Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Rosalyn Abbott.
Despite developing prenatally, the adipose tissue is unique in its ability to undergo drastic growth even after reaching its mature size. ThProper adis pose tissue development and subsequent maintenance rely on the properrelies on tightly regulated processes that require careful coordination and cooperation between the vascular nichemany different cell types and the adipose compartmentir matrix cues.
- white adipose tissue
- brown adipose tissue
- angiogenesis
- adipogenesis
- extracellular matrix remodeling
- adipose development
- mechanics
- paracrine signaling
- proteolysis
- cell shape
1. Introduction
While classically viewed as merely a storage depot, adipose tissue (fat) has many roles and is classified as a connective, thermogenic, and endocrine organ. Healthy adipose tissue is required to regulate systemic energy homeostasis, modulate inflammatory responses, store and metabolize steroids, protect internal organs, and maintain body temperature (both as an insulating organ and through non-shivering thermogenesis) [1,2,3,4,5,6][1][2][3][4][5][6]. Proper adipose tissue development relies on tightly regulated processes that require careful coordination and cooperation between many different cell types and their matrix cues.
Full-term human infants are composed of 11 to 28% fat [7], which is classified as either brown or white fat. Brown fat, which fully develops and matures prenatally, plays a pertinent role in neonatal thermal regulation, as it contains ample mitochondria to convert the many, small lipids stored in the brown multilocular adipocytes into thermal energy [8]. However, brown adipose tissue proceeds from being 5% of the human body mass at birth to a mere 1.5% of the adult human mass and is replaced by white adipose tissue as the primary adipose mass [9,10][9][10]. On the other hand, white adipose tissue fluctuates in mass throughout both prepubescent and adult life. White adipose tissue serves as the primary energy reservoir in the human body, with approximately 90% being classified as subcutaneous (underneath the skin) and 10% being visceral (surrounding the internal organs/viscera), although there have been quantitative differences shown to be dependent on sex, menopausal state, and disease state [11,12,13][11][12][13].
White adipose tissue is a heterogenous mixture of cells, with mature adipocytes being the primary metabolic component and accounting for approximately 90% of the tissue’s volume [14,15][14][15]. These mature adipocytes arise from precursor preadipocytes that exist alongside many other cells, including adipose stem cells, stromal cells, endothelial cells, and resident immune cells. Despite taking up only 10% of the tissue’s