Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Rosalyn Abbott and Version 2 by Vivi Li.
Despite developing prenatally, the adipose tissue is unique in its ability to undergo drastic growth even after reaching its mature size. Proper adThipose tissue s development relies on tightly regulated processes that require careful c and subsequent maintenance rely on the proper coordination and cooperation between many different cell typesthe vascular niche and their matrix cues adipose compartment.
- white adipose tissue
- brown adipose tissue
- angiogenesis
- adipogenesis
- extracellular matrix remodeling
- adipose development
- mechanics
- paracrine signaling
- proteolysis
- cell shape
1. Introduction
While classically viewed as merely a storage depot, adipose tissue (fat) has many roles and is classified as a connective, thermogenic, and endocrine organ. Healthy adipose tissue is required to regulate systemic energy homeostasis, modulate inflammatory responses, store and metabolize steroids, protect internal organs, and maintain body temperature (both as an insulating organ and through non-shivering thermogenesis) [1][2][3][4][5][6][1,2,3,4,5,6]. Proper adipose tissue development relies on tightly regulated processes that require careful coordination and cooperation between many different cell types and their matrix cues.
Full-term human infants are composed of 11 to 28% fat [7], which is classified as either brown or white fat. Brown fat, which fully develops and matures prenatally, plays a pertinent role in neonatal thermal regulation, as it contains ample mitochondria to convert the many, small lipids stored in the brown multilocular adipocytes into thermal energy [8]. However, brown adipose tissue proceeds from being 5% of the human body mass at birth to a mere 1.5% of the adult human mass and is replaced by white adipose tissue as the primary adipose mass [9][10][9,10]. On the other hand, white adipose tissue fluctuates in mass throughout both prepubescent and adult life. White adipose tissue serves as the primary energy reservoir in the human body, with approximately 90% being classified as subcutaneous (underneath the skin) and 10% being visceral (surrounding the internal organs/viscera), although there have been quantitative differences shown to be dependent on sex, menopausal state, and disease state [11][12][13][11,12,13].
White adipose tissue is a heterogenous mixture of cells, with mature adipocytes being the primary metabolic component and accounting for approximately 90% of the tissue’s volume [14][15][14,15]. These mature adipocytes arise from precursor preadipocytes that exist alongside many other cells, including adipose stem cells, stromal cells, endothelial cells, and resident immune cells. Despite taking up only 10% of the tissue’s space, these cells, termed stromal vascular cells, play an important role in regulating vascular innervation and the immune response within the tissue [16]. The proper development of white adipose tissue relies on highly coordinated spatial and temporal communication amongst all of these cells and their microenvironment. There are several reviews published that focus on the interplay between adipose and endothelial cells during obesity-driven adipogenesis and angiogenesis [17][18][17,18]. However, the process of adipose organogenesis relies not only on cellular crosstalk but also on cellular self-assembly, which is highly dependent upon both extracellular and intracellular forces in order to control the cell’s fate, matrix remodeling and mechanics, and the development of tissue patterns [19]. There is accumulating evidence indicating the importance of mechanics given that it affects fundamental factors of proliferation, migration, and differentiation [20][21][22][23][20,21,22,23]. With the bulk modulus of adipose tissues being one of the lowest in the body, around 0.5–1 kPa [24], proper cell fate relies on controlled proliferation and differentiation amidst softer materials. Furthermore, with adipose tissue being physiologically exposed to a range of bulk physiological forces (compressive, tensile, and shear) due to body weight [25][26][25,26], there must be adaptive modalities on a cellular level to maintain bulk tissue health and integrity.
