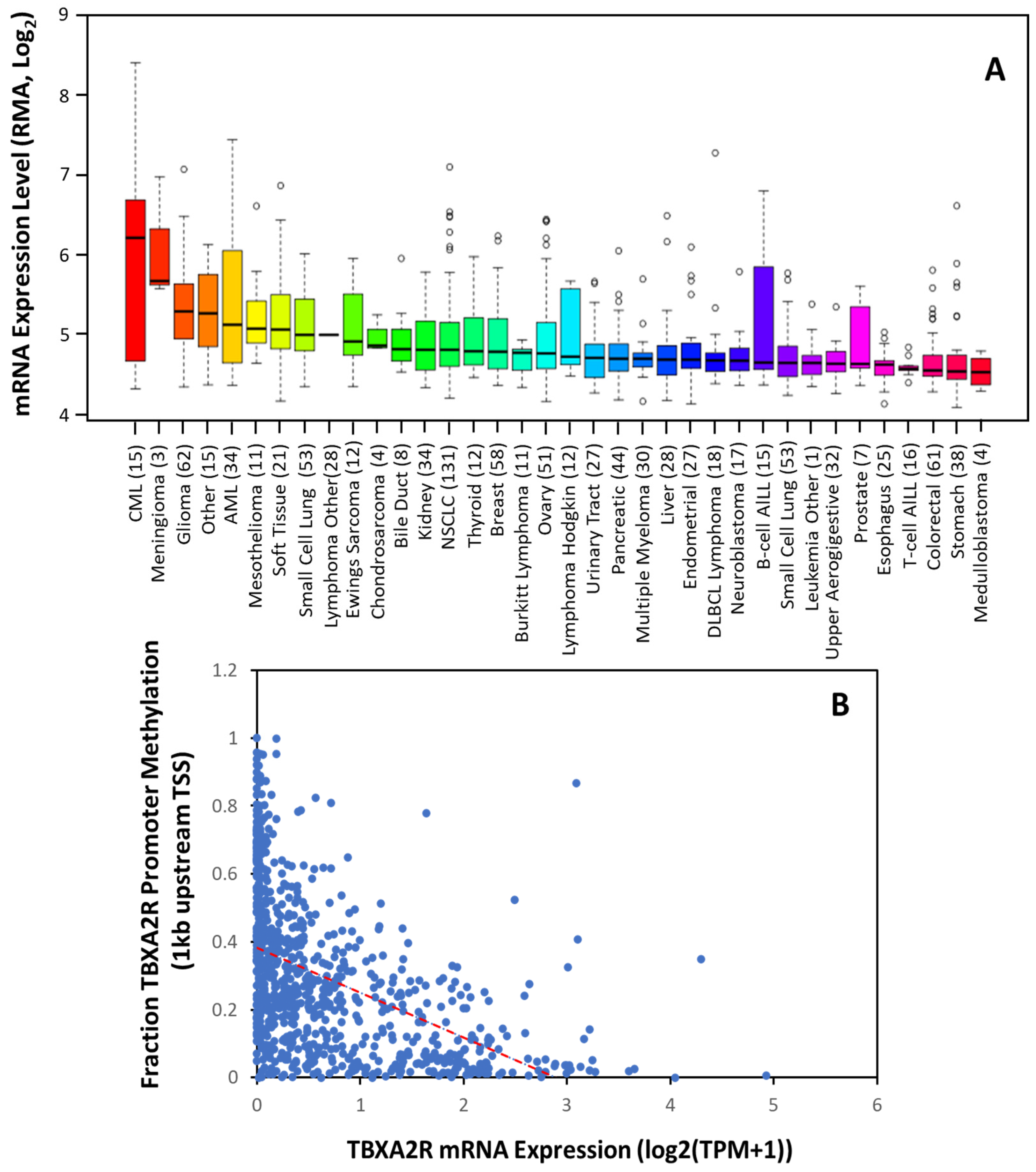
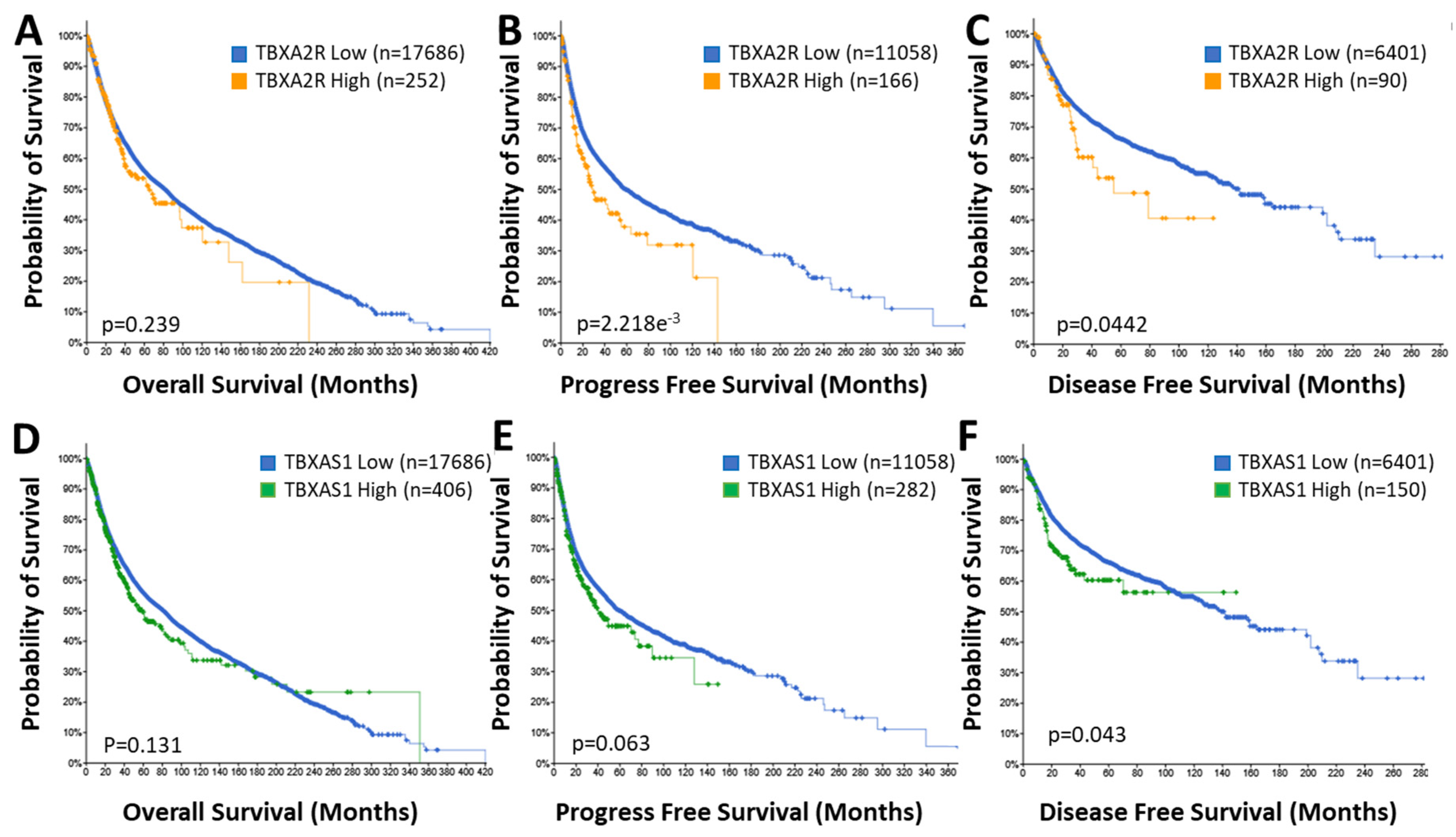
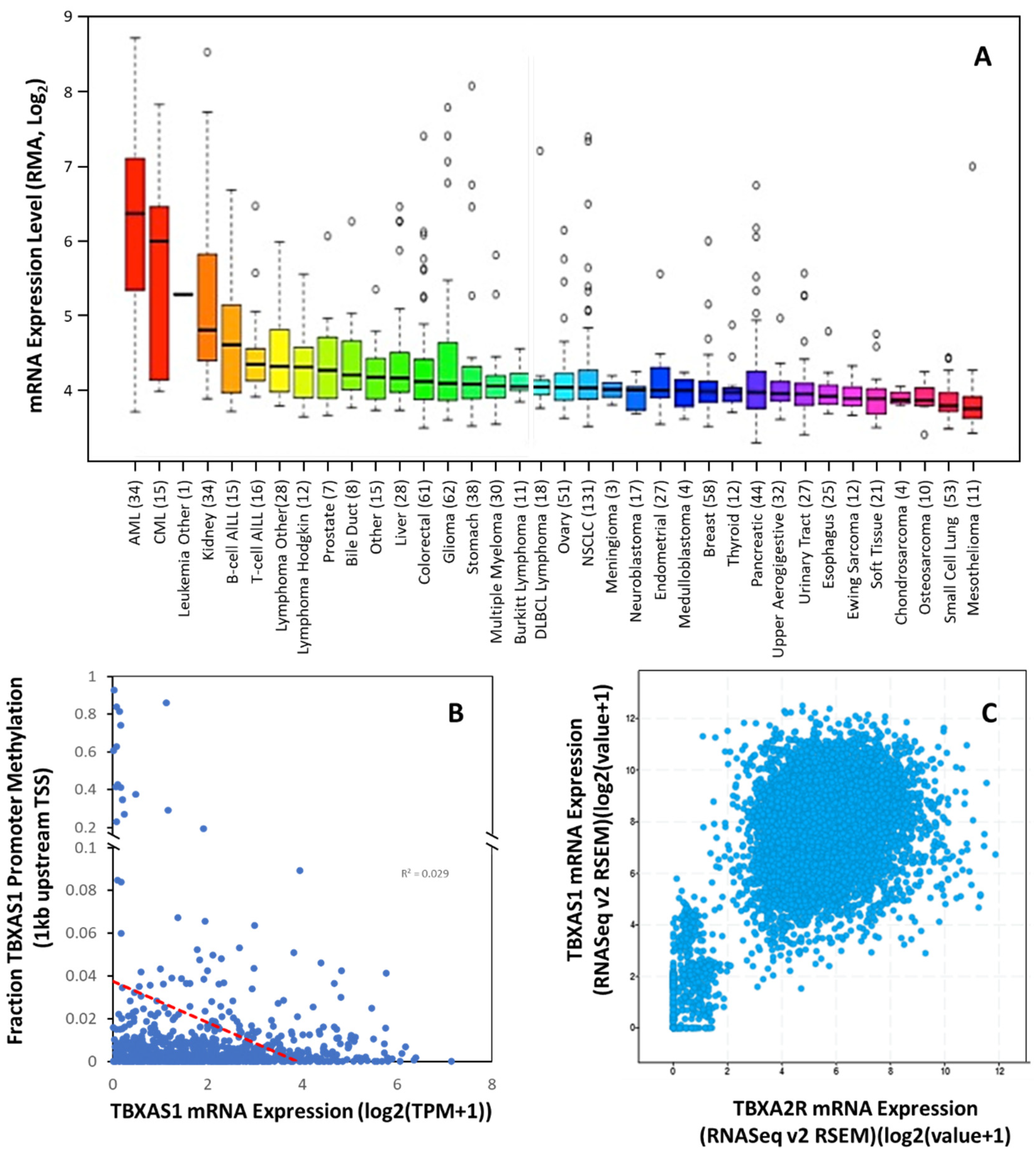
2.2. TBXAS1 in Cancer
References
- Hirata, M.; Hayashi, Y.; Ushikubi, F.; Yokota, Y.; Kageyama, R.; Nakanishi, S.; Narumiya, S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 1991, 349, 617–620.
- Kinsella, B.T. Thromboxane A2 signalling in humans: A ‘Tail’ of two receptors. Biochem. Soc. Trans. 2001, 29, 641–654.
- Nakahata, N. Thromboxane A2: Physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol. Ther. 2008, 118, 18–35.
- Nüsing, R.M.; Hirata, M.; Kakizuka, A.; Eki, T.; Ozawa, K.; Narumiya, S. Characterization and chromosomal mapping of the human thromboxane A2 receptor gene. J. Biol. Chem. 1993, 268, 25253–25259.