space, these cells, termed stromal vascular cells, play an important role in regulating vascular innervation and the immune response within the tissue [16]. The proper development of white adipose tissue relies on highly coordinated spatial and temporal communication amongst all of these cells and their microenvironment. There are several reviews published that focus on the interplay between adipose and endothelial cells during obesity-driven adipogenesis and angiogenesis [17,18][17][18]. However, the process of adipose organogenesis relies not only on cellular crosstalk but also on cellular self-assembly, which is highly dependent upon both extracellular and intracellular forces in order to control the cell’s fate, matrix remodeling and mechanics, and the development of tissue patterns [19]. There is accumulating evidence indicating the importance of mechanics given that it affects fundamental factors of proliferation, migration, and differentiation [20,21,22,23][20][21][22][23]. With the bulk modulus of adipose tissues being one of the lowest in the body, around 0.5–1 kPa [24], proper cell fate relies on controlled proliferation and differentiation amidst softer materials. Furthermore, with adipose tissue being physiologically exposed to a range of bulk physiological forces (compressive, tensile, and shear) due to body weight [25[25][26],26], there must be adaptive modalities on a cellular level to maintain bulk tissue health and integrity.
2. Adipose Tissue Remodeling during Fetal Development
2.1. Ultrastructural Changes during Adipose Tissue Development
Early studies piloted by Hausman et al. and Poissonet et al. described the complexities of fetal subcutaneous white adipose tissue development using early microscopical and immunohistochemical techniques [27,28,29,30,31,32,33,34,35][27][28][29][30][31][32][33][34][35]. Collectively, these studies describe fetal adipose tissue growth through five defined morphometric stages, as shown in Figure 1. Notably, these developmental stages are highly dependent upon primitive matrix deposition and vessel outgrowth and organization.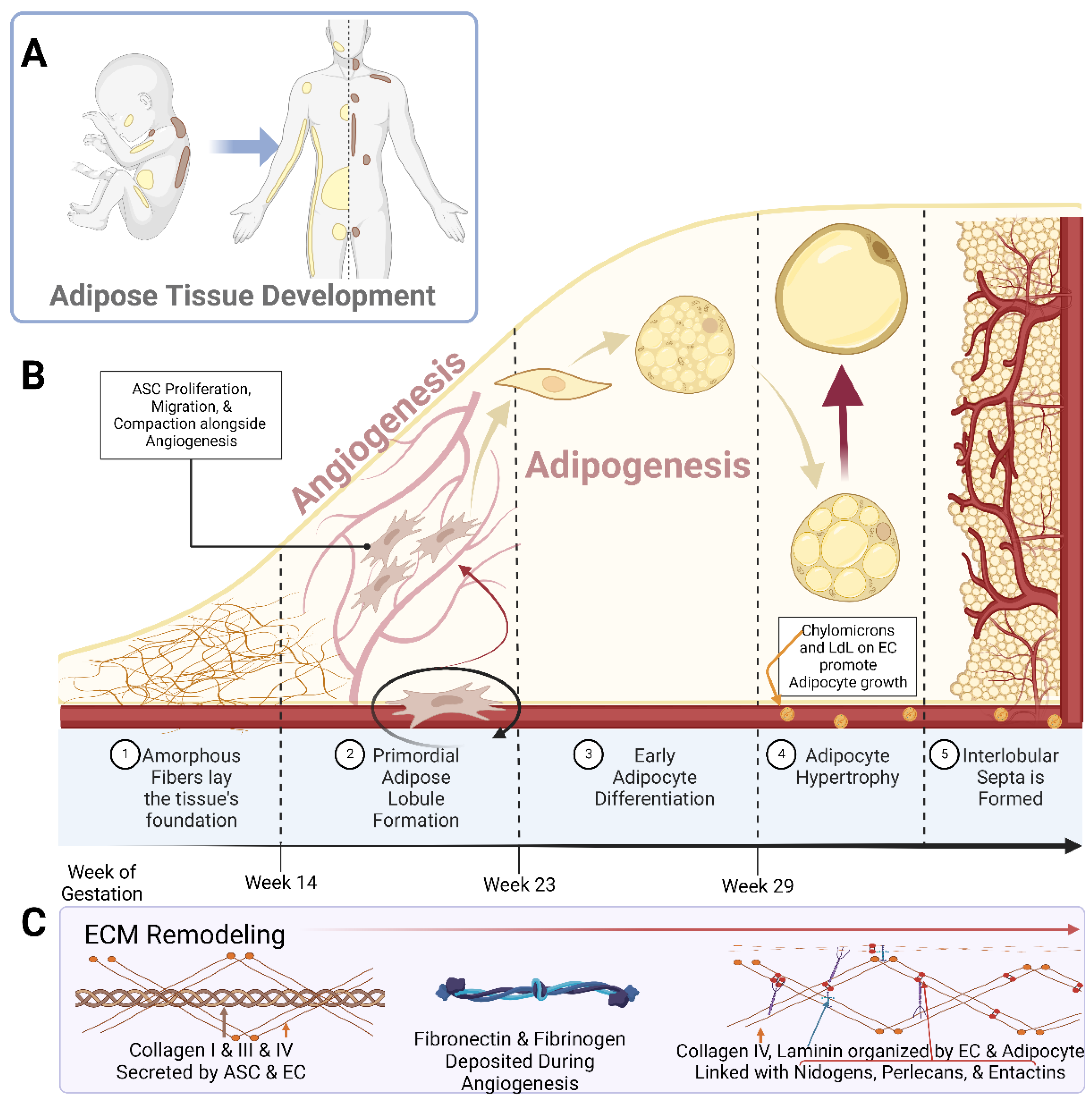
Figure 1. Schematic representations of (A) the localization of white adipose tissue (yellow) and brown adipose tissue (brown) in a developing neonate and adult human; (B) the breakdown of adipose tissue development into the 5 specified stages; (C) the prominent Extracellular Matrix (ECM) components over the course of adipose tissue development. Abbreviations: adipose stem cell (ASC), low-density lipoprotein (LdL), and endothelial cell (EC). Created with Biorender.com.
2.2. Remodeling during Angiogenesis and Adipogenesis
2.2.1. Angiogenesis
