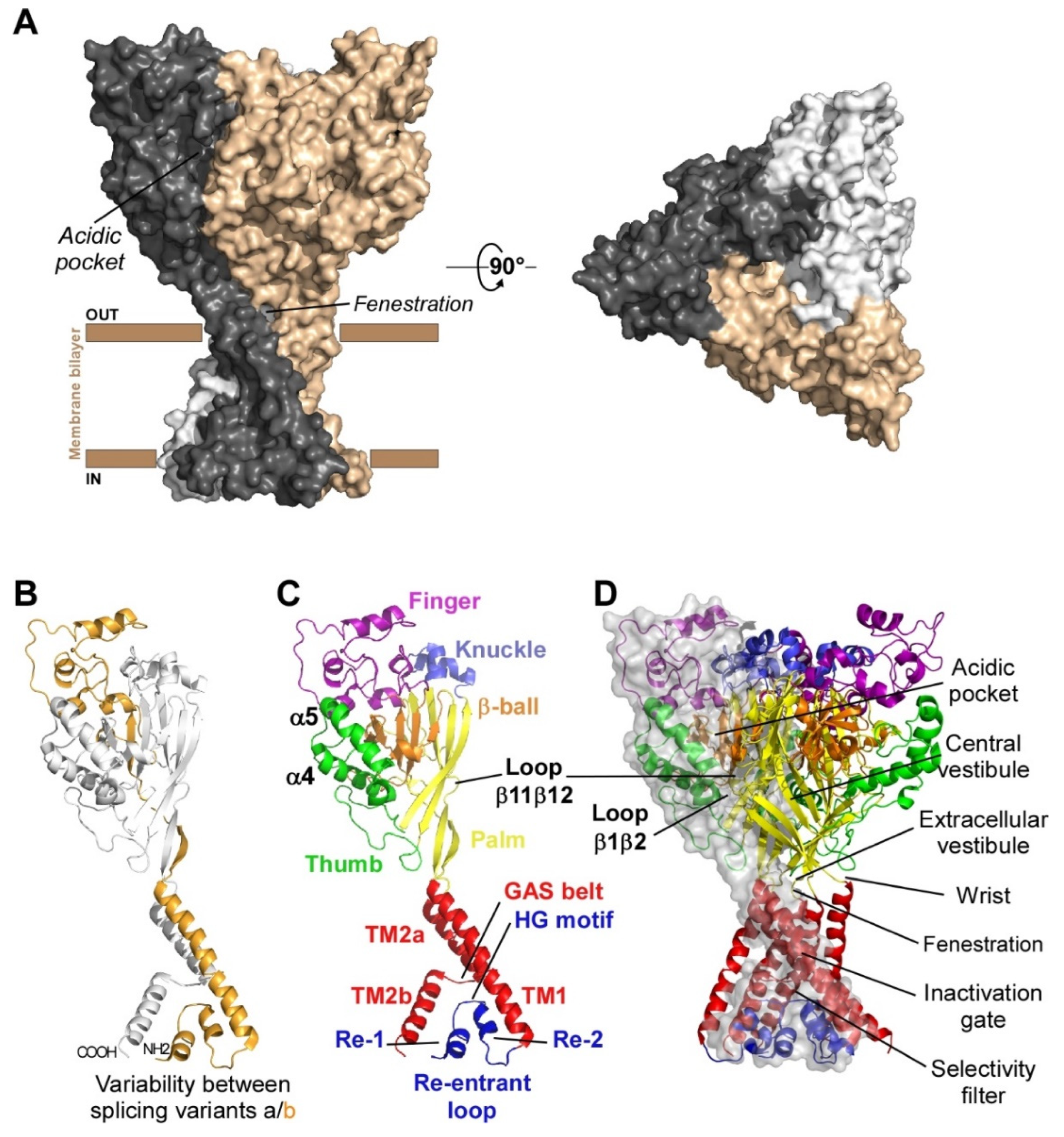
Acid-sensing ion channels (ASICs) are voltage-independent H+-gated cation channels largely expressed in the nervous system of rodents and humans, and involved in pain sensing and associated pathologies. At least six isoforms (ASIC1a, 1b, 2a, 2b, 3 and 4) associate into homotrimers or heterotrimers to form functional channels with highly pH-dependent gating properties.
