Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Nina D. Anfinogenova.
Ascending thoracic aortic aneurysm is a life-threatening disease, which is difficult to detect prior to the occurrence of a catastrophe. Epidemiology patterns of ascending thoracic aortic dilations/aneurysms remain understudied, whereas the risk assessment of it may be improved.
- ascending thoracic aortic aneurysm
- risk prediction
- open aortic repair
- imaging
1. Introduction
Ascending thoracic aortic aneurysm is a life-threatening and insidious disease that is difficult to detect prior to the occurrence of a catastrophe [1,2][1][2]. A single-center study, which was based on computed tomography (CT) scans that included the chest, which were performed in the Yale University School of Medicine, allowed for the authors to evaluate the prevalence of the incidentally detected ascending thoracic aortic dilations with the ascending thoracic aorta diameter ≥4.0 cm: 2.1% of the total, and 3.2% and 0.9% in men and women, respectively, and 2.8% in people who were aged ≥50 years [3]. A population-based retrospective cohort study, which was carried out in Ontario, Canada, showed that the incidence rate of type A aortic dissections (TAD) is 1.5 per 100,000, and total rate of the incidentally detected thoracic aortic aneurysms is 7.6 per 100,000 [4]. However, the true incidence of ascending thoracic aortic dilations including the undetected cases in the population remains poorly studied.
The timely diagnosis and pre-emptive management of it are essential, and patients may benefit from an elective aortic repair if a twelve-month risk of the aneurysm rupture/dissection exceeds the combined risks of the perioperative morbidity and mortality. The patients with thoracic aortic aneurysms who receive later elective surgeries experience significantly worse outcomes when they are compared with the medically triaged candidates [5]. The current guidelines suggest that an aortic diameter-based assessment of the thoracic aortic aneurysm risk should be conducted [6] and recommend pre-emptive open aortic repair if the aortic diameter reaches 55 mm because greater diameters are associated with a significantly higher risk of dissection or rupture. However, most of the ascending aorta dissections occur at a size that is lower than the recommended threshold.
The questions on the personalization of the indications for the pre-emptive open repair and potential left-shift of diameter-based criterion continue to be discussed in the literature. Moreover, alternative approaches to assessing the risk of ascending aortic aneurysm dissection/rupture are currently under development, and they may potentially result in the elaboration of more precise tools to select the patients for pre-emptive open aortic repair. A personalized risk assessment may be the most promising strategy for establishing adequate indications for the pre-emptive open repair of ascending thoracic aorta.
2. Biomechanical Properties of Ascending Aorta
2.1. In Vivo Evaluation of Elastic/Biomechanical Properties of Ascending Aorta
The biomechanical properties of the ascending aorta have been studied using various approaches. An intraoperative video-based method was proposed to assess the local biaxial strains of the ascending thoracic aorta in patients who were undergoing open-chest surgery. Repeated biaxial strain measurements were obtained at low- and high-pressure conditions with reliable precision. This method may be used to further study the biomechanical properties of the ascending thoracic aorta in various relevant populations of patients who are undergoing open-chest surgery. The method builds a foundation for clinically relevant biomechanical modeling and mechanobiological profiling [26][7].
The imaging-based monitoring of the biomechanical properties of the aortic wall may help to identify the patients with a borderline aortic dilatation that require pre-emptive surgery. The biomechanical parameters characterizing the distensibility and mechanical elasticity of the ascending aorta may be assessed using the data of the aortic deformations during the cardiac cycle in routine practice based on an electrocardiogram (ECG)-synchronized MRI. Serial cardiac magnetic resonance (CMR) measurements demonstrate that ascending aortic distensibility declined in BAV patients even faster than it did in a comparison group with connective tissue disorders [27][8]. A prospective assessment of the biomechanical and elastic aortic properties based on the data of echocardiography, CT, and CMR imaging is promising for risk stratification [28[9][10][11],29,30], but it should be integrated with other predictors.
2.2. Young’s Elastic Modulus
Young’s elastic modulus is the ratio of the tensile stress to strain. Higher values of tensile strength and Young’s modulus are present in patients with an ascending aortic aneurysm [31][12], but studies focusing on Young’s modulus as a criterion for determining the timing of pre-emptive open aortic repair in patients with a borderline dilatation of the ascending thoracic aorta are almost lacking. WThe researchers here encourage researchers to consider the Young’s elastic modulus of the ascending aorta for the risk assessment of it because it may be easily calculated based on widely available ECG-synchronized MRI aortography as follows (1) (Figure 1) [28][9]:
where
E = ((ddiast2 × (1 − 0.25) × ABPpulse)/(2 × h × ∆dpulse)) × 133.3
-
E—Young’s elastic modulus (Pa);
-
ddiast—diastolic aortic diameter;
-
∆dpulse—an increase in aortic diameter during systole;
-
0.25—squared Poisson ratio for the aorta wall (Poisson ratio is known to be 0.5);
-
h—aorta wall thickness during diastole;
-
ABPpulse—pulse arterial blood pressure;
-
133.3—mmHg to Pa conversion factor.