In embryonic development, the main vasculature of the human body is formed through de novo vasculogenesis, whereby stem cells undergo differentiation towards endothelial progenitor cells, also known as angioblasts, and then towards endothelial cells to form tubule structures [38]. However, in specified organogenesis, vessels branch off of existing vasculature through controlled sprouting or non-sprouting angiogenesis (Figure 2), with the assistance of existing mesenchymal cells [39]. As previously discussed, the primitive vasculature in adipose tissue has developed prior to week 14 [27,28][27][28]. In order for angiogenesis to occur, matrix metalloproteinases (MMP) must first break down the basement membrane that is currently supporting the vessel. In these early stages, MMP2, MMP3, and MMP9 tend to start this process allowing for the release of fibrinogen and fibronectin, which lay the provisional foundation for the migration of additional endothelial cells [40,41,42,43,44][40][41][42][43][44]. Chemotactic signals, such as vascular endothelial growth factor (VEGF), encourage the proliferation and migration of existing endothelial cells towards the prepared void [45,46,47][45][46][47]. Furthermore, platelet-derived growth factor-β (PDGF-β) recruits pericytes/smooth muscle cells to stabilize the vessel [45]. From then on, transforming growth factor beta (TGF-β) stimulates the differentiation of mesenchymal stem cells to fibroblasts and mural cells in order to increase extracellular matrix (ECM) production to promote vessel maturation [40,48,49][40][48][49]. While the general composition of the vessel’s basement membrane is rather consistent: Laminin and Type IV Collagen linked with Nidogen and Perlecans [35,50[35][50][51],51], the isoform profile and quantity of each ECM will be unique to the specification of the vessel, whether it be arterial, venule, or capillary. For example, Laminin α4 exists on all vessels while Laminin α5 is more exclusive to capillaries [39,40][39][40]. Furthermore, Laminin 10 is present at birth, but gradually, it is replaced with Laminin 8 [39], showing that not only does each cell type have a unique matrix signature but also that this signature changes over time.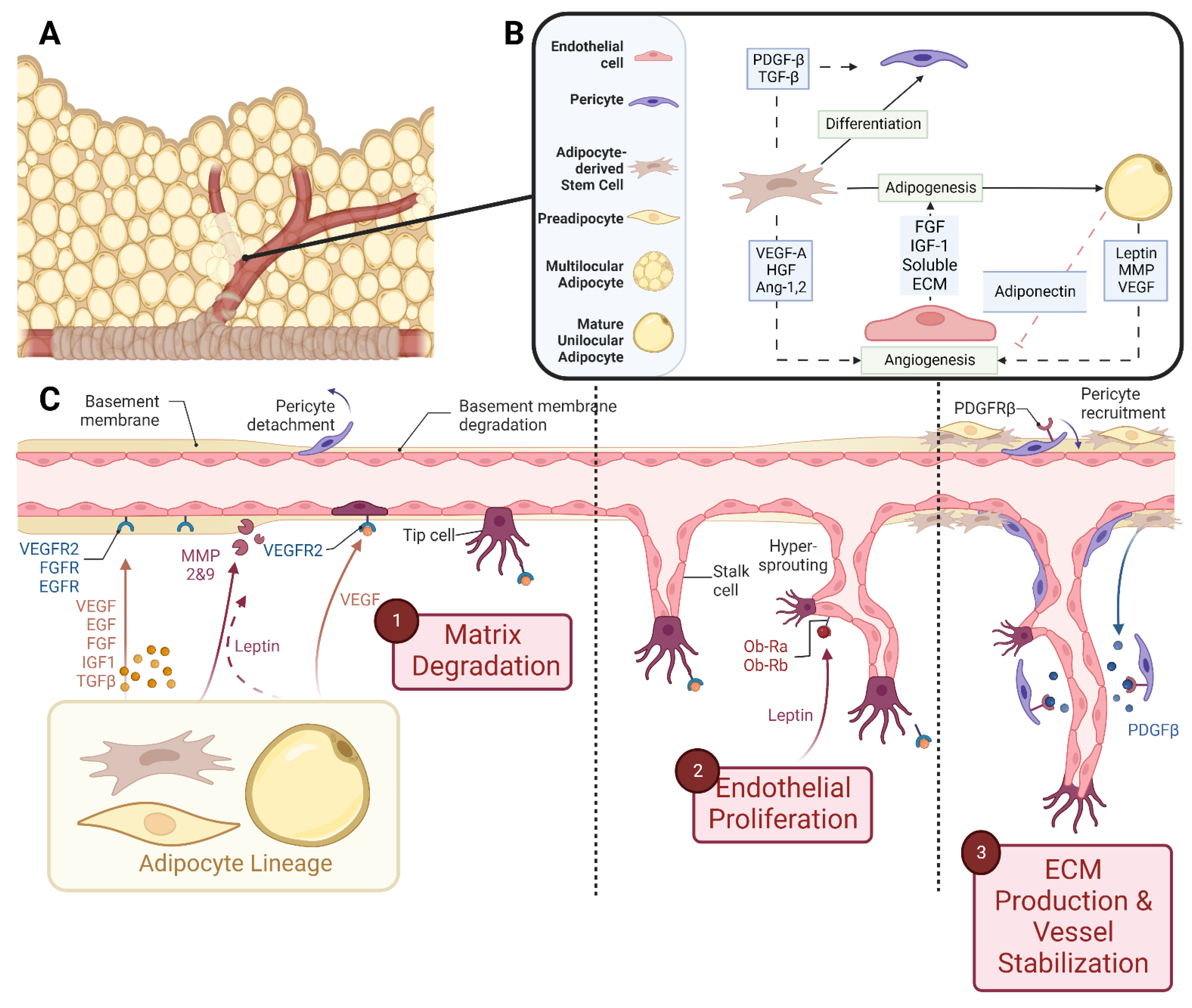
Figure 2. Schematic representations of (A) vascularized adipose tissue and (B) paracrine interactions between both the vascular and adipose lineages. (C) Stepwise breakdown of cellular and paracrine activity during angiogenesis. Abbreviations: Platelet-derived growth factor-β (PDGF-β), transforming growth factor-β (TGF-β), vascular endothelial growth factor-A (VEGF-A), hepatocyte growth factor (HGF), angiopoietin-1,2 (Ang-1,2), fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF-1), extracellular matrix (ECM), and matrix metalloproteinase (MMP). Created with Biorender.com.
