Inflammatory bowel disease (IBD) is a gastrointestinal disease that involves chronic mucosal or submucosal lesions that affect tissue integrity. Although IBD is not life-threatening, it sometimes causes severe complications, such as colon cancer. The exact etiology of IBD remains unclear, but several risk factors, such as pathogen infection, stress, diet, age, and genetics, have been involved in the occurrence and aggravation of IBD. Immune system malfunction with the over-production of inflammatory cytokines and associated oxidative stress are the hallmarks of IBD. Dietary intervention and medical treatment suppressing abnormal inflammation and oxidative stress are recommended as potential therapies. Thymol, a natural monoterpene phenol that is mostly found in thyme, exhibits multiple biological functions as a potential adjuvant for IBD. The purpose of this review is to summarize current findings on the protective effect of thymol on intestinal health in the context of specific animal models of IBD, describe the role of thymol in the modulation of inflammation, oxidative stress, and gut microbiota against gastrointestinal disease, and discuss the potential mechanism for its pharmacological activity.
- gastrointestinal disease
- gut health
- natural anti-oxidant
1. Introduction
2. Pharmacokinetics and Pharmacological Properties of Thymol
Thymol is the secondary metabolite produced by the aromatization of γ-terpinene to p-cymene, followed by the hydroxylation of p-cymene in plants [19][18] (Figure 1), including Thymus zygis [20][19], Thymbra capitata [21][20], Thymus vulgaris [22][21], Satureja thymbra [23][22], Nigella sativa seeds [24][23], and Monarda didyma [25][24]. Pure thymol has low solubility in water, high volatility, and a strong bitter/irritating taste [26][25]; thus, it is usually encapsulated in electrospun nanofibers to enhance its water solubility and high temperature stability [27][26]. Emulsification is also used for thymol processing. Both encapsulation and emulsification have been shown to improve the anti-oxidant activity of thymol [28][27].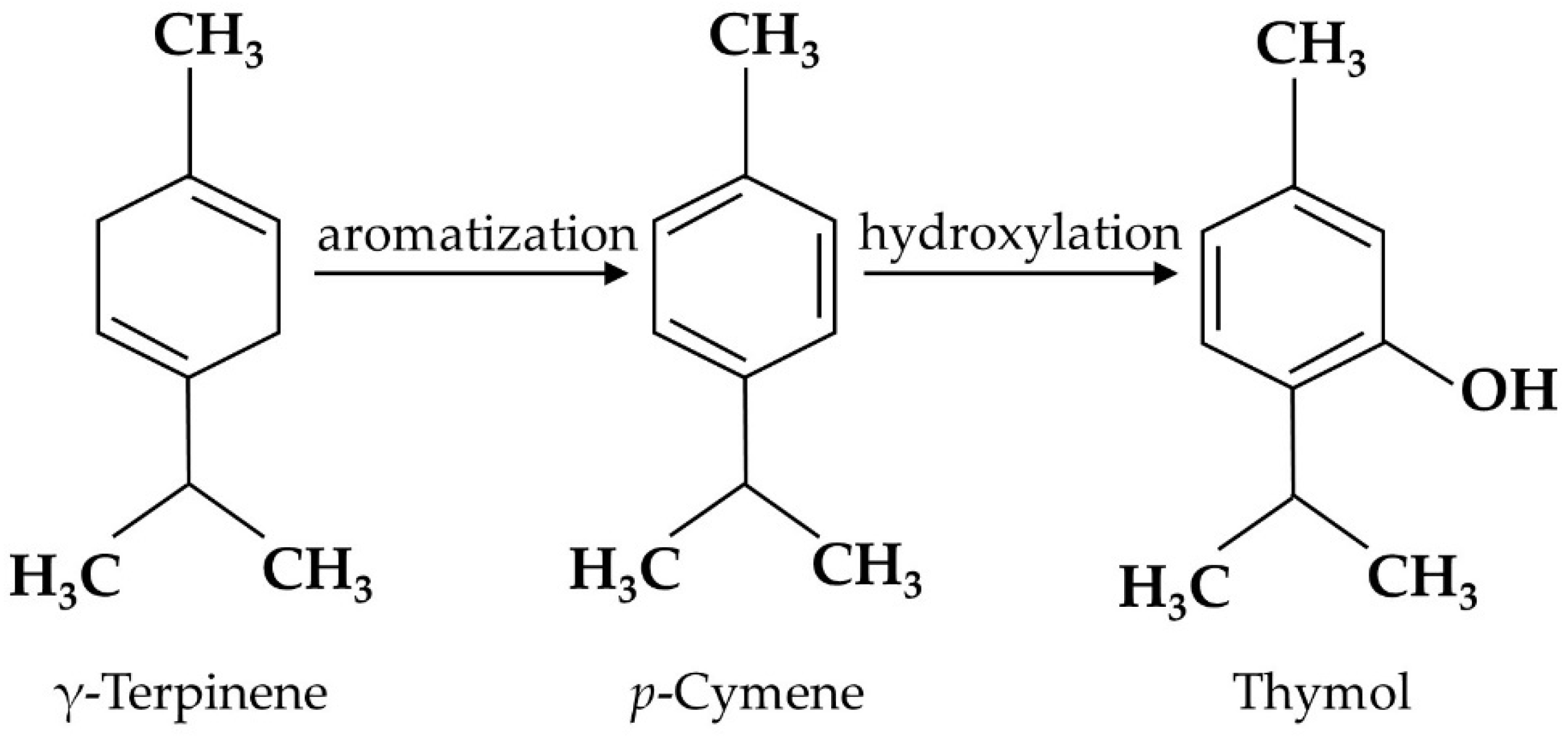
3. Thymol Protects Intestinal Barrier Function against IBD
The intestinal barrier, mainly composed of intestinal epithelial cells (IECs) and immune cells, maintains the balance between the luminal contents and mucosa. The disturbance of this balance has been associated with gastrointestinal diseases, such as IBD [42][41]. Although the exact pathogenesis of IBD remains unclear, a “leaky gut” with impaired intestinal barrier function is the main feature. The intestinal barrier is the first line of defense against pathogen infection, and injury to the intestinal barrier aggravates the disease. Thus, understanding how thymol protects the intestinal barrier is important for relieving IBD. Thymol has exhibited a protective function for the intestinal barrier in both in vivo and in vitro studies, as illustrated in Table 1, and its specific mechanism is shown in Figure 2. Thymol attenuates weaning stress-induced diarrheal and intestinal barrier dysfunction in weanling pigs by reducing the serum diamine oxidase level, an indicator of intestinal integrity, and increasing the expression of the tight junction protein zonula occludens-1 (ZO-1) and occludins [43][42]. Thymol alleviates dextran sulfate sodium (DSS)-induced intestinal damage and increases tight junction claudin-3 expression [44][43]. Increased plasma endotoxin and D-lactic acid levels are markers of increased intestinal permeability. The latest study found that dietary thymol reduced