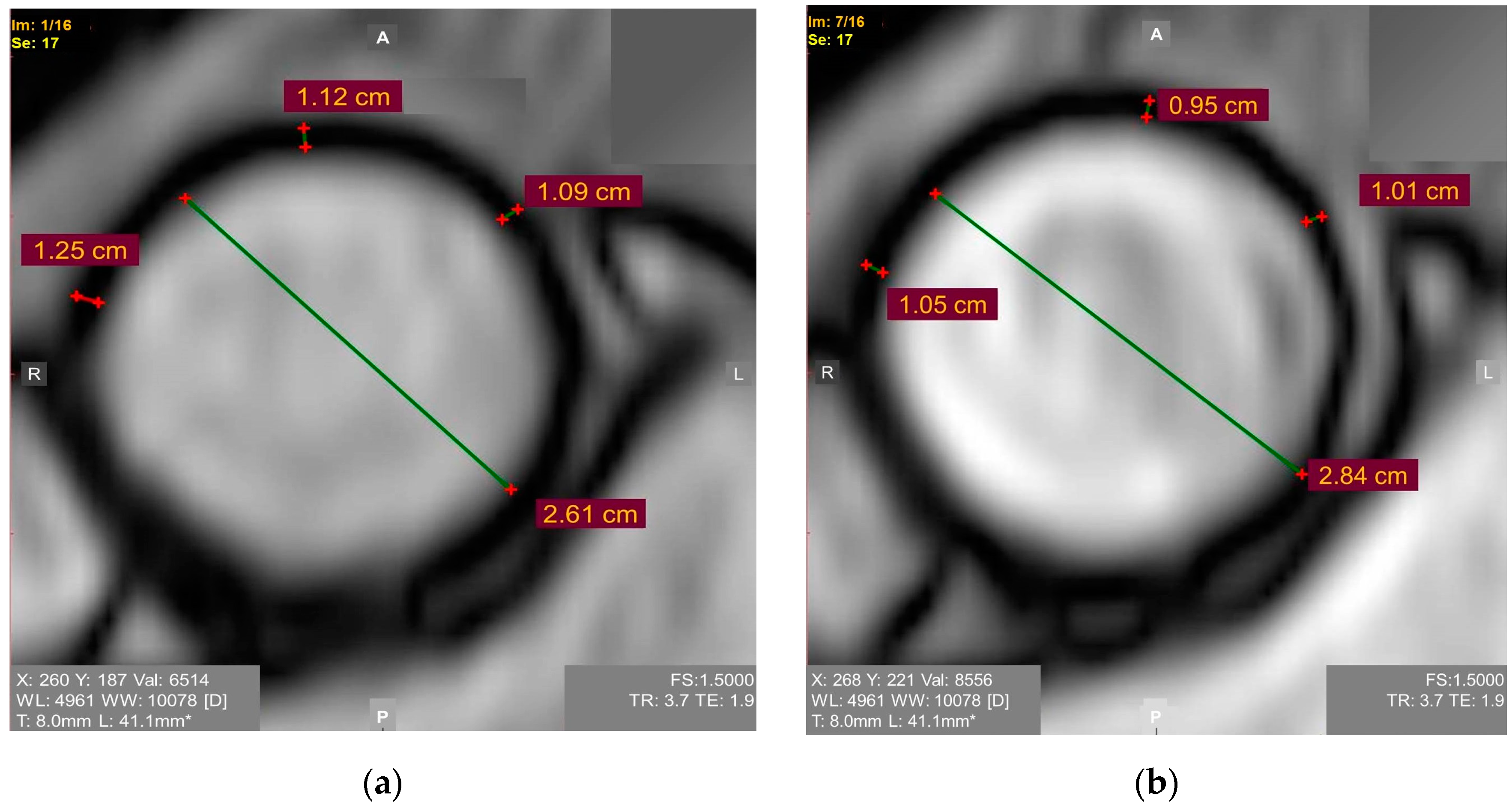
Figure 1. ECG-synchronized magnetic resonance aortography images used for Young’s modulus calculation in the ascending aorta: (a) Diastole. Mean diastolic aortic wall thickness = 1.15 mm; (b) Systole. Mean systolic aortic wall thickness = 1.05 mm.