- ASIC
- sodium channels
- toxins
- peptide
- PcTx1
- APETx2
- MitTx
1. Subunits Diversity and Structure
| Isoform | Species | % Identity | Size (aa) | Name in Genbank | Sequence ID |
|---|---|---|---|---|---|
| ASIC1a | Rattus norvegicus | 98.11% | 526 | ASIC1 | NP_077068.1 |
| Homo sapiens | 528 | ASIC1 isoform b | NP_001086.2 | ||
| ASIC1b | Rattus norvegicus | 93.24% | 559 | ASIC1 isoform X5 | XP_006257440.1 |
| Homo sapiens | 562 | ASIC1 isoform c | NP_001243759.1 | ||
| ASIC2a | Rattus norvegicus | 99.02% | 512 | ASIC2 isoform MDEG1 | NP_001029186.1 |
| Homo sapiens | 512 |
| Cloned Channel | ACTIVATION | SSD | ||||
|---|---|---|---|---|---|---|
| Test pH Threshold/max |
pH | 0.5 | Conditioning pH Threshold/max |
pH | 0.5 | |
| rASIC1a | 7.0/5.5 | 6.4–5.8 | chimnqtwxyz | 7.4/6.8 | 7.3–7.1 | cehimtyz |
| rASIC1b | 6.4/5.6 | 6.3–5.7 | fitwxy# | 7.3/6.6 | 7.0–6.5 | fit# |
| m/rASIC2a | 6.0/3.0 | 5.0–3.8 | bnqwxz | 7.0/4.5 | 6.3–5.6 | mz |
| m/rASIC3 | 7.2/5.5 | 6.8–6.3 | otwy | 7.4/6.8 | 7.2–7.0 | sty |
| rASIC1a/2a | 6.3/4.5 | 5.6–4.8 | nqrw | |||
| ASIC2 isoform MDEG1 | NP_001085.2 | |||||
| ASIC2b | Rattus norvegicus | 98.83% | 563 | ASIC2 isoform MDEG2 | NP_037024.2 | |
| Homo sapiens | 563 | ASIC2 isoform MDEG2 | NP_899233.1 | |||
| ASIC3 | Rattus norvegicus | 83.68% | 533 | ASIC3 | NP_775158.1 | |
| Homo sapiens | 531 | ASIC3 isoform a | NP_004760.1 | |||
| ASIC4 | Rattus norvegicus | 97.22% | 539 | ASIC4 | NP_071570.2 | |
| Homo sapiens | 539 | ASIC4 isoform 1 | NP_061144.4 | |||
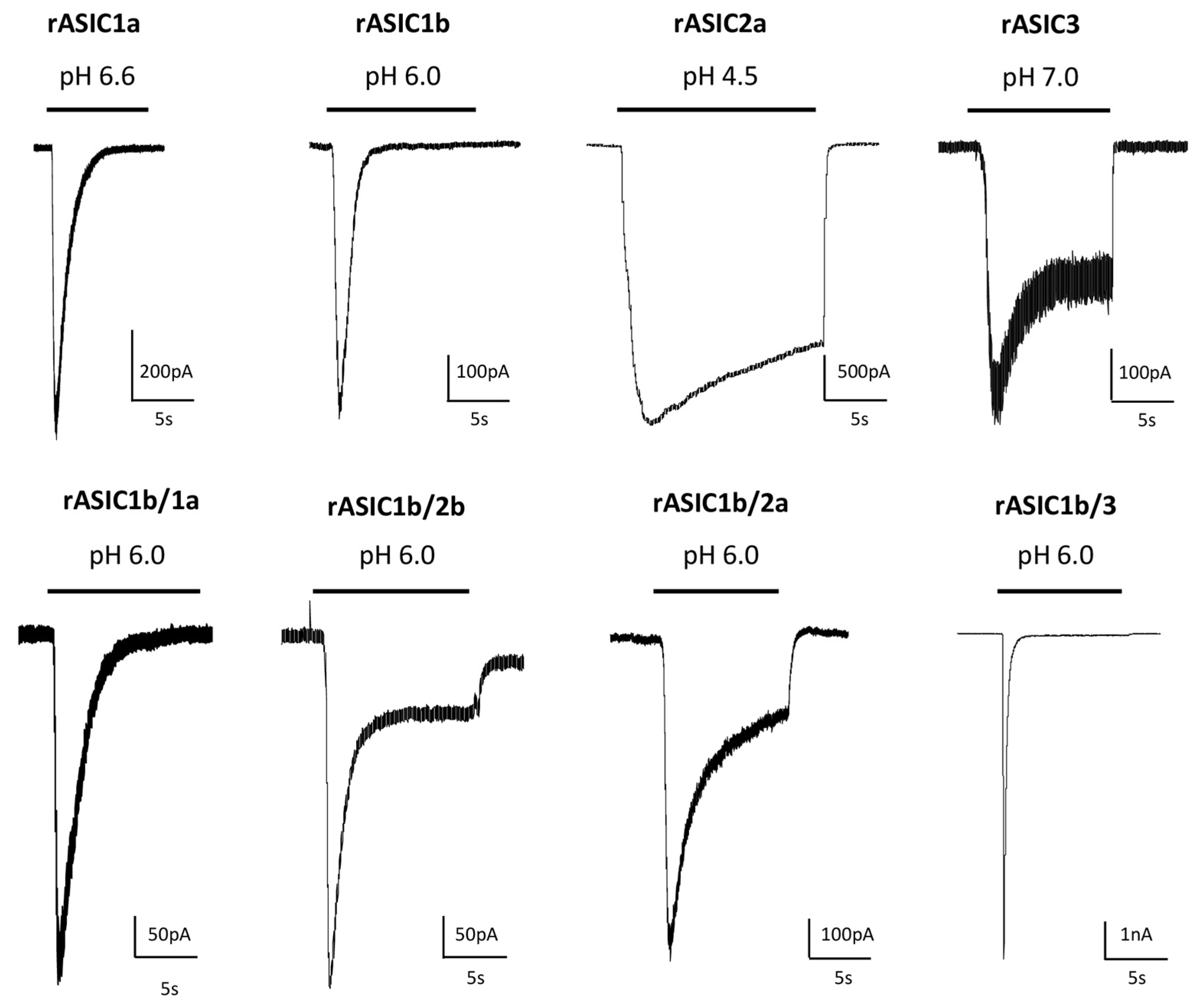
2. pH-Dependency
| m/rASIC1a/2b | ||||||
| 6.8/6.0 | ||||||