the plasma endotoxin and D-lactic acid concentrations on days 7 and 14 post-weaning [45][44]. The intestinal mucus layer is the first line of defense maintaining bacterial symbiosis with the host and preventing bacterial penetration into epithelial cells [46][45]. Thymol increases mucus secretion to relieve ethanol-induced ulcer mucosal damage in rats [47][46]. In IPEC-J2 cells, thymol alleviates lipopolysaccharide (LPS)-induced decrease in trans-epithelial electrical resistance (TEER), indicating an increase in the integrity of the single cell layer [48][47]. In Caco-2 cells, thymol increases the integrity of the tight junction and up-regulates cyclooxygenase-1 (COX1) activity to maintain GIT homeostasis, which is beneficial for intestinal health [49][48]. In addition, thymol changes the expressions of 120 and 59 genes in the oxyntic and pyloric mucosa, respectively, which are associated with gastric epithelium proliferation and maturation activities in weaned pigs [29][28].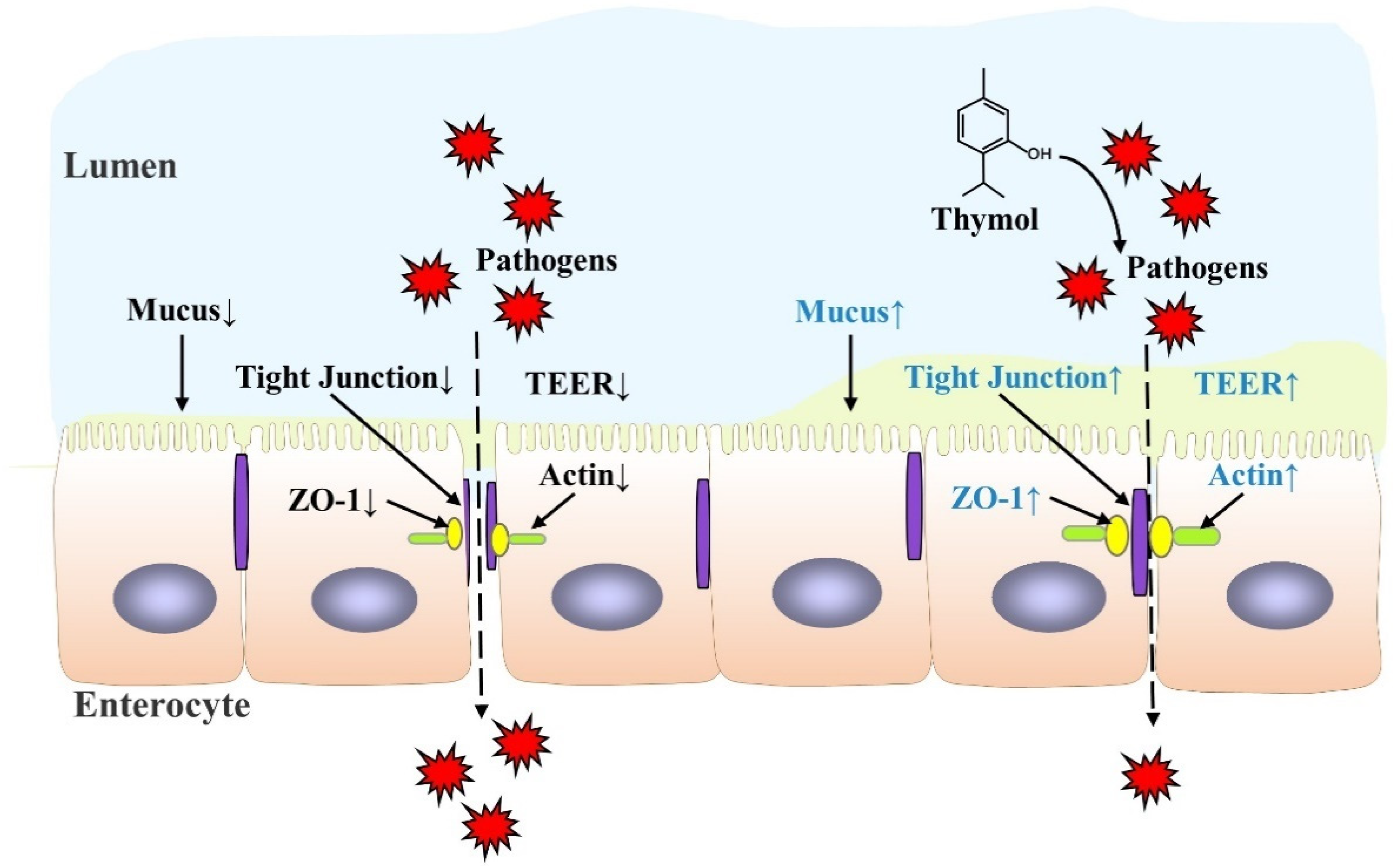
| Model | Thymol Dose | Effects | Ref. |
|---|---|---|---|
| In vivo | |||
| Weaned pigs | 50 mg/kg diet | Enrichment of 120 and 59 gene sets in oxyntic and pyloric mucosa↓ |
[29][28] |
| Ethanol-induced acute ulcer | 10–100 mg/kg diet | Mucosal damage↓ Amount of mucus↑ |
[47][46] |
| Chicken infected with Clostridium perfringens |
30 mg/kg diet | Intestinal lesions and mortality↓ Lactobacillus salivarius and L. johnsonii↓ L. crispatus, L. agilis, and Escherichia coli↑ |
[50][49] |
| Weaned pigs | 100 mg/kg diet | Expression of ZO-1 and occludins in jejunal mucosa↓ Enterococcus genus and E. coli↓ Plasma diamine oxidase concentration↓ Weaning-induced intestinal OS↓ |
[43][42] |
| In vitro | |||
| IPEC-J2 | 50 μM | TEER↑ Cell permeability↓ ZO-1and actin staining↑ |