- Coyle, A.T.; Kinsella, B.T. Characterization of promoter 3 of the human thromboxane A receptor gene. A functional AP-1 and octamer motif are required for basal promoter activity. Febs J. 2005, 272, 1036–1053.
- Coyle, A.T.; Miggin, S.M.; Kinsella, B.T. Characterization of the 5’ untranslated region of alpha and beta isoforms of the human thromboxane A2 receptor (TP). Differential promoter utilization by the TP isoforms. Eur. J. Biochem. 2002, 269, 4058–4073.
- Miggin, S.M.; Kinsella, B.T. Expression and tissue distribution of the mRNAs encoding the human thromboxane A2 receptor (TP) alpha and beta isoforms. Biochim. Biophys. Acta 1998, 1425, 543–559.
- Habib, A.; FitzGerald, G.A.; Maclouf, J. Phosphorylation of the thromboxane receptor alpha, the predominant isoform expressed in human platelets. J. Biol. Chem. 1999, 274, 2645–2651.
- Wilson, S.J.; McGinley, K.; Huang, A.J.; Smyth, E.M. Heterodimerization of the alpha and beta isoforms of the human thromboxane receptor enhances isoprostane signaling. Biochem. Biophys. Res. Commun. 2007, 352, 397–403.
- Bambi-Nyanguile, S.M.; Hanson, J.; Ooms, A.; Alpan, L.; Kolh, P.; Dogne, J.M.; Pirotte, B. Synthesis and pharmacological evaluation of 2-aryloxy/arylamino-5-cyanobenzenesulfonylureas as novel thromboxane A(2) receptor antagonists. Eur. J. Med. Chem. 2013, 65, 32–40.
- Qiao, N.; Reynaud, D.; Demin, P.; Halushka, P.V.; Pace-Asciak, C.R. The thromboxane receptor antagonist PBT-3, a hepoxilin stable analog, selectively antagonizes the TPalpha isoform in transfected COS-7 cells. J. Pharmacol. Exp. Ther. 2003, 307, 1142–1147.
- Hanson, J.; Reynaud, D.; Qiao, N.; Devel, P.; Moray, A.L.; Renard, J.F.; Kelley, L.P.; Winum, J.Y.; Montero, J.L.; Kinsella, B.T.; et al. Synthesis and pharmacological evaluation of novel nitrobenzenic thromboxane modulators as antiplatelet agents acting on both the alpha and beta isoforms of the human thromboxane receptor. J. Med. Chem. 2006, 49, 3701–3709.
- Hanson, J.; Dogné, J.M.; Ghiotto, J.; Moray, A.L.; Kinsella, B.T.; Pirotte, B. Design, synthesis, and SAR study of a series of N-alkyl-N’-ureas and -cyanoguanidine as selective antagonists of the TPalpha and TPbeta isoforms of the human thromboxane A2 receptor. J. Med. Chem. 2007, 50, 3928–3936.
- Halushka, P.V. Thromboxane A(2) recept.tors: Where have you gone? Prostaglandins Other Lipid Mediat. 2000, 60, 175–189.
- Zhang, L.; DiLizio, C.; Kim, D.; Smyth, E.M.; Manning, D.R. The G12 family of G proteins as a reporter of thromboxane A2 receptor activity. Mol. Pharmacol. 2006, 69, 1433–1440.
- Vezza, R.; Habib, A.; FitzGerald, G.A. Differential signaling by the thromboxane receptor isoforms via the novel GTP-binding protein, Gh. J. Biol. Chem. 1999, 274, 12774–12779.
- Hirata, T.; Ushikubi, F.; Kakizuka, A.; Okuma, M.; Narumiya, S. Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J. Clin. Investig. 1996, 97, 949–956.
- Gao, Y.; Tang, S.; Zhou, S.; Ware, J.A. The thromboxane A2 receptor activates mitogen-activated protein kinase via protein kinase C-dependent Gi coupling and Src-dependent phosphorylation of the epidermal growth factor receptor. J. Pharmacol. Exp. Ther. 2001, 296, 426–433.