A group of patients underwent ECG-synchronized MRI-based aortography for months before some of them developed an ascending aortic aneurysm. The values of the Young’s elastic modulus in all of the patients who later developed the aneurysms exceeded 0.67 mPa, though the study was limited by its retrospective nature and small sample size [28][9]. The involvement of the aorta wall thickness in the formula agrees with the report revealing the association between this parameter and the adverse ascending aortic events. It is expected that increased the aortic wall thickness may be the early warning sign requiring patient monitoring [32][13]. Considering that the biomechanical properties of the ascending aorta are anisotropic [33][14], it is essential to develop a well-defined standardized approach to assessing the Young’s elastic modulus.
2.3. Windkessel Function
The capacitance or the Windkessel function characterizes the aorta acting as an elastic buffering chamber behind the heart. The aorta stores about 50% of the left ventricular stroke volume during systole, and it releases the elastic forces during diastole. Energy loss is the measure of the Windkessel function. Recently, the biomechanics of normal, dilated, and dissecting ascending aortas were studied via destructive testing [34,35][15][16]. The energy loss under the biaxial tensile loading was shown to be independent of the strain rate, strain magnitude, and strain pre-load over a wide range of physiological values except at the low-strain regions. However, the energy loss was significantly correlated with the delamination strength. Energy loss may be considered a biomarker of aortic dissection risk in patients with ascending aortic aneurysms. [34,35][15][16].
2.4. Fluid Hemodynamics and Vortical Patterns in Proximal Aorta for Risk Assessment
One of the potential areas of growth in identifying the candidates for pre-emptive open aortic repair is the study of fluid hemodynamics and vortical patterns in the proximal aorta because the ascending thoracic aortic aneurysm progression may be partially flow-mediated [21][17]. The first attempts to understand hemodynamics in the aorta date as early as 500 years ago. Leonardo da Vinci depicted (~1512–13) multiple systolic flow vortices through the ascending aorta. Bissell et al. encoded 3D vector fields to map the aortic root blood flow using time-resolved magnetic resonance techniques and questioned the existence of secondary vortices which were postulated by Leonardo da Vinci in the normal aorta [36][18]. However, multiple vortices in the sinuses and proximal ascending aorta, which were postulated by Leonardo, still may be involved in aortic dilatation, hypertension, and valvular diseases [37][19].
New hemodynamic tools allow uthe researchers to study the vorticity scores, the qualitative helicity, the quantitative vorticity, the local normalized helicity, the abnormally directed velocity volume, and the wall shear stress (WSS) surface in ascending aorta disease [38][20]. A WSS topological skeleton may reveal the promising indicators of local wall abnormalities [39][21]. According to a 4D flow CMR study, the helices and vortices in the thoracic aortic blood flow are present in aortic dilatations and aneurysms [39][21]. Aortic valve abnormalities cause aberrant 3D hemodynamics, and an altered WSS may potentially trigger an aortic dilatation and aortopathy [40,41][22][23].
A valuable tool was developed to study the hemodynamic alterations in the diseased aorta depending on the aortic shape and the aortic valve variations which were based on statistical shape modeling. The tool was designed to support the risk assessment in patients with thoracic ascending aortic disease over time. The authors created the ascending thoracic aortic aneurysm template, which may be modelled to see the differences that it produces in the flow patterns, blood pressure, and fluid shear forces when they are deformed. The authors performed the exquisite atlas-based analyses to understand the mechanistic link between the shape and the pathophysiology [42][24].
The hemodynamic patterns depend on the blood flow eccentricity at the aortic root regardless of the aortic valve phenotype [43][25]. The blood flow vortices produce the excessive mechanical stresses at certain aortic wall locations, which further become the foci of the dilatation and, eventually, they become the entry sites for acute aortic dissections in patients with aortic arch variants. The wall stress/strength relationships may be used for the risk assessment [44][26]. A turbulent blood flow may trigger signaling cascades in the aortic wall, and this is linked to an elevated eNOS expression at the concave wall in dilated aortas [45][27].
Regional hemodynamic and biomechanical characteristics depend on different rotational positions of the normal aortic root [46][28]. The velocity and therefore, the stresses over the aortic arch can be significantly reduced by a reversed flow and vortex formation as shown in both the normal and aortic arch aneurysm models [47][29].
Computational fluid dynamics enable uthe researchers to evaluate the vorticity, the helicity index, a disturbed laminar flow, the flow streamline, the flow velocity, the WSS, and the recirculation regions in untreated and treated aortic diseases, and other fluid dynamic parameters [44,47,48,49][26][29][30][31].
References
- Zafar, M.A.; Chen, J.F.; Wu, J.; Li, Y.; Papanikolaou, D.; Abdelbaky, M.; Faggion Vinholo, T.; Rizzo, J.A.; Ziganshin, B.A.; Mukherjee, S.K.; et al. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2021, 161, 498–511.e1.