2.2.2. Adipogenesis
Adipogenesis is a highly regulated, dynamic process whereby adipose stem cells undergo stepwise differentiation towards an adipocyte. One notable change in white adipocyte development includes the gradual morphological change from the fibroblastic shape to a multilocular adipocyte and then finally a spherical unilocular adipocyte, designed to store triglycerides efficiently and effectively [52]. Throughout this adipogenic process, the cells experience alterations in their ECM secretions and network organization, which ultimately affect the rigidity of the environment [53,54][53][54]. Antras et al. found that during the differentiation of adipocytes, there was an obvious slowdown and halt in the synthesis of actin and other cytoskeletal proteins to accommodate the expansion and accumulation of lipid droplets intracellularly [55]. One notable change in the ECM profile of adipose-derived stem cells (ASCs) cultured in growth media versus differentiation media is the gradual loss of fibronectin and increase in the secretion of laminin of ASCs undergoing adipogenesis [56]. Several studies have shown that the utilization of decellularized matrix from differentiated adipocytes increased the adipogenic potential of ASCs when compared to the adipogenic potential of stem cells cultured with the decellularized matrix of ASCs grown in growth media, indicating that the matrix contains adipogenic cues [57,58][57][58]. Considering their pre-established commitment to the adipocyte lineage, 3T3-L1 cells are a common model to study adipogenesis. In their undifferentiated state, 3T3-L1 cells have a fibroblast-like morphology and secretome [59]. It has been shown that these fibroblasts primarily secrete fibrillar Type I and III Collagen when exogenously stimulated with ascorbic acid [60,61][60][61]. In addition to their usage as an adipogenic model, this ability to secrete fibrous matrix has previously made them very beneficial as feeder cells to more fragile cells [62,63][62][63]. In these adipogenic models, there is an early peak of fibrillar proteins (Types I, II, and III collagen) by day 4 of differentiation, followed by a transient decline that is offset by a gradual increase in basement membrane proteins, including Type IV Collagen, Laminin, and Entactin [64,65,66,67,68,69][64][65][66][67][68][69].3. Cellular Crosstalk and Paracrine Signaling during Simultaneous Adipogenesis and Angiogenesis
It is apparent that there is a macrostructural dependence between developing adipocytes and their vasculature, with the vasculature defining the architecture of the lobule and supplying the necessary materials to promote adipogenesis [31,32][31][32]. However, there are also paracrine and contact-dependent interactions that promote the development of vascularized adipose tissue. It is known that ASCs, which are mesenchymal stem cells not yet committed to the adipocyte lineage, have the ability to differentiate into multiple cell lineages, including adipocytes (white or brown) and endothelial cells [70,71,72,73][70][71][72][73]. There has been previous histological evidence to suggest that adipocytes and adipose-derived endothelial cells share a common ancestor residing in the vascular niche [34,74,75][34][74][75]. This common precursor was verified and defined by Tang et al. through the utilization of a Green Fluorescent Protein (GFP)-Peroxisome Proliferator-activated Receptor Gamma (PPAR- γ) mouse model [76]. This model suggested that there are PPAR-γ positive cells lining the vasculature that also express Smooth Muscle Actin (SMA), PDGF-β, and neuron-glial antigen 2 (NG2), indicating that they are of perivascular origin [76,77][76][77]. After isolation, it was shown that these cells took up Bromodeoxyuridine (BrdU), showing their proliferative capacity, while also having immense adipogenic potential [76,78,79][76][78][79]. Together, these findings demonstrate that many adipocytes are derived from progenitors that reside within the mural cells’ compartment, surrounding the vasculature [76,77][76][77]. However, there are many regulators that can determine the fate and function of these progenitor cells, including paracrine or mechanical signals. It should be firstly noted that there is immense crosstalk within the stromal vascular fraction (SVF) that influences both adipogenesis and angiogenesis. One prominent stromal cell, the fibroblast, has demonstrated its importance in adipose tissue development and maintenance. Fibroblast Specific Protein-1 (FSP-1)+ fibroblasts reside adjacent to preadipocytes and regulate PDGF signaling and MMP expression in order to promote adipogenesis [80]. As regulators of matrix construction, these stromal cells also support angiogenesis [81]. ASCs from the SVF promote endothelial colony-forming cell proliferation and differentiation by secreting proangiogenic factors such as VEGF [16,82][16][82]. Moreover, those stem cells can then differentiate into pericytes to stabilize the newly formed vessel structure [82]. When in indirect coculture, ASCs release VEGF and Angiopoietin 1 and 2 in order to promote the tubule formation of endothelial progenitor cells which then upregulate their expression of Tunica Interna Endothelial Cell Kinase 2 (TIE2) and VEGFR1. In this sentudry, VEGFR2 was not detected, suggesting a more prominent role in Angiopoeitin-TIE2-mediated angiogenesis in endothelial progenitor cells [83]. Endothelial cells within the SVF are also a source of mitogenic and adipogenic components. Conditioned medium from microvascular endothelial cell cultures promoted the proliferation of preadipocytes [84]. It is likely that this media contained heparin-binding Fibroblast Growth Factor (FGF) and Insulin-like