- Parent, J.L.; Labrecque, P.; Orsini, M.J.; Benovic, J.L. Internalization of the TXA2 receptor alpha and beta isoforms. Role of the differentially spliced cooh terminus in agonist-promoted receptor internalization. J. Biol. Chem. 1999, 274, 8941–8948.
- Reid, H.M.; Kinsella, B.T. Palmitoylation of the TPbeta isoform of the human thromboxane A2 receptor. Modulation of G protein: Effector coupling and modes of receptor internalization. Cell Signal. 2007, 19, 1056–1070.
- Foley, J.F.; Kelley, L.P.; Kinsella, B.T. Prostaglandin D(2) receptor-mediated desensitization of the alpha isoform of the human thromboxane A(2) receptor. Biochem. Pharmacol. 2001, 62, 229–239.
- Walsh, M.T.; Foley, J.F.; Kinsella, B.T. The alpha, but not the beta, isoform of the human thromboxane A2 receptor is a target for prostacyclin-mediated desensitization. J. Biol. Chem. 2000, 275, 20412–20423.
- Walsh, M.T.; Kinsella, B.T. Regulation of the human prostanoid TPalpha and TPbeta receptor isoforms mediated through activation of the EP(1) and IP receptors. Br. J. Pharmacol. 2000, 131, 601–609.
- Yamamoto, S.; Yan, F.; Zhou, H.; Tai, H.H. Serine 331 is the major site of receptor phosphorylation induced by agents that activate protein kinase G in HEK 293 cells overexpressing thromboxane receptor alpha. Arch. Biochem. Biophys. 2001, 393, 97–105.
- Wang, G.R.; Zhu, Y.; Halushka, P.V.; Lincoln, T.M.; Mendelsohn, M.E. Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 4888–4893.
- Spurney, R.F. Regulation of thromboxane receptor (TP) phosphorylation by protein phosphatase 1 (PP1) and PP2A. J. Pharmacol. Exp. Ther. 2001, 296, 592–599.
- Hamelin, E.; Thériault, C.; Laroche, G.; Parent, J.L. The intracellular trafficking of the G protein-coupled receptor TPbeta depends on a direct interaction with Rab11. J. Biol. Chem. 2005, 280, 36195–36205.
- Rochdi, M.D.; Laroche, G.; Dupré, E.; Giguère, P.; Lebel, A.; Watier, V.; Hamelin, E.; Lépine, M.C.; Dupuis, G.; Parent, J.L. Nm23-H2 interacts with a G protein-coupled receptor to regulate its endocytosis through an Rac1-dependent mechanism. J. Biol. Chem. 2004, 279, 18981–18989.
- Ekambaram, P.; Lambiv, W.; Cazzolli, R.; Ashton, A.W.; Honn, K.V. The thromboxane synthase and receptor signaling pathway in cancer: An emerging paradigm in cancer progression and metastasis. Cancer Metastasis Rev. 2011, 30, 397–408.
- Gannon, A.M.; Kinsella, B.T. Regulation of the human thromboxane A2 receptor gene by Sp1, Egr1, NF-E2, GATA-1, and Ets-1 in megakaryocytes. J. Lipid Res. 2008, 49, 2590–2604.
- Orr, K.; Buckley, N.E.; Haddock, P.; James, C.; Parent, J.L.; McQuaid, S.; Mullan, P.B. Thromboxane A2 receptor (TBXA2R) is a potent survival factor for triple negative breast cancers (TNBCs). Oncotarget 2016, 7, 55458–55472.
- Keating, G.L.; Reid, H.M.; Eivers, S.B.; Mulvaney, E.P.; Kinsella, B.T. Transcriptional regulation of the human thromboxane A2 receptor gene by Wilms’ tumor (WT)1 and hypermethylated in cancer (HIC) 1 in prostate and breast cancers. Biochim. Biophys. Acta 2014, 1839, 476–492.
- Mulvaney, E.P.; Shilling, C.; Eivers, S.B.; Perry, A.S.; Bjartell, A.; Kay, E.W.; Watson, R.W.; Kinsella, B.T. Expression of the TPα and TPβ isoforms of the thromboxane prostanoid receptor (TP) in prostate cancer: Clinical significance and diagnostic potential. Oncotarget 2016, 7, 73171–73187.