| 6.4–6.2 | pw | 7.4/7.1 | 7.3 | p | ||
| rASIC1a/1b | 6.3–5.8 | w | ||||
| rASIC1a/3 | 7.0/5.5 | 6.7–6.3 | rtw | 7.0/6.8 | 7.1 | t |
| rASIC1b/3 | 6.6/5.9 | 6.7–6.2 | tw | 6.9/6.6 | 6.8 | t |
| rASIC1b/2a | 4.9 | w | ||||
| rASIC2a/3 | 7.2/4.5 | 6.1–5.6 | rw | |||
| m/rASIC2a/2b | 4.8 | bw | ||||
| rASIC2b/3 | 6.5 | w | ||||
| m/rASIC1a/2a/3 | 6.4–5.1 | rw | ||||
| rASIC1a/2b/3 | 6.3 | w | ||||
| rASIC1b/2a/3 | 4.9 | w | ||||
| hASIC1a | 6.8/6.0 | 6.6–6.3 | dgikov | 7.0/6.7 | 7.2–6.9 | degiko |
| hASIC1b | 6.5/5.5 | 5.9–5.7 | gi | 6.7/6.4 | 6.5–6.1 | gi |
| hASIC2a | 6.8/3.5 | 5.7 | u | 6.0/4.7 | 5.5 | u |
| hASIC3a | 7.0/5.5 | 6.6–6.2 | aj | 7.0/7.9 | 7.7–7.5 | as |
| cASIC1 | 6.8/6.3 | 6.6 | l | 7.4/7.1 | 7.3 | l |
Representative pH ranges (threshold/max) and pH0.5 values for pH-dependent activation of cellular ASIC currents activated from conditioning pH 7.4 to variable test pHs, and for pH-dependent steady state desensitization (SSD) of currents maximally activated from variable conditioning pHs with rat (r), mouse (m), chicken (c) and human (h) homotrimeric and heterotrimeric ASICs heterologously expressed in Xenopus oocytes or mammalian cell lines. The corresponding current noted rASIC1a/2a, for example, results from the co-expression of rASIC1a and rASIC2a subunits. References: a [27][17], b [28][18], c [29][19], d [30][20], e [31][21], f [32][22], g [19][9], h [33][23], i [34][24], j [22][12], k [35][25], l [36][26], m [37][27], n [38][28], o [39][29], p [40][30], q [41][31], r [42][32], s [43][33], t [44][34], u [45][35], v [46][36], w [14][3], x [47][37], y [48][38], z [49][39], # unpublished data.