Growth Factor-1 (IGF-1), as shown in Figure 2 [85,86][85][86]. Furthermore, when endothelial cell-derived ECM components, including Fibronectin, Laminin, and Collagen IV, were applied as soluble entities to differentiating preadipocytes in vitro, there was a four-fold increase in triglyceride accumulation, indicating that a strong paracrine signal from endothelial cells is communicated via the ECM that they produce [51]. Fukumura et al. used an in vivo fat pad formation murine model in conjunction with an in vitro preadipocyte differentiation assay to monitor simultaneous angiogenesis and adipogenesis as well as unravel the intricacies of the paracrine signaling existing between preadipocytes and endothelial cells [87]. When PPAR-γ is inhibited in vivo, 3T3 cells remained undifferentiated, which corresponded to a reduction in vessel infiltration. Furthermore, when VEGFR2 was blocked, there was a reduction in both angiogenesis and 3T3-F442A differentiation. It was found that this was due to the paracrine interaction between endothelial cells and preadipocytes, which is dependent upon endothelial treatments with VEGF, as the treatment with VEGF and a VEGF blocker on preadipocytes did not directly affect differentiation in vitro [87]. This sentudry highlights the importance of cell-specific paracrine signaling in simultaneous adipogenesis and angiogenesis. In embryonic development and prior to the maturation of white adipocytes, preadipocytes take on a hormonal role and secrete adipokines, including adiponectin [88]. These secretions have been shown to have an imperative paracrine effect in assisting angiogenesis [89]. Leptin, another adipose hormone known to play a regulatory role in satiety, has displayed an important role in regulating fetal growth and organogenesis [90], although its angiogenic implications remain unclear. In vitro studies with human umbilical vein endothelial cells (HUVECs) and porcine aortic endothelial cells demonstrated that HUVECs displayed Ob-Ra and Ob-Rb, two isoforms of the Leptin receptor, and responded to Leptin by increasing their proliferative rate and forming capillary-like tubes when in Fibrin gels [91]. This functionality was attributed to the activation of the Mitogen-activated Protein Kinase (MAPK) pathway [91,92][91][92]. Other studies have shown the Leptin-mediated upregulation of angiogenic factors FGF-2 and VEGF, further supporting this pro-angiogenic response [93,94][93][94]. However, more recently, a study reported that metreleptin, a recombinant leptin analog, does not induce endothelial sprouting in a 3D gel and it also does not affect circulating angiogenic factor levels [89,95][89][95]. Additional research shows that adipocyte-derived leptin induces endothelial apoptosis through Ang-2, but angiogenesis occurs through VEGF and FGF-2 (also produced by adipocytes) [93,96,97][93][96][97], demonstrating situationally dependent responses.References
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949.
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 1–30.
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104.
- Gregory, E.L. Thermoregulatory aspects of adipose tissue. Clin. Dermatol. 1989, 7, 78–92.
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44.
- Widdowson, E.M. Chemical composition of newly born mammals. Nature 1950, 166, 626–628.
- Gilsanz, V.; Smith, M.L.; Goodarzian, F.; Kim, M.; Wren, T.A.L.; Hu, H.H. Changes in Brown Adipose Tissue in Boys and Girls during Childhood and Puberty. J. Pediatr. 2012, 160, 604–609.e1.
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654.
- Symonds, M.E.; Lomax, M.A. Maternal and environmental influences on thermoregulation in the neonate. Proc. Nutr. Soc. 1992, 51, 165–172.
- Harrington, T.A.M.; Thomas, E.L.; Modi, N.; Frost, G.; Coutts, G.A.; Bell, J.D. Fast and reproducible method for the direct quantitation of adipose tissue in newborn infants. Lipids 2002, 37, 95–100.
- Harrington, T.A.M.; Thomas, E.L.; Frost, G.; Modi, N.; Bell, J.D. Distribution of Adipose Tissue in the Newborn. Pediatr. Res. 2004, 55, 437–441.
- Mittal, B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J. Med. Res. 2019, 149, 571–573.
- Sun, M.; He, Y.; Zhou, T.; Zhang, P.; Gao, J.; Lu, F. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel Secretes Angiogenic Factors and Enhances Skin Wound Healing in a Murine Model. BioMed Res. Int. 2017, 2017, 3105780.
- Yoshimura, K.; Suga, H.; Eto, H. Adipose-derived stem/progenitor cells: Roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen. Med. 2009, 4, 265–273.
- Sun, Y.; Chen, S.; Zhang, X.; Pei, M. Significance of Cellular Cross-Talk in Stromal Vascular Fraction of Adipose Tissue in Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1034–1044.
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2021, 11, 1–9.
- Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013, 18, 478–489.
- Mammoto, T.; Mammoto, A.; Ingber, D.E. Mechanobiology and Developmental Control. Annu. Rev. Cell Dev. Biol. 2013, 29, 27–61.
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689.
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling Self-Renewal and Differentiation of Stem Cells via Mechanical Cues. J. Biomed. Biotechnol. 2012, 2012, 797410.
- Zhang, Z.; Cai, J.; Li, Y.; He, Y.; Dong, Z.; Dai, J.; Lu, F. External Volume Expansion Adjusted Adipose Stem Cell by Shifting the Ratio of Fibronectin to Laminin. Tissue Eng. Part A 2020, 26, 66–77.
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15.
- Linder-Ganz, E.; Shabshin, N.; Itzchak, Y.; Gefen, A. Assessment of mechanical conditions in sub-dermal tissues during sitting: A combined experimental-MRI and finite element approach. J. Biomech. 2007, 40, 1443–1454.