[48][47] |
| Caco-2 cells | 15 mg/L | COX1 transcription↑ COX1:COX2 ratio↑ TEER↑ |
[49][48] |
4. Thymol Alleviates Intestinal Inflammation in IBD
The dysregulation of innate and adaptive immune responses leads to chronic intestinal inflammation in IBD patients [51,52,53][50][51][52]. Transcription factor nuclear factor κB (NF-κB) is the key mediator regulating the inflammatory response. The activation of NF-κB signaling induces the expression of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), inductible nitric oxide synthase (iNOS), interleukin (IL-1β), and IL-6 [54][53]. The over-production and accumulation of inflammatory cytokines cause intestinal epithelial apoptosis and the disruption of intestinal homeostasis, resulting in the dysfunction of the intestinal epithelial barrier in IBD patients [55][54]. Currently, the suppression of inflammation is the mainstay of IBD treatment. Thymol shows strong anti-inflammatory properties in in vivo and in vitro studies [56][55]. A schematic diagram of thymol’s action is depicted in Figure 3. Toll-like receptors (TLRs) are the main sensors used to detect various dangerous signals and activate innate immune responses. The classic TLR signaling pathway activates NF-κB to regulate the expressions of a series of cytokines [57][56]. In mice and macrophages, thymol inhibits TLR4 expression and then inhibits the activation of NF-κB signaling, which reduces the production of inflammatory cytokines, such as TNF-α and IL-1β [58,59][57][58]. NF-κB is a master mediator of inflammatory responses. Inactive NF-κB binds to IκB, an inhibitory subunit of NF-κB, and presents in the cytoplasm. When activated by a variety of signals, such as cytokine receptors and pattern-recognition receptors (PRRs), IκB is phosphorylated and