2. Adipose Tissue Remodeling during Fetal Development
2.1. Ultrastructural Changes during Adipose Tissue Development
Early studies piloted by Hausman et al. and Poissonet et al. described the complexities of fetal subcutaneous white adipose tissue development using early microscopical and immunohistochemical techniques [27][28][29][30][31][32][33][34][35][27,28,29,30,31,32,33,34,35]. Collectively, these studies describe fetal adipose tissue growth through five defined morphometric stages, as shown in Figure 1. Notably, these developmental stages are highly dependent upon primitive matrix deposition and vessel outgrowth and organization.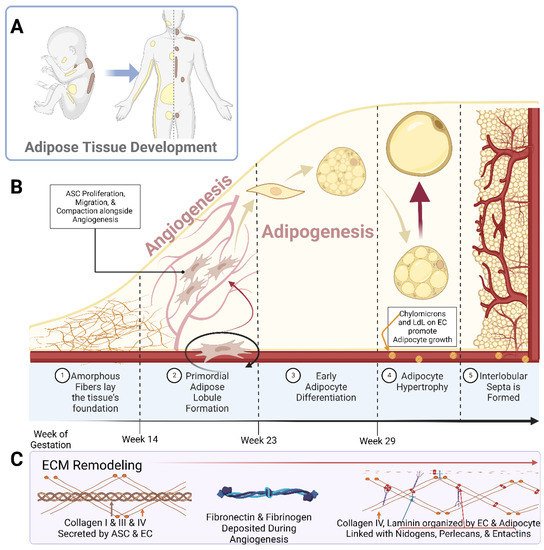
Figure 1. Schematic representations of (A) the localization of white adipose tissue (yellow) and brown adipose tissue (brown) in a developing neonate and adult human; (B) the breakdown of adipose tissue development into the 5 specified stages; (C) the prominent Extracellular Matrix (ECM) components over the course of adipose tissue development. Abbreviations: adipose stem cell (ASC), low-density lipoprotein (LdL), and endothelial cell (EC). Created with Biorender.com.
2.2. Remodeling during Angiogenesis and Adipogenesis
2.2.1. Angiogenesis
In embryonic development, the main vasculature of the human body is formed through de novo vasculogenesis, whereby stem cells undergo differentiation towards endothelial progenitor cells, also known as angioblasts, and then towards endothelial cells to form tubule structures [38]. However, in specified organogenesis, vessels branch off of existing vasculature through controlled sprouting or non-sprouting angiogenesis (Figure 2), with the assistance of existing mesenchymal cells [39]. As previously discussed, the primitive vasculature in adipose tissue has developed prior to week 14 [27][28][27,28]. In order for angiogenesis to occur, matrix metalloproteinases (MMP) must first break down the basement membrane that is currently supporting the vessel. In these early stages, MMP2, MMP3, and MMP9 tend to start this process allowing for the release of fibrinogen and fibronectin, which lay the provisional foundation for the migration of additional endothelial cells [40][41][42][43][44][40,41,42,43,44]. Chemotactic signals, such as vascular endothelial growth factor (VEGF), encourage the proliferation and migration of existing endothelial cells towards the prepared void [45][46][47][45,46,47]. Furthermore, platelet-derived growth factor-β (PDGF-β) recruits pericytes/smooth muscle cells to stabilize the vessel [45]. From then on, transforming growth factor beta (TGF-β) stimulates the differentiation of mesenchymal stem cells to fibroblasts and mural cells in order to increase extracellular matrix (ECM) production to promote vessel maturation [40][48][49][40,48,49]. While the general composition of the vessel’s basement membrane is rather consistent: Laminin and Type IV Collagen linked with Nidogen and Perlecans [35][50][51][35,50,51], the isoform profile and quantity of each ECM will be unique to the specification of the vessel, whether it be arterial, venule, or capillary. For example, Laminin α4 exists on all vessels while Laminin α5 is more exclusive to capillaries [39][40][39,40]. Furthermore, Laminin 10 is present at birth, but gradually, it is replaced with Laminin 8 [39], showing that not only does each cell type have a unique matrix signature but also that this signature changes over time.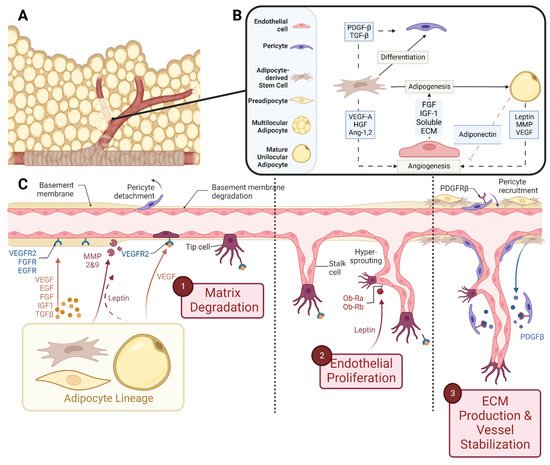
Figure 2. Schematic representations of (A) vascularized adipose tissue and (B) paracrine interactions between both the vascular and adipose lineages. (C) Stepwise breakdown of cellular and paracrine activity during angiogenesis. Abbreviations: Platelet-derived growth factor-β (PDGF-β), transforming growth factor-β (TGF-β), vascular endothelial growth factor-A (VEGF-A), hepatocyte growth factor (HGF), angiopoietin-1,2 (Ang-1,2), fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF-1), extracellular matrix (ECM), and matrix metalloproteinase (MMP). Created with Biorender.com.