- Slomka, N.; Or-Tzadikario, S.; Sassun, D.; Gefen, A. Membrane-stretch-induced cell death in deep tissue injury: Computer model studies. Cell. Mol. Bioeng. 2009, 2, 118–132.
- Poissonnet, C.M.; Burdi, A.R.; Bookstein, F.L. Growth and development of human adipose tissue during early gestation. Early Hum. Dev. 1983, 8, 1–11.
- Poissonnet, C.M.; Burdi, A.R.; Garn, S.M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 1984, 10, 1–11.
- Hausman, G.J.; Martin, R.J. Subcutaneous Adipose Tissue Development in Yorkshire (Lean) and Ossabaw (Obese) Pigs. J. Anim. Sci. 1981, 52, 1442–1449.
- Hausman, G.J.; Richardson, L.R. Histochemical and Ultrastructural Analysis of Developing Adipocytes in the Fetal Pig. Cells Tissues Organs 1982, 114, 228–247.
- Hausman, G.J.; Richardson, R.L. Cellular and vascular development in immature rat adipose tissue. J. Lipid Res. 1983, 24, 522–532.
- Hausman, G.J.; Thomas, G.B. Enzyme histochemical differentiation of white adipose tissue in the rat. Am. J. Anat. 1984, 169, 315–326.
- Hausman, G.J.; Thomas, G.B. Differentiation of blood vessels in the adipose tissue of lean and obese fetal pigs, studied by differential enzyme histochemistry. Acta Anat. 1985, 123, 137–144.
- Wright, J.T.; Hausman, G.J. Adipose tissue development in the fetal pig examined using monoclonal antibodies. J. Anim. Sci. 1990, 68, 1170–1175.
- Hausman, G.J.; Wright, J.T.; Thomas, G.B. Vascular and cellular development in fetal adipose tissue: Lectin binding studies and immunocytochemistry for laminin and type IV collagen. Microvasc. Res. 1991, 41, 111–125.
- Hausman, G.J.; Wright, J.T. Cytochemical studies of adipose tissue-associated blood vessels in untreated and thyroxine-treated hypophysectomized pig fetuses. J. Anim. Sci. 1996, 74, 354–362.
- Estève, D.; Boulet, N.; Belles, C.; Zakaroff-Girard, A.; Decaunes, P.; Briot, A.; Veeranagouda, Y.; Didier, M.; Remaury, A.; Guillemot, J.C.; et al. Lobular architecture of human adipose tissue defines the niche and fate of progenitor cells. Nat. Commun. 2019, 10, 2549.
- Asahara, T.; Kawamoto, A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 2004, 287, C572–C579.
- Hallmann, R.; Horn, N.; Selg, M.; Wendler, O.; Pausch, F.; Sorokin, L.M. Expression and Function of Laminins in the Embryonic and Mature Vasculature. Physiol. Rev. 2005, 85, 979–1000.
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693.
- Cleaver, O.; Melton, D.A. Endothelial signaling during development. Nat. Med. 2003, 9, 661–668.
- Bouloumié, A.; Sengenès, C.; Portolan, G.; Galitzky, J.; Lafontan, M. Adipocyte produces matrix metalloproteinases 2 and 9: Involvement in adipose differentiation. Diabetes 2001, 50, 2080–2086.
- Bourlier, V.; Zakaroff-Girard, A.; De Barros, S.; Pizzacalla, C.; de Saint Front, V.D.; Lafontan, M.; Bouloumié, A.; Galitzky, J. Protease inhibitor treatments reveal specific involvement of matrix metalloproteinase-9 in human adipocyte differentiation. J. Pharm. Exp. 2005, 312, 1272–1279.
- Lijnen, H.R.; Maquoi, E.; Demeulemeester, D.; Van Hoef, B.; Collen, D. Modulation of fibrinolytic and gelatinolytic activity during adipose tissue development in a mouse model of nutritionally induced obesity. Thromb. Haemost. 2002, 88, 345–353.
- Evensen, L.; Micklem, D.R.; Blois, A.; Berge, S.V.; Aarsæther, N.; Littlewood-Evans, A.; Wood, J.; Lorens, J.B. Mural Cell Associated VEGF Is Required for Organotypic Vessel Formation. PLoS ONE 2009, 4, e5798.
- Leung, D.W.; Cachianes, G.; Kuang, W.-J.; Goeddel, D.V.; Ferrara, N. Vascular Endothelial Growth Factor Is a Secreted Angiogenic Mitogen. Science 1989, 246, 1306–1309.
- Igarashi, J.; Erwin, P.A.; Dantas, A.P.V.; Chen, H.; Michel, T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 10664–10669.
- Pepper, M.S. Transforming growth factor-beta: Vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997, 8, 21–43.
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Rosendahl, A.; Sideras, P.; ten Dijke, P. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 2002, 21, 1743–1753.
- Laurie, G.W.; Leblond, C.P.; Martin, G.R. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J. Cell Biol. 1982, 95, 340–344.
- Varzaneh, F.E.; Shillabeer, G.; Wong, K.L.; Lau, D.C.W. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism 1994, 43, 906–912.