- Kanwal, R.; Gupta, S. Epigenetic modifications in cancer. Clin. Genet. 2012, 81, 303–311.
- Moussa, O.; Ashton, A.W.; Fraig, M.; Garrett-Mayer, E.; Ghoneim, M.A.; Halushka, P.V.; Watson, D.K. Novel role of thromboxane receptors beta isoform in bladder cancer pathogenesis. Cancer Res. 2008, 68, 4097–4104.
- Honma, S.; Saika, M.; Ohkubo, S.; Kurose, H.; Nakahata, N. Thromboxane A2 receptor-mediated G12/13-dependent glial morphological change. Eur. J. Pharmacol. 2006, 545, 100–108.
- Nie, D.; Guo, Y.; Yang, D.; Tang, Y.; Chen, Y.; Wang, M.T.; Zacharek, A.; Qiao, Y.; Che, M.; Honn, K.V. Thromboxane A2 receptors in prostate carcinoma: Expression and its role in regulating cell motility via small GTPase Rho. Cancer Res. 2008, 68, 115–121.
- Kelly, P.; Stemmle, L.N.; Madden, J.F.; Fields, T.A.; Daaka, Y.; Casey, P.J. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J. Biol. Chem. 2006, 281, 26483–26490.
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104.
- Yan, Y.; Huang, H. Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 319–331.
- Sobolesky, P.M.; Halushka, P.V.; Garrett-Mayer, E.; Smith, M.T.; Moussa, O. Regulation of the tumor suppressor FOXO3 by the thromboxane-A2 receptors in urothelial cancer. PLoS ONE 2014, 9, e107530.
- Huang, R.Y.; Li, M.Y.; Hsin, M.K.; Underwood, M.J.; Ma, L.T.; Mok, T.S.; Warner, T.D.; Chen, G.G. 4-Methylnitrosamino-1-3-pyridyl-1-butanone (NNK) promotes lung cancer cell survival by stimulating thromboxane A2 and its receptor. Oncogene 2011, 30, 106–116.
- Li, X.; Tai, H.H. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis 2009, 30, 1606–1613.
- O’Sullivan, A.G.; Mulvaney, E.P.; Hyland, P.B.; Kinsella, B.T. Protein kinase C-related kinase 1 and 2 play an essential role in thromboxane-mediated neoplastic responses in prostate cancer. Oncotarget 2015, 6, 26437–26456.
- Turner, E.C.; Kavanagh, D.J.; Mulvaney, E.P.; McLean, C.; Wikström, K.; Reid, H.M.; Kinsella, B.T. Identification of an interaction between the TPalpha and TPbeta isoforms of the human thromboxane A2 receptor with protein kinase C-related kinase (PRK) 1: Implications for prostate cancer. J. Biol. Chem. 2011, 286, 15440–15457.
- Huang, R.Y.; Li, M.Y.; Ng, C.S.; Wan, I.Y.; Kong, A.W.; Du, J.; Long, X.; Underwood, M.J.; Mok, T.S.; Chen, G.G. Thromboxane A2 receptor α promotes tumor growth through an autoregulatory feedback pathway. J. Mol. Cell Biol. 2013, 5, 380–390.
- Watkins, G.; Douglas-Jones, A.; Mansel, R.E.; Jiang, W.G. Expression of thromboxane synthase, TBXAS1 and the thromboxane A2 receptor, TBXA2R, in human breast cancer. Int. Semin. Surg. Oncol. 2005, 2, 23.
- Li, H.; Lee, M.H.; Liu, K.; Wang, T.; Song, M.; Han, Y.; Yao, K.; Xie, H.; Zhu, F.; Grossmann, M.; et al. Inhibiting breast cancer by targeting the thromboxane A(2) pathway. NPJ Precis. Oncol. 2017, 1, 8.
- Gustafsson, A.; Hansson, E.; Kressner, U.; Nordgren, S.; Andersson, M.; Lönnroth, C.; Lundholm, K. Prostanoid receptor expression in colorectal cancer related to tumor stage, differentiation and progression. Acta Oncol. 2007, 46, 1107–1112.