degraded to release RelA (p65) from the NF-κB complex. p65 is then translocated to the nucleus to induce pro-inflammatory cytokine expression as a transcription factor [60][59]. Thymol has been shown to inhibit NF-κB activation by reducing p65 translocation and abundance in the colons of acetic acid-induced colitis rats and in LPS-activated macrophages, respectively, with decreased cytokine production [61,62][60][61]. The activation of NF-κB induces the expressions of iNOS and COX-2, which further promote vigorous inflammation. In ulcerative colitis rats, thymol reduces the COX-2 expression and nitric oxide (NO) levels produced by iNOS in the rats’ colon [63][62]. These studies showed the anti-inflammatory function of thymol through the inhibition of the NF-κB signaling pathway.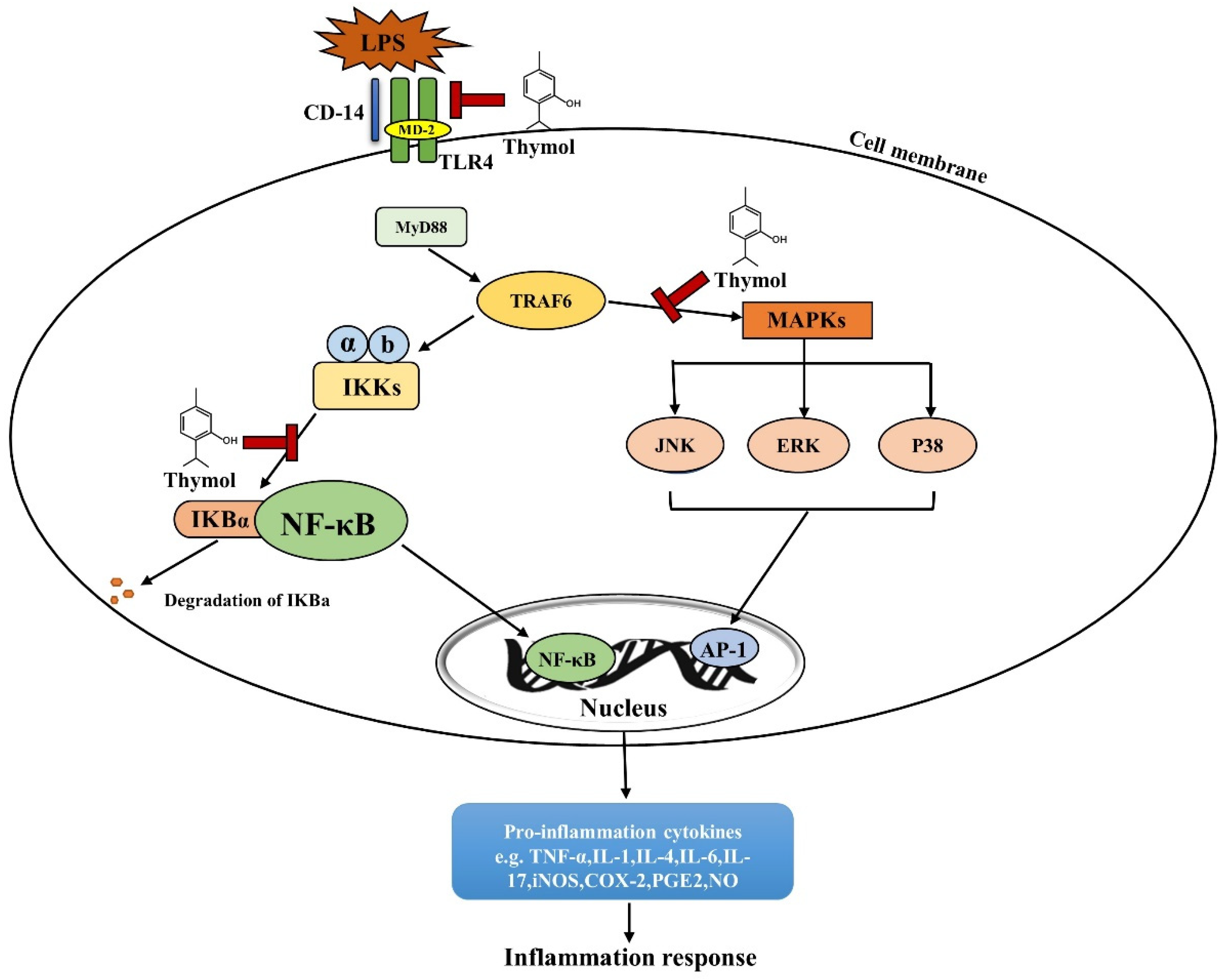
References
- Duerr, R.H.; Taylor, K.D.; Brant, S.R.; Rioux, J.D.; Silverberg, M.S.; Daly, M.J.; Steinhart, A.H.; Abraham, C.; Regueiro, M.; Griffiths, A.; et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006, 314, 1461–1463.
- Sudabeh Alatab, S.G.S. Kevin Ikuta, Homayoon Vahedi, Catherine Bisignano. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 5, 17–30.
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621.
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517.
- Ng, S.C. Epidemiology of inflammatory bowel disease: Focus on Asia. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 363–372.
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.
- Corridoni, D.; Arseneau, K.O.; Cominelli, F. Inflammatory bowel disease. Immunol. Lett. 2014, 161, 231–235.
- Walfish, A.; Sachar, D. Phenotype classification in IBD: Is there an impact on therapy? Inflamm. Bowel Dis. 2007, 13, 1573–1575.
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10.