- Abuhattum, S.; Gefen, A.; Weihs, D. Ratio of total traction force to projected cell area is preserved in differentiating adipocytes. Integr. Biol. 2015, 7, 1212–1217.
- Luo, W.; Shitaye, H.; Friedman, M.; Bennett, C.N.; Miller, J.; Macdougald, O.A.; Hankenson, K.D. Disruption of cell-matrix interactions by heparin enhances mesenchymal progenitor adipocyte differentiation. Exp. Cell Res. 2008, 314, 3382–3391.
- Shoham, N.; Girshovitz, P.; Katzengold, R.; Shaked, N.T.; Benayahu, D.; Gefen, A. Adipocyte stiffness increases with accumulation of lipid droplets. Biophys. J. 2014, 106, 1421–1431.
- Antras, J.; Hilliou, F.; Redziniak, G.; Pairault, J. Decreased biosynthesis of actin and cellular fibronectin during adipose conversion of 3T3-F442A cells. Reorganization of the cytoarchitecture and extracellular matrix fibronectin. Biol. Cell 1989, 66, 247–254.
- Rodríguez Fernández, J.L.; Ben-Ze’ev, A. Regulation of fibronectin, integrin and cytoskeleton expression in differentiating adipocytes: Inhibition by extracellular matrix and polylysine. Differentiation 1989, 42, 65–74.
- Chen, Y.; Lee, K.; Chen, Y.; Yang, Y.; Kawazoe, N.; Chen, G. Preparation of Stepwise Adipogenesis-Mimicking ECM-Deposited PLGA–Collagen Hybrid Meshes and Their Influence on Adipogenic Differentiation of hMSCs. ACS Biomater. Sci. Eng. 2019, 5, 6099–6108.
- Zhang, Z.; Qu, R.; Fan, T.; Ouyang, J.; Lu, F.; Dai, J. Stepwise Adipogenesis of Decellularized Cellular Extracellular Matrix Regulates Adipose Tissue-Derived Stem Cell Migration and Differentiation. Stem. Cells Int. 2019, 2019, 1845926.
- Spiegelman, B.M.; Ginty, C.A. Fibronectin modulation of cell shape and lipogenic gene expression in 3t3-adipocytes. Cell 1983, 35, 657–666.
- Paganelli, A.; Benassi, L.; Rossi, E.; Magnoni, C. Extracellular matrix deposition by adipose-derived stem cells and fibroblasts: A comparative study. Arch. Dermatol. Res. 2020, 312, 295–299.
- Zhou, Z.Q.; Chen, Y.; Chai, M.; Tao, R.; Lei, Y.H.; Jia, Y.Q.; Shu, J.; Ren, J.; Li, G.; Wei, W.X.; et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose-derived stem cells into fibroblasts. Int. J. Mol. Med. 2019, 43, 890–900.
- Alitalo, K.; Kuismanen, E.; Myllylä, R.; Kiistala, U.; Asko-Seljavaara, S.; Vaheri, A. Extracellular matrix proteins of human epidermal keratinocytes and feeder 3T3 cells. J. Cell Biol. 1982, 94, 497–505.
- Ejaz, A.; Hatzmann, F.M.; Hammerle, S.; Ritthammer, H.; Mattesich, M.; Zwierzina, M.; Waldegger, P.; Zwerschke, W. Fibroblast feeder layer supports adipogenic differentiation of human adipose stromal/progenitor cells. Adipocyte 2019, 8, 178–189.
- Nakajima, I.; Yamaguchi, T.; Ozutsumi, K.; Aso, H. Adipose tissue extracellular matrix: Newly organized by adipocytes during differentiation. Differentiation 1998, 63, 193–200.
- Nakajima, I.; Muroya, S.; Tanabe, R.; Chikuni, K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biol. Cell 2002, 94, 197–203.
- Nakajima, I.; Muroya, S.; Tanabe, R.-i.; Chikuni, K. Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes. Differentiation 2002, 70, 84–91.
- Aratani, Y.; Kitagawa, Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J. Biol. Chem. 1988, 263, 16163–16169.
- Weiner, F.R.; Shah, A.; Smith, P.J.; Rubin, C.S.; Zern, M.A. Regulation of collagen gene expression in 3T3-L1 cells. Effects of adipocyte differentiation and tumor necrosis factor alpha. Biochemistry 1989, 28, 4094–4099.
- Wang, P.; Mariman, E.; Keijer, J.; Bouwman, F.; Noben, J.P.; Robben, J.; Renes, J. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. Cell. Mol. Life Sci. CMLS 2004, 61, 2405–2417.
- Wang, T.; Hill, R.C.; Dzieciatkowska, M.; Zhu, L.; Infante, A.M.; Hu, G.; Hansen, K.C.; Pei, M. Site-Dependent Lineage Preference of Adipose Stem Cells. Front. Cell Dev. Biol. 2020, 8, 1–16.
- Li, H.; Zimmerlin, L.; Marra, K.G.; Donnenberg, V.S.; Donnenberg, A.D.; Rubin, J.P. Adipogenic potential of adipose stem cell subpopulations. Plast. Reconstr. Surg. 2011, 128, 663–672.