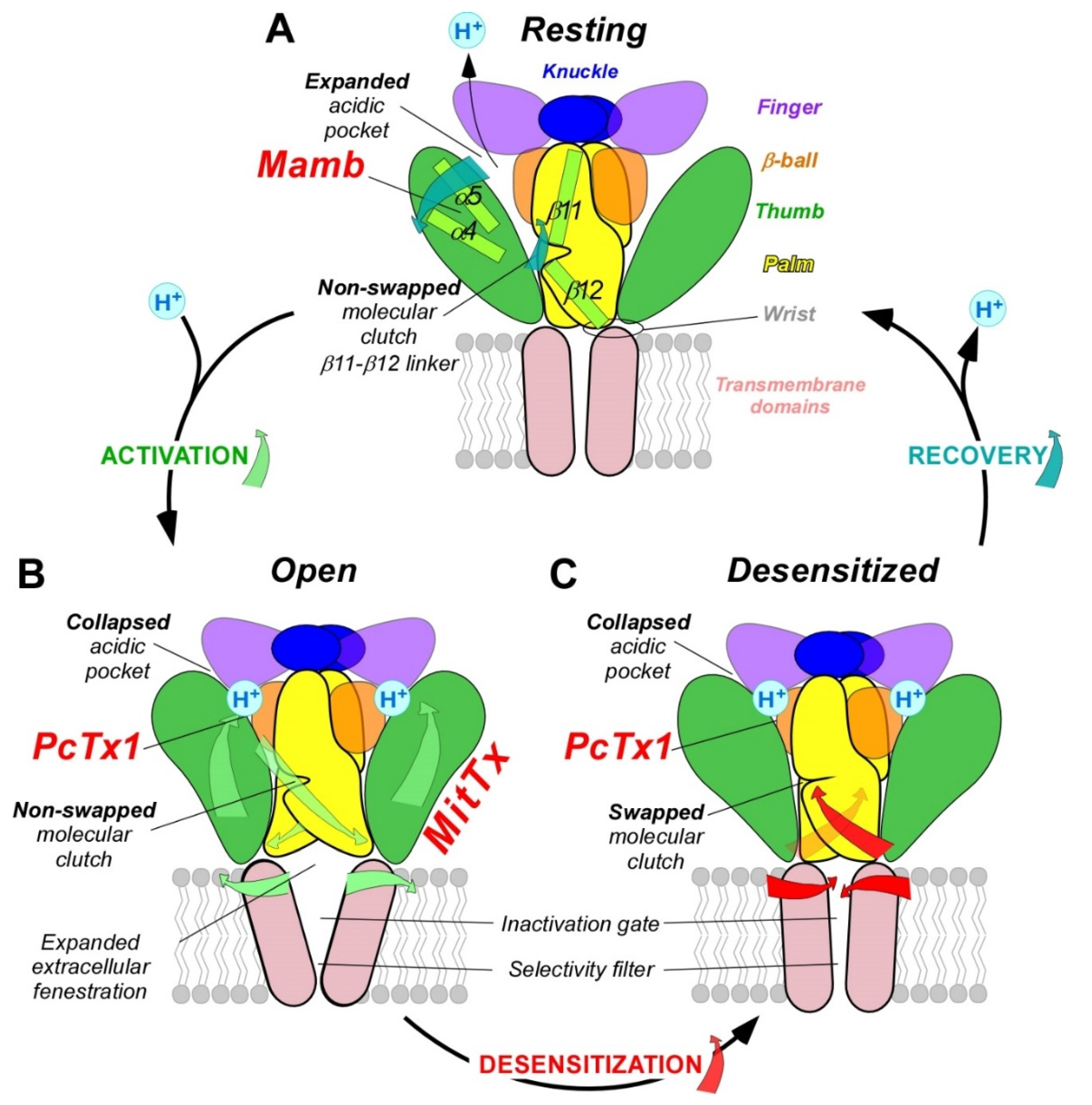
3. pH-Dependent Gating
4. Pathophysiological Roles in Pain Sensing
References
- Jasti, J.; Furukawa, H.; Gonzales, E.B.; Gouaux, E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 2007, 449, 316–323.
- Lingueglia, E.; de Weille, J.R.; Bassilana, F.; Heurteaux, C.; Sakai, H.; Waldmann, R.; Lazdunski, M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 1997, 272, 29778–29783.
- Hesselager, M.; Timmermann, D.B.; Ahring, P.K. pH Dependency and Desensitization Kinetics of Heterologously Expressed Combinations of Acid-sensing Ion Channel Subunits. J. Biol. Chem. 2004, 279, 11006–11015.
- Gonzales, E.B.; Kawate, T.; Gouaux, E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 2009, 460, 599–604.
- Wu, Y.; Chen, Z.; Sigworth, F.J.; Canessa, C.M. Structure and analysis of nanobody binding to the human ASIC1a ion channel. eLife 2021, 10, e67115.
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589.
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 1997, 386, 173–177.
- Bassler, E.L.; Ngo-Anh, T.J.; Geisler, H.S.; Ruppersberg, J.P.; Grunder, S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J. Biol. Chem. 2001, 276, 33782–33787.
- Hoagland, E.N.; Sherwood, T.W.; Lee, K.G.; Walker, C.J.; Askwith, C.C. Identification of a Calcium Permeable Human Acid-sensing Ion Channel 1 Transcript Variant. J. Biol. Chem. 2010, 285, 41852–41862.