- Van Limbergen, J.; Russell, R.K.; Nimmo, E.R.; Satsangi, J. The genetics of inflammatory bowel disease. Am. J. Gastroenterol. 2007, 102, 2820–2831.
- Zhao, Y.; Guo, Q.; Zhu, Q.; Tan, R.; Bai, D.; Bu, X.; Lin, B.; Zhao, K.; Pan, C.; Chen, H.; et al. Flavonoid VI-16 protects against DSS-induced colitis by inhibiting Txnip-dependent NLRP3 inflammasome activation in macrophages via reducing oxidative stress. Mucosal Immunol. 2019, 12, 1150–1163.
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55.
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106.
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786.
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006, 54, 6314–6321.
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706.
- Geyikoglu, F.; Yilmaz, E.G.; Erol, H.S.; Koc, K.; Cerig, S.; Ozek, N.S.; Aysin, F. Hepatoprotective Role of Thymol in Drug-Induced Gastric Ulcer Model. Ann. Hepatol. 2018, 17, 980–991.
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of gamma-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314.
- Ocaña, A.; Reglero, G. Effects of Thyme Extract Oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on Cytokine Production and Gene Expression of oxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 104706.
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Cavaleiro, C.; Custódio, J.B.; Sousa Mdo, C. Anti-Giardia activity of phenolic-rich essential oils: Effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitol. Res. 2010, 106, 1205–1215.
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190.
- Karousou, R.; Koureas, D.N.; Kokkini, S. Essential oil composition is related to the natural habitats: Coridothymus capitatus and Satureja thymbra in NATURA 2000 sites of Crete. Phytochemistry 2005, 66, 2668–2673.
- Maulidiani, M.; Sheikh, B.Y.; Mediani, A.; Wei, L.S.; Ismail, I.S.; Abas, F.; Lajis, N.H. Differentiation of Nigella sativa seeds from four different origins and their bioactivity correlations based on NMR-metabolomics approach. Phytochem. Lett. 2015, 13, 308–318.
- Laquale, S.; Avato, P.; Argentieri, M.P.; Bellardi, M.G.; D’Addabbo, T. Nematotoxic activity of essential oils from Monarda species. J. Pest. Sci. 2018, 91, 1115–1125.
- Nieddu, M.; Rassu, G.; Boatto, G.; Bosi, P.; Trevisi, P.; Giunchedi, P.; Carta, A.; Gavini, E. Improvement of thymol properties by complexation with cyclodextrins: In vitro and in vivo studies. Carbohydr. Polym. 2014, 102, 393–399.
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290.
- Sedaghat Doost, A.; Van Camp, J.; Dewettinck, K.; Van der Meeren, P. Production of thymol nanoemulsions stabilized using Quillaja Saponin as a biosurfactant: Antioxidant activity enhancement. Food Chem. 2019, 293, 134–143.
- Colombo, M.; Priori, D.; Gandolfi, G.; Boatto, G.; Nieddu, M.; Bosi, P.; Trevisi, P. Effect of free thymol on differential gene expression in gastric mucosa of the young pig. Animal 2014, 8, 786–791.
- Pisarčíková, J.; Oceľová, V.; Faix, Š.; Plachá, I.; Calderón, A.I. Identification and quantification of thymol metabolites in plasma, liver and duodenal wall of broiler chickens using UHPLC-ESI-QTOF-MS. Biomed. Chromatogr. 2017, 31, e3881.
- Lugarà, R.; Grześkowiak, Ł.; Zentek, J.; Meese, S.; Kreuzer, M.; Giller, K. A High-Energy Diet and Spirulina Supplementation during Pre-Gestation, Gestation, and Lactation do Not Affect the Reproductive and Lactational Performance of Primiparous Sows. Animals 2022, 12, 1171.
- Pandur, E.; Micalizzi, G.; Mondello, L.; Horvath, A.; Sipos, K.; Horvath, G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants 2022, 11, 1330.