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663.
- Zannettino, A.C.; Paton, S.; Arthur, A.; Khor, F.; Itescu, S.; Gimble, J.M.; Gronthos, S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J. Cell Physiol. 2008, 214, 413–421.
- Cinti, S.; Cigolini, M.; Bosello, O.; Björntorp, P. A morphological study of the adipocyte precursor. J. Submicrosc. Cytol. 1984, 16, 243–251.
- Iyama, K.; Ohzono, K.; Usuku, G. Electron microscopical studies on the genesis of white adipocytes: Differentiation of immature pericytes into adipocytes in transplanted preadipose tissue. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1979, 31, 143–155.
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science 2008, 322, 583–586.
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215.
- Castillo, G.; Hauser, S.; Rosenfield, J.K.; Spiegelman, B.M. Role and Regulation of PPARγ During Adipogenesis. J. Anim. Sci. 1999, 77, 9–15.
- Otto, T.C.; Lane, M.D. Adipose development: From stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 229–242.
- Zhang, R.; Gao, Y.; Zhao, X.; Gao, M.; Wu, Y.; Han, Y.; Qiao, Y.; Luo, Z.; Yang, L.; Chen, J.; et al. FSP1-positive fibroblasts are adipogenic niche and regulate adipose homeostasis. PLoS Biol. 2018, 16, e2001493.
- Bi, H.; Li, H.; Zhang, C.; Mao, Y.; Nie, F.; Xing, Y.; Sha, W.; Wang, X.; Irwin, D.M.; Tan, H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem. Cell Res. Ther. 2019, 10, 302.
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wąsiatycz, G.; Antosik, P.; et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790.
- Gan, F.; Liu, L.; Zhou, Q.; Huang, W.; Huang, X.; Zhao, X. Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis. PLoS ONE 2022, 17, e0261498.
- Hutley, L.J.; Herington, A.C.; Shurety, W.; Cheung, C.; Vesey, D.A.; Cameron, D.P.; Prins, J.B. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1037–E1044.
- Ramsay, T.G.; Rao, S.V.; Wolverton, C.K. In Vitro Systems for the Analysis of the Development of Adipose Tissue in Domestic Animals. J. Nutr. 1992, 122, 806–817.
- Crandall, D.L.; Hausman, G.J.; Kral, J.G. A Review of the Microcirculation of Adipose Tissue: Anatomic, Metabolic, and Angiogenic Perspectives. Microcirculation 1997, 4, 211–232.
- Fukumura, D.; Ushiyama, A.; Duda, D.G.; Xu, L.; Tam, J.; Krishna, V.; Chatterjee, K.; Garkavtsev, I.; Jain, R.K. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 2003, 93, e88–e97.
- Shao, M.; Hepler, C.; Vishvanath, L.; MacPherson, K.A.; Busbuso, N.C.; Gupta, R.K. Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol. Metab. 2017, 6, 111–124.
- Nigro, E.; Mallardo, M.; Polito, R.; Scialò, F.; Bianco, A.; Daniele, A. Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9. Int. J. Mol. Sci. 2021, 22, 7516.
- Briffa, J.F.; McAinch, A.J.; Romano, T.; Wlodek, M.E.; Hryciw, D.H. Leptin in pregnancy and development: A contributor to adulthood disease? Am. J. Physiol. Endocrinol. Metab. 2015, 308, E335–E350.
- Bouloumie, A.; Drexler, H.C.; Lafontan, M.; Busse, R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 1998, 83, 1059–1066.
- Sierra-Honigmann, M.R.o.; Nath Anjali, K.; Murakami, C.; García-Cardeña, G.; Papapetropoulos, A.; Sessa William, C.; Madge Lisa, A.; Schechner Jeffrey, S.; Schwabb Michael, B.; Polverini Peter, J.; et al. Biological Action of Leptin as an Angiogenic Factor. Science 1998, 281, 1683–1686.
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395.
- Park, H.-Y.; Kwon, H.M.; Lim, H.J.; Hong, B.K.; Lee, J.Y.; Park, B.E.; Jang, Y.S.; Cho, S.Y.; Kim, H.-S. Potential role of leptin in angiogenesis: Leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 2001, 33, 95–102.
- Aronis, K.; Diakopoulos, K.; Fiorenza, C.; Chamberland, J.; Mantzoros, C. Leptin administered in physiological or pharmacological doses does not regulate circulating angiogenesis factors in humans. Diabetologia 2011, 54, 2358–2367.
- Cohen, B.; Barkan, D.; Levy, Y.; Goldberg, I.; Fridman, E.; Kopolovic, J.; Rubinstein, M. Leptin induces angiopoietin-2 expression in adipose tissues. J. Biol. Chem. 2001, 276, 7697–7700.
- Zhang, Q.X.; Magovern, C.J.; Mack, C.A.; Budenbender, K.T.; Ko, W.; Rosengart, T.K. Vascular endothelial growth factor is the major angiogenic factor in omentum: Mechanism of the omentum-mediated angiogenesis. J. Surg. Res. 1997, 67, 147–154.
More