- Jacquot, F.; Khoury, S.; Labrum, B.; Delanoe, K.; Pidoux, L.; Barbier, J.; Delay, L.; Bayle, A.; Aissouni, Y.; Barriere, D.A.; et al. Lysophosphatidylcholine 16: 0 mediates chronic joint pain associated to rheumatic diseases through acid-sensing ion channel 3. Pain 2022, 163, 1999–2013.
- Marra, S.; Ferru-Clément, R.; Breuil, V.; Delaunay, A.; Christin, M.; Friend, V.; Sebille, S.; Cognard, C.; Ferreira, T.; Roux, C.; et al. Non-acidic activation of pain-related Acid-Sensing Ion Channel 3 by lipids. EMBO J. 2016, 35, 414–428.
- Delaunay, A.; Gasull, X.; Salinas, M.; Noël, J.; Friend, V.; Lingueglia, E.; Deval, E. Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc. Natl. Acad. Sci. USA 2012, 109, 13124–13129.
- Deval, E.; Salinas, M.; Baron, A.; Lingueglia, E.; Lazdunski, M. ASIC2b-dependent Regulation of ASIC3, an Essential Acid-sensing Ion Channel Subunit in Sensory Neurons via the Partner Protein PICK-1. J. Biol. Chem. 2004, 279, 19531–19539.
- Sivils, A.; Yang, F.; Wang, J.Q.; Chu, X.-P. Acid-Sensing Ion Channel 2: Function and Modulation. Membranes 2022, 12, 113.
- Salinas, M.; Lazdunski, M.; Lingueglia, E. Structural Elements for the Generation of Sustained Currents by the Acid Pain Sensor ASIC3. J. Biol. Chem. 2009, 284, 31851–31859.
- Bartoi, T.; Augustinowski, K.; Polleichtner, G.; Gründer, S.; Ulbrich, M.H. Acid-sensing ion channel (ASIC) 1a/2a heteromers have a flexible 2:1/1:2 stoichiometry. Proc. Natl. Acad. Sci. USA 2014, 111, 8281–8286.
- de Weille, J.R.; Bassilana, F.; Lazdunski, M.; Waldmann, R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett. 1998, 433, 257–260.
- Askwith, C.C.; Wemmie, J.A.; Price, M.P.; Rokhlina, T.; Welsh, M.J. Acid-sensing Ion Channel 2 (ASIC2) Modulates ASIC1 H+-activated Currents in Hippocampal Neurons. J. Biol. Chem. 2004, 279, 18296–18305.
- Chen, X.; Kalbacher, H.; Grunder, S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J. Gen. Physiol. 2005, 126, 71–79.
- Sherwood, T.W.; Askwith, C.C. Endogenous Arginine-Phenylalanine-Amide-related Peptides Alter Steady-state Desensitization of ASIC1a. J. Biol. Chem. 2008, 283, 1818–1830.
- Cristofori-Armstrong, B.; Saez, N.J.; Chassagnon, I.R.; King, G.F.; Rash, L.D. The modulation of acid-sensing ion channel 1 by PcTx1 is pH-, subtype- and species-dependent: Importance of interactions at the channel subunit interface and potential for engineering selective analogues. Biochem. Pharmacol. 2019, 163, 381–390.
- Chen, X.; Kalbacher, H.; Grunder, S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J. Gen. Physiol. 2006, 127, 267–276.
- Diochot, S.; Baron, A.; Salinas, M.; Douguet, D.; Scarzello, S.; Dabert-Gay, A.-S.; Debayle, D.; Friend, V.; Alloui, A.; Lazdunski, M.; et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012, 490, 552–555.
- Cristofori-Armstrong, B.; Budusan, E.; Rash, L.D. Mambalgin-3 potentiates human acid-sensing ion channel 1b under mild to moderate acidosis: Implications as an analgesic lead. Proc. Natl. Acad. Sci. USA 2021, 118, e2021581118.
- Vaithia, A.; Vullo, S.; Peng, Z.; Alijevic, O.; Kellenberger, S. Accelerated Current Decay Kinetics of a Rare Human Acid-Sensing ion Channel 1a Variant That Is Used in Many Studies as Wild Type. Front. Mol. Neurosci. 2019, 12, 133.
- Rook, M.L.; Miaro, M.; Couch, T.; Kneisley, D.L.; Musgaard, M.; MacLean, D.M. Mutation of a conserved glutamine residue does not abolish desensitization of acid-sensing ion channel 1. J. Gen. Physiol. 2021, 153, e202012855.
- Baron, A.; Voilley, N.; Lazdunski, M.; Lingueglia, E. Acid sensing ion channels in dorsal spinal cord neurons. J. Neurosci. 2008, 28, 1498–1508.