- Dassesse, T.; de Leval, X.; de Leval, L.; Pirotte, B.; Castronovo, V.; Waltregny, D. Activation of the thromboxane A2 pathway in human prostate cancer correlates with tumor Gleason score and pathologic stage. Eur. Urol. 2006, 50, 1021–1031, discussion 1031.
- Coyle, A.T.; O’Keeffe, M.B.; Kinsella, B.T. 15-deoxy Delta12,14-prostaglandin J2 suppresses transcription by promoter 3 of the human thromboxane A2 receptor gene through peroxisome proliferator-activated receptor gamma in human erythroleukemia cells. Febs J. 2005, 272, 4754–4773.
- Coyle, A.T.; Kinsella, B.T. Synthetic peroxisome proliferator-activated receptor gamma agonists rosiglitazone and troglitazone suppress transcription by promoter 3 of the human thromboxane A2 receptor gene in human erythroleukemia cells. Biochem. Pharmacol. 2006, 71, 1308–1323.
- Valentin, F.; Field, M.C.; Tippins, J.R. The mechanism of oxidative stress stabilization of the thromboxane receptor in COS-7 cells. J. Biol. Chem. 2004, 279, 8316–8324.
- Valentin, F.; Tippins, J.R.; Field, M.C. The role of alternative splicing and C-terminal amino acids in thromboxane receptor stabilization. Biochem. Biophys. Res. Commun. 2005, 329, 898–904.
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197.
- Werfel, T.A.; Hicks, D.J.; Rahman, B.; Bendeman, W.E.; Duvernay, M.T.; Maeng, J.G.; Hamm, H.; Lavieri, R.R.; Joly, M.M.; Pulley, J.M.; et al. Repurposing of a Thromboxane Receptor Inhibitor Based on a Novel Role in Metastasis Identified by Phenome-Wide Association Study. Mol. Cancer Ther. 2020, 19, 2454–2464.
- Chen, G.G.; Lee, T.W.; Yip, J.H.; Xu, H.; Lee, I.K.; Mok, T.S.; Warner, T.D.; Yim, A.P. Increased thromboxane B(2) levels are associated with lipid peroxidation and Bcl-2 expression in human lung carcinoma. Cancer Lett. 2006, 234, 193–198.
- Karmali, R.A.; Welt, S.; Thaler, H.T.; Lefevre, F. Prostaglandins in breast cancer: Relationship to disease stage and hormone status. Br. J. Cancer 1983, 48, 689–696.
- Moussa, O.; Ciupek, A.; Watson, D.K.; Halushka, P.V. Urinary thromboxane B2 and thromboxane receptors in bladder cancer: Opportunity for detection and monitoring. Prostaglandins Other Lipid Mediat. 2011, 96, 41–44.
- Kajita, S.; Ruebel, K.H.; Casey, M.B.; Nakamura, N.; Lloyd, R.V. Role of COX-2, thromboxane A2 synthase, and prostaglandin I2 synthase in papillary thyroid carcinoma growth. Mod. Pathol. 2005, 18, 221–227.
- Sakai, H.; Suzuki, T.; Takahashi, Y.; Ukai, M.; Tauchi, K.; Fujii, T.; Horikawa, N.; Minamimura, T.; Tabuchi, Y.; Morii, M.; et al. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Lett. 2006, 580, 3368–3374.
- Moussa, O.; Yordy, J.S.; Abol-Enein, H.; Sinha, D.; Bissada, N.K.; Halushka, P.V.; Ghoneim, M.A.; Watson, D.K. Prognostic and functional significance of thromboxane synthase gene overexpression in invasive bladder cancer. Cancer Res. 2005, 65, 11581–11587.
- Kuo, H.L.; Lien, J.C.; Chung, C.H.; Chang, C.H.; Lo, S.C.; Tsai, I.C.; Peng, H.C.; Kuo, S.C.; Huang, T.F. NP-184, a novel orally active antithrombotic agent with dual antiplatelet and anticoagulant activities. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 495–505.
- Cathcart, M.C.; Gately, K.; Cummins, R.; Drakeford, C.; Kay, E.W.; O’Byrne, K.J.; Pidgeon, G.P. Thromboxane synthase expression and correlation with VEGF and angiogenesis in non-small cell lung cancer. Biochim. Biophys. Acta 2014, 1842, 747–755.