- Ahmed, O.M.; Galaly, S.R.; Mostafa, M.M.A.; Eed, E.M.; Ali, T.M.; Fahmy, A.M.; Zaky, M.Y. Thyme Oil and Thymol Counter Doxorubicin-Induced Hepatotoxicity via Modulation of Inflammation, Apoptosis, and Oxidative Stress. Oxid. Med. Cell. Longev. 2022, 2022, 6702773.
- Yin, L.; Liang, C.; Wei, W.; Huang, S.; Ren, Y.; Geng, Y.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; et al. The Antibacterial Activity of Thymol Against Drug-Resistant Streptococcus iniae and Its Protective Effect on Channel Catfish (Ictalurus punctatus). Front. Microbiol. 2022, 13, 914868.
- Yao, Z.; Feng, L.; Zhao, Y.; Zhang, X.; Chen, L.; Wang, L.; Zhang, Y.; Sun, Y.; Zhou, T.; Cao, J. Thymol Increases Sensitivity of Clinical Col-R Gram-Negative Bacteria to Colistin. Microbiol. Spectr. 2022, 10, e0018422.
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823.
- Hassan, H.F.H.; Mansour, A.M.; Salama, S.A.; El-Sayed, E.M. The chemopreventive effect of thymol against dimethylhydrazine and/or high fat diet-induced colon cancer in rats: Relevance to NF-κB. Life Sci. 2021, 274, 119335.
- Mendes, S.S.; Bomfim, R.R.; Jesus, H.C.; Alves, P.B.; Blank, A.F.; Estevam, C.S.; Antoniolli, A.R.; Thomazzi, S.M. Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. J. Ethnopharmacol. 2010, 129, 391–397.
- Mohammadi, A.; Mahjoub, S.; Ghafarzadegan, K.; Nouri, H.R. Immunomodulatory effects of Thymol through modulation of redox status and trace element content in experimental model of asthma. Biomed. Pharmacother. 2018, 105, 856–861.
- Raghuvanshi, D.S.; Verma, N.; Singh, S.V.; Khare, S.; Pal, A.; Negi, A.S. Synthesis of thymol-based pyrazolines: An effort to perceive novel potent-antimalarials. Bioorg. Chem. 2019, 88, 102933.
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219.
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305.
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201.
- Mueller, K.; Blum, N.M.; Mueller, A.S. Examination of the Anti-Inflammatory, Antioxidant, and Xenobiotic-Inducing Potential of Broccoli Extract and Various Essential Oils during a Mild DSS-Induced Colitis in Rats. ISRN Gastroenterol. 2013, 2013, 710856.
- Wang, Y.; Yang, Z.; Zhou, Y.; Tan, J.; Sun, H.; Sun, D.; Mu, Y.; Peng, J.; Wei, H. Effects of different amino acid levels and a carvacrol-thymol blend on growth performance and intestinal health of weaned pigs. J. Anim. Sci. Biotechnol. 2022, 13, 22.
- Fernández-Tomé, S.; Ortega Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 10224.
- Ribeiro, A.R.S.; Diniz, P.B.F.; Pinheiro, M.S.; Albuquerque-Júnior, R.L.C.; Thomazzi, S.M. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, ATP-sensitive K+ channels, and gastric mucus secretion. Chem. Biol. Interact. 2016, 244, 121–128.
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol Improves Barrier Function and Attenuates Inflammatory Responses in Porcine Intestinal Epithelial Cells during Lipopolysaccharide (LPS)-Induced Inflammation. J. Agric. Food Chem. 2019, 67, 615–624.
- Putaala, H.; Nurminen, P.; Tiihonen, K. Effects of cinnamaldehyde and thymol on cytotoxicity, tight junction barrier resistance, and cyclooxygenase-1 and -2 expression in Caco-2 cells. J. Anim. Sci. 2017, 26, 274–284.
- Yin, D.; Du, E.; Yuan, J.; Gao, J.; Wang, Y.; Aggrey, S.E.; Guo, Y. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci. Rep. 2017, 7, 7334.
- Choy, M.C.; Visvanathan, K.; Cruz, P.D. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2–13.
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336, 1314–1317.