- Baron, A.; Schaefer, L.; Lingueglia, E.; Champigny, G.; Lazdunski, M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J. Biol. Chem. 2001, 276, 35361–35367.
- Chen, Z.; Kuenze, G.; Meiler, J.; Canessa, C.M. An arginine residue in the outer segment of hASIC1a TM1 affects both proton affinity and channel desensitization. J. Gen. Physiol. 2021, 153, e202012802.
- Sherwood, T.W.; Lee, K.G.; Gormley, M.G.; Askwith, C.C. Heteromeric Acid-Sensing Ion Channels (ASICs) Composed of ASIC2b and ASIC1a Display Novel Channel Properties and Contribute to Acidosis-Induced Neuronal Death. J. Neurosci. 2011, 31, 9723–9734.
- Bassilana, F.; Champigny, G.; Waldmann, R.; de Weille, J.R.; Heurteaux, C.; Lazdunski, M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J. Biol. Chem. 1997, 272, 28819–28822.
- Hattori, T.; Chen, J.; Harding, A.M.S.; Price, M.P.; Lu, Y.; Abboud, F.M.; Benson, C.J. ASIC2a and ASIC3 Heteromultimerize to Form pH-Sensitive Channels in Mouse Cardiac Dorsal Root Ganglia Neurons. Circ. Res. 2009, 105, 279–286.
- Osmakov, D.I.; Koshelev, S.G.; Andreev, Y.A.; Kozlov, S.A. Endogenous Isoquinoline Alkaloids Agonists of Acid-Sensing Ion Channel Type 3. Front. Mol. Neurosci. 2017, 10, 282.
- Chen, X.; Paukert, M.; Kadurin, I.; Pusch, M.; Gründer, S. Strong modulation by RFamide neuropeptides of the ASIC1b/3 heteromer in competition with extracellular calcium. Neuropharmacology 2006, 50, 964–974.
- Malysz, J.; Scott, V.E.; Faltynek, C.; Gopalakrishnan, M. Characterization of human ASIC2a homomeric channels stably expressed in murine Ltk− cells. Life Sci. 2008, 82, 30–40.
- Cho, J.-H.; Askwith, C.C. Potentiation of acid-sensing ion channels by sulfhydryl compounds. Am. J. Physiol.-Cell. Physiol. 2007, 292, C2161–C2174.
- Salinas, M.; Rash, L.D.; Baron, A.; Lambeau, G.; Escoubas, P.; Lazdunski, M. The receptor site of the spider toxin PcTx1 on the proton-gated cation channel ASIC1a. J. Physiol. 2006, 570, 339–354.
- Sutherland, S.P.; Benson, C.J.; Adelman, J.P.; McCleskey, E.W. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 711–716.
- Salinas, M.; Besson, T.; Delettre, Q.; Diochot, S.; Boulakirba, S.; Douguet, D.; Lingueglia, E. Binding Site and Inhibitory Mechanism of the Mambalgin-2 Pain-relieving Peptide on Acid-sensing Ion Channel 1a. J. Biol. Chem. 2014, 289, 13363–13373.
- Yoder, N.; Yoshioka, C.; Gouaux, E. Gating mechanisms of acid-sensing ion channels. Nature 2018, 555, 397–401.
- Baconguis, I.; Bohlen, C.J.; Goehring, A.; Julius, D.; Gouaux, E. X-Ray Structure of Acid-Sensing Ion Channel 1–Snake Toxin Complex Reveals Open State of a Na+-Selective Channel. Cell 2014, 156, 717–729.
- Sun, D.; Liu, S.; Li, S.; Zhang, M.; Yang, F.; Wen, M.; Shi, P.; Wang, T.; Pan, M.; Chang, S.; et al. Structural insights into human acid-sensing ion channel 1a inhibition by snake toxin mambalgin1. eLife 2020, 9, e57096.
- Yoder, N.; Gouaux, E. Divalent cation and chloride ion sites of chicken acid sensing ion channel 1a elucidated by x-ray crystallography. PLoS ONE 2018, 13, e0202134.
- Li, T.; Yang, Y.; Canessa, C.M. Interaction of the Aromatics Tyr-72/Trp-288 in the Interface of the Extracellular and Transmembrane Domains Is Essential for Proton Gating of Acid-sensing Ion Channels. J. Biol. Chem. 2009, 284, 4689–4694.
- Yoder, N.; Gouaux, E. The His-Gly motif of acid-sensing ion channels resides in a reentrant ‘loop’ implicated in gating and ion selectivity. eLife 2020, 9, e56527.
- Bargeton, B.; Kellenberger, S. The Contact Region between Three Domains of the Extracellular Loop of ASIC1a Is Critical for Channel Function. J. Biol. Chem. 2010, 285, 13816–13826.