- Huang, R.Y.; Chu, Y.L.; Jiang, Z.B.; Chen, X.M.; Zhang, X.; Zeng, X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell Physiol. Biochem. 2014, 33, 375–388.
- Kurzel, F.; Hagel, C.; Zapf, S.; Meissner, H.; Westphal, M.; Giese, A. Cyclo-oxygenase inhibitors and thromboxane synthase inhibitors differentially regulate migration arrest, growth inhibition and apoptosis in human glioma cells. Acta Neurochir. 2002, 144, 71–87.
- Leung, K.C.; Hsin, M.K.; Chan, J.S.; Yip, J.H.; Li, M.; Leung, B.C.; Mok, T.S.; Warner, T.D.; Underwood, M.J.; Chen, G.G. Inhibition of thromboxane synthase induces lung cancer cell death via increasing the nuclear p27. Exp. Cell Res. 2009, 315, 2974–2981.
- Leung, K.C.; Li, M.Y.; Leung, B.C.; Hsin, M.K.; Mok, T.S.; Underwood, M.J.; Chen, G.G. Thromboxane synthase suppression induces lung cancer cell apoptosis via inhibiting NF-kappaB. Exp. Cell Res. 2010, 316, 3468–3477.
- Liu, Q.; Tao, B.; Liu, G.; Chen, G.; Zhu, Q.; Yu, Y.; Yu, Y.; Xiong, H. Thromboxane A2 Receptor Inhibition Suppresses Multiple Myeloma Cell Proliferation by Inducing p38/c-Jun N-terminal Kinase (JNK) Mitogen-activated Protein Kinase (MAPK)-mediated G2/M Progression Delay and Cell Apoptosis. J. Biol. Chem. 2016, 291, 4779–4792.
- Li, X.; Tai, H.H. Increased expression of matrix metalloproteinases mediates thromboxane A2-induced invasion in lung cancer cells. Curr. Cancer Drug Targets 2012, 12, 703–715.
- Li, X.; Tai, H.H. Activation of thromboxane A2 receptor (TP) increases the expression of monocyte chemoattractant protein -1 (MCP-1)/chemokine (C-C motif) ligand 2 (CCL2) and recruits macrophages to promote invasion of lung cancer cells. PLoS ONE 2013, 8, e54073.
- Matsui, Y.; Amano, H.; Ito, Y.; Eshima, K.; Suzuki, T.; Ogawa, F.; Iyoda, A.; Satoh, Y.; Kato, S.; Nakamura, M.; et al. Thromboxane A₂ receptor signaling facilitates tumor colonization through P-selectin-mediated interaction of tumor cells with platelets and endothelial cells. Cancer Sci. 2012, 103, 700–707.
- Xu, R.; Yan, Y.; Zheng, X.; Zhang, H.; Chen, W.; Li, H.; Dong, Z. Aspirin suppresses breast cancer metastasis to lung by targeting anoikis resistance. Carcinogenesis 2022, 43, 104–114.
- Giese, A.; Hagel, C.; Kim, E.L.; Zapf, S.; Djawaheri, J.; Berens, M.E.; Westphal, M. Thromboxane synthase regulates the migratory phenotype of human glioma cells. Neuro. Oncol. 1999, 1, 3–13.
- Schauff, A.K.; Kim, E.L.; Leppert, J.; Nadrowitz, R.; Wuestenberg, R.; Brockmann, M.A.; Giese, A. Inhibition of invasion-associated thromboxane synthase sensitizes experimental gliomas to gamma-radiation. J. Neuro. Oncol. 2009, 91, 241–249.
- Abraham, J.E.; Harrington, P.; Driver, K.E.; Tyrer, J.; Easton, D.F.; Dunning, A.M.; Pharoah, P.D. Common polymorphisms in the prostaglandin pathway genes and their association with breast cancer susceptibility and survival. Clin. Cancer Res. 2009, 15, 2181–2191.
- Lee, K.D.; Baek, S.J.; Shen, R.F. Multiple factors regulating the expression of human thromboxane synthase gene. Biochem. J. 1996, 319 Pt 3, 783–791.