- Roy, U.; Gálvez, E.J.C.; Iljazovic, A.; Lesker, T.R.; Błażejewski, A.J.; Pils, M.C.; Heise, U.; Huber, S.; Flavell, R.A.; Strowig, T. Distinct Microbial Communities Trigger Colitis Development upon Intestinal Barrier Damage via Innate or Adaptive Immune Cells. Cell. Rep. 2017, 21, 994–1008.
- Natarajan, K.; Abraham, P.; Kota, R.; Isaac, B. NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food. Chem. Toxicol. 2018, 118, 766–783.
- Salim, S.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381.
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565.
- Peng, Y.; Zhang, X.; Zhang, T.; Grace, P.M.; Li, H.; Wang, Y.; Li, H.; Chen, H.; Watkins, L.R.; Hutchinson, M.R.; et al. Lovastatin inhibits Toll-like receptor 4 signaling in microglia by targeting its co-receptor myeloid differentiation protein 2 and attenuates neuropathic pain. Brain Behav. Immun. 2019, 82, 432–444.
- Khazdair, M.R.; Ghorani, V.; Alavinezhad, A.; Boskabady, M.H. Pharmacological effects of Zataria multiflora Boiss L. and its constituents focus on their anti-inflammatory, antioxidant, and immunomodulatory effects. Fundam. Clin. Pharmacol. 2018, 32, 26–50.
- Gholijani, N.; Gharagozloo, M.; Farjadian, S.; Amirghofran, Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol. 2016, 13, 157–164.
- Jin, B.R.; Chung, K.S.; Cheon, S.Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.J.; An, H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7, 46252.
- Chamanara, M.; Abdollahi, A.; Rezayat, S.M.; Ghazi-Khansari, M.; Dehpour, A.; Nassireslami, E.; Rashidian, A. Thymol reduces acetic acid-induced inflammatory response through inhibition of NF-kB signaling pathway in rat colon tissue. Inflammopharmacology 2019, 27, 1275–1283.
- Liu, D.-M.; Zhou, C.-Y.; Meng, X.-L.; Wang, P.; Li, W. Thymol exerts anti-inflammatory effect in dextran sulfate sodium-induced experimental murine colitis. Trop.J. Pharm. Res. 2018, 17, 1803–1810.
- Tahmasebi, P.; Abtahi Froushani, S.M.; Afzale Ahangaran, N. Thymol has beneficial effects on the experimental model of ulcerative colitis. Avicenna J. Phytomed. 2019, 9, 538–550.
- Waetzig, G.H.; Seegert, D.; Rosenstiel, P.; Nikolaus, S.; Schreiber, S. p38 Mitogen-Activated Protein Kinase Is Activated and Linked to TNF-α Signaling in Inflammatory Bowel Disease. J. Immunol. 2002, 168, 5342–5351.
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222.
- Chen, J.; Li, D.-L.; Xie, L.-N.; Ma, Y.-R.; Wu, P.-P.; Li, C.; Liu, W.-F.; Zhang, K.; Zhou, R.-P.; Xu, X.-T.; et al. Synergistic anti-inflammatory effects of silibinin and thymol combination on LPS-induced RAW264.7 cells by inhibition of NF-κB and MAPK activation. Phytomedicine 2020, 78, 153309.
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118.
- Namdari, H.; Izad, M.; Rezaei, F.; Amirghofran, Z. Thymol as a reciprocal regulator of T cell differentiation: Promotion of regulatory T cells and suppression of Th1/Th17 cells. Int. Immunopharmacol. 2019, 67, 417–426.
- Gholijani, N.; Amirghofran, Z. Effects of thymol and carvacrol on T-helper cell subset cytokines and their main transcription factors in ovalbumin-immunized mice. J. Immunotoxicol. 2016, 13, 729–737.
- Amirghofran, Z.; Ahmadi, H.; Karimi, M.H.; Kalantar, F.; Gholijani, N.; Malek-Hosseini, Z. In vitro inhibitory effects of thymol and carvacrol on dendritic cell activation and function. Pharm. Biol. 2016, 54, 1125–1132.