- Zafar, M.A.; Farkas, E.A.; Javier, A.; Anderson, M.; Gilani, O.; Elefteriades, J.A. Are thromboembolic and bleeding complica-tions a drawback for composite aortic root replacement? Ann. Thorac. Surg. 2012, 94, 737–743.
- Mori, M.; Bin Mahmood, S.U.; Yousef, S.; Shioda, K.; Faggion Vinholo, T.; Mangi, A.A.; Elefteriades, J.A.; Geirsson, A. Prevalence of incidentally identified thoracic aortic dilations: Insights for screening criteria. Can. J. Cardiol. 2019, 35, 892–898.
- McClure, R.S.; Brogly, S.B.; Lajkosz, K.; Payne, D.; Hall, S.F.; Johnson, A.P. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: A population-based study. J. Thorac. Cardiovasc. Surg. 2018, 155, 2254–2264.e4.
- Saeyeldin, A.A.; Velasquez, C.A.; Mahmood, S.U.B.; Brownstein, A.J.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Thoracic aortic an-eurysm: Unlocking the “silent killer” secrets. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 1–11.
- Borger, M.A.; Fedak, P.W.M.; Stephens, E.H.; Gleason, T.G.; Girdauskas, E.; Ikonomidis, J.S.; Khoynezhad, A.; Siu, S.C.; Verma, S.; Hope, M.D.; et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J. Thorac. Car-Diovasc. Surg. 2018, 156, e41–e74.
- Parikh, S.; Ganizada, B.; Debeij, G.; Natour, E.; Maessen, J.; Spronck, B.; Schurgers, L.; Delhaas, T.; Huberts, W.; Bidar, E.; et al. Intra-Operative Video-Based Measurement of Biaxial Strains of the Ascending Thoracic Aorta. Biomedicines 2021, 9, 670.
- Perez-Casares, A.; Dionne, A.; Gauvreau, K.; Prakash, A. Rapid ascending aorta stiffening in bicuspid aortic valve on serial cardiovascular magnetic resonance evaluation: Comparison with connective tissue disorders. J. Cardiovasc. Magn. Reason. 2021, 23, 11.
- Ussov, W.Y.; Ignatenko, G.A.; Bergen, T.A.; Shelkovnikova, T.A.; Bril, K.R.; Khovrin, V.V.; Maksimova, A.S.; Belichenko, O.I.; Trufanov, G.E. Computational evaluation of mechano-elastic properties and of paramagnetic contrast enhancement of thoracic aortic wall in acute myocardial infarction and in non-coronarogenic myocardial damage, from the data of dynamic ECG-gated MRI (MR-elastometry). Transl. Med. = Transl. Med. 2021, 8, 43–58.
- Ussov, W.Y.; Ryumshina, N.I.; Bagriy, A.E.; Sukhareva, A.E.; Maksimova, A.S.; Sinitsyn, V.E.; Falkovskaya, A.Y.; Mordovin, V.F.; Belichenko, O.I. Magnetic resonance con-trast-enhanced imaging of the aortic wall as risk index for acute ischemic cerebral stroke in patients with resistant arterial hypertension. Russ. Electron. J. Radiol. 2020, 10, 108–119. (In Russian)
- Maksimova, A.S.; Lishmanov, Y.B.; Usov, W.Y. Role of magnetic resonance imaging in studies of atherosclerosis of aorta. Russ. Electron. J. Radiol. 2018, 8, 184–193.
- Kozuń, M.; Płonek, T.; Jasiński, M.; Filipiak, J. Effect of dissection on the mechanical properties of human ascending aorta and human ascending aorta aneurysm. Acta Bioeng. Biomech. 2019, 21, 127–134.
- Saeyeldin, A.; Zafar, M.A.; Baldassarre, L.A.; Mojibian, H.; Ziganshin, B.A.; Mukherjee, S.K.; Elefteriades, J.A. Aortic delamination-a pos-sible precursor of impending catastrophe. Int. J. Angiol. 2021, 30, 160–164.
- Subramaniam, D.R.; Gutmark, E.; Andersen, N.; Nielsen, D.; Mortensen, K.; Gravholt, C.; Backeljauw, P.; Gutmark-Little, I. Influence of material model and aortic root motion in finite element analysis of two exemplary cases of proximal aortic dissection. J. Biomech. Eng. 2021, 143, 014504.
- Chung, J.C.; Wong, E.; Tang, M.; Eliathamby, D.; Forbes, T.L.; Butany, J.; Simmons, C.A.; Ouzounian, M. Biomechanics of Aortic Dissection: A Comparison