- Paukert, M.; Babini, E.; Pusch, M.; Gründer, S. Identification of the Ca2+ Blocking Site of Acid-sensing Ion Channel (ASIC) 1. J. Gen. Physiol. 2004, 124, 383–394.
- Paukert, M.; Chen, X.; Polleichtner, G.; Schindelin, H.; Gründer, S. Candidate Amino Acids Involved in H+ Gating of Acid-sensing Ion Channel 1a. J. Biol. Chem. 2008, 283, 572–581.
- Klipp, R.C.; Bankston, J.R. Structural determinants of acid-sensing ion channel potentiation by single chain lipids. J. Gen. Physiol. 2022, 154, e202213156.
- Baconguis, I.; Gouaux, E. Structural plasticity and dynamic selectivity of acid-sensing ion channel–spider toxin complexes. Nature 2012, 489, 400–405.
- Wu, Y.; Chen, Z.; Canessa, C.M. A valve-like mechanism controls desensitization of functional mammalian isoforms of acid-sensing ion channels. eLife 2019, 8, e45851.
- Li, T.; Yang, Y.; Canessa, C.M. Asn415 in the β11-β12 Linker Decreases Proton-dependent Desensitization of ASIC1. J. Biol. Chem. 2010, 285, 31285–31291.
- Rook, M.L.; Williamson, A.; Lueck, J.D.; Musgaard, M.; Maclean, D.M. β11-12 linker isomerization governs acid-sensing ion channel desensitization and recovery. eLife 2020, 9, e51111.
- Rook, M.L.; Ananchenko, A.; Musgaard, M.; MacLean, D.M. Molecular Investigation of Chicken Acid-Sensing Ion Channel 1 β11-12 Linker Isomerization and Channel Kinetics. Front. Cell. Neurosci. 2021, 15, 761813.
- Rook, M.L.; Musgaard, M.; MacLean, D.M. Coupling structure with function in acid-sensing ion channels: Challenges in pursuit of proton sensors. J. Physiol. 2020, 599, 417–430.
- Cushman, K.A.; Marsh-Haffner, J.; Adelman, J.P.; McCleskey, E.W. A Conformation Change in the Extracellular Domain that Accompanies Desensitization of Acid-sensing Ion Channel (ASIC) 3. J. Gen. Physiol. 2007, 129, 345–350.
- Della Vecchia, M.C.; Rued, A.C.; Carattino, M.D. Gating Transitions in the Palm Domain of ASIC1a*. J. Biol. Chem. 2013, 288, 5487–5495.
- Krauson, A.J.; Carattino, M.D. The Thumb Domain Mediates Acid-sensing Ion Channel Desensitization. J. Biol. Chem. 2016, 291, 11407–11419.
- Kusama, N.; Gautam, M.; Harding, A.M.S.; Snyder, P.M.; Benson, C.J. Acid-sensing ion channels (ASICs) are differentially modulated by anions dependent on their subunit composition. Am. J. Physiol.-Cell. Physiol. 2013, 304, C89–C101.
- Kusama, N.; Harding, A.M.S.; Benson, C.J. Extracellular Chloride Modulates the Desensitization Kinetics of Acid-sensing Ion Channel 1a (ASIC1a). J. Biol. Chem. 2010, 285, 17425–17431.
- Ruan, Z.; Osei-Owusu, J.; Du, J.; Qiu, Z.; Lu, W. Structures and pH-sensing mechanism of the proton-activated chloride channel. Nature 2020, 588, 350–354.
- Osei-Owusu, J.; Kots, E.; Ruan, Z.; Mihaljevic, L.; Chen, K.H.; Tamhaney, A.; Ye, X.; Lu, W.; Weinstein, H.; Qiu, Z. Molecular determinants of pH sensing in the proton-activated chloride channel. Proc. Natl. Acad. Sci. USA 2022, 119, e2200727119.
- Hu, M.; Li, P.; Wang, C.; Feng, X.; Geng, Q.; Chen, W.; Marthi, M.; Zhang, W.; Gao, C.; Reid, W.; et al. Parkinson’s disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes. Cell 2022, 185, 2292–2308.e2220.
- Aneiros, E.; Cao, L.; Papakosta, M.; Stevens, E.B.; Phillips, S.; Grimm, C. The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 2011, 30, 994–1002.
- Marchesi, A.; Arcangeletti, M.; Mazzolini, M.; Torre, V. Proton transfer unlocks inactivation in cyclic nucleotide-gated A1 channels. J. Physiol. 2015, 593, 857–870.
- Feliciangeli, S.; Chatelain, F.C.; Bichet, D.; Lesage, F. The family of K2P channels: Salient structural and functional properties. J. Physiol. 2015, 593, 2587–2603.
- Kellenberger, S.; Schild, L.; Ohlstein, E.H. International Union of Basic and Clinical Pharmacology. XCI. Structure, Function, and Pharmacology of Acid-Sensing Ion Channels and the Epithelial Na+ Channel. Pharmacol. Rev. 2015, 67, 1–35.
- Karsan, N.; Gonzales, E.B.; Dussor, G. Targeted Acid-Sensing Ion Channel Therapies for Migraine. Neurotherapeutics 2018, 15, 402–414.
- Lee, C.H.; Chen, C.C. Roles of ASICs in Nociception and Proprioception. Adv. Exp. Med. Biol. 2018, 1099, 37–47.
- Deval, E.; Lingueglia, E. Acid-Sensing Ion Channels and nociception in the peripheral and central nervous systems. Neuropharmacology 2015, 94, 49–57.
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-Sensing Ion Channels: Focus on Physiological and Some Pathological Roles in the Brain. Curr. Neuropharmacol. 2021, 19, 1570–1589.
- Heusser, S.A.; Pless, S.A. Acid-sensing ion channels as potential therapeutic targets. Trends Pharmacol. Sci. 2021, 42, 1035–1050.
- Lin, S.-H.; Sun, W.-H.; Chen, C.-C. Genetic exploration of the role of acid-sensing ion channels. Neuropharmacology 2015, 94, 99–118.
- Lin, J.-H.; Hung, C.-H.; Han, D.-S.; Chen, S.-T.; Lee, C.-H.; Sun, W.-Z.; Chen, C.-C. Sensing acidosis: Nociception or sngception? J. Biomed. Sci. 2018, 25, 85.
- Dulai, J.S.; Smith, E.S.J.; Rahman, T. Acid-sensing ion channel 3: An analgesic target. Channels 2021, 15, 94–127.
- Ritzel, R.M.; He, J.; Li, Y.; Cao, T.; Khan, N.; Shim, B.; Sabirzhanov, B.; Aubrecht, T.; Stoica, B.A.; Faden, A.I.; et al. Proton extrusion during oxidative burst in microglia exacerbates pathological acidosis following traumatic brain injury. Glia 2020, 69, 746–764.
- Lin, L.-H.; Jones, S.; Talman, W.T. Cellular Localization of Acid-Sensing Ion Channel 1 in Rat Nucleus Tractus Solitarii. Cell. Mol. Neurobiol. 2017, 38, 219–232.
- Wood, J.N.; Stein, C.; Gaveriaux-Ruff, C. Opioids and Pain. In The Oxford Handbook of the Neurobiology of Pain; Oxford University Press: Oxford, UK, 2020; pp. 727–769.
- Chesler, M. Regulation and Modulation of pH in the Brain. Physiol. Rev. 2003, 83, 1183–1221.
- Ugawa, S.; Ueda, T.; Ishida, Y.; Nishigaki, M.; Shibata, Y.; Shimada, S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J. Clin. Invest. 2002, 110, 1185–1190.
- Jones, N.G.; Slater, R.; Cadiou, H.; McNaughton, P.; McMahon, S.B. Acid-induced pain and its modulation in humans. J. Neurosci. 2004, 24, 10974–10979.
- Ruan, N.; Tribble, J.; Peterson, A.M.; Jiang, Q.; Wang, J.Q.; Chu, X.-P. Acid-Sensing Ion Channels and Mechanosensation. Int. J. Mol. Sci. 2021, 22, 4810.
- Wang, Y.; O’Bryant, Z.; Wang, H.; Huang, Y. Regulating Factors in Acid-Sensing Ion Channel 1a Function. Neurochem. Res. 2015, 41, 631–645.
- Cullinan, M.M.; Klipp, R.C.; Bankston, J.R. Regulation of acid-sensing ion channels by protein binding partners. Channels 2021, 15, 635–647.
- Deval, E.; Noël, J.; Lay, N.; Alloui, A.; Diochot, S.; Friend, V.; Jodar, M.; Lazdunski, M.; Lingueglia, E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008, 27, 3047–3055.
- Du, J.; Reznikov, L.R.; Price, M.P.; Zha, X.-m.; Lu, Y.; Moninger, T.O.; Wemmie, J.A.; Welsh, M.J. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc. Natl. Acad. Sci. USA 2014, 111, 8961–8966.
- Huang, Y.; Jiang, N.; Li, J.; Ji, Y.-H.; Xiong, Z.-G.; Zha, X.-m. Two aspects of ASIC function: Synaptic plasticity and neuronal injury. Neuropharmacology 2015, 94, 42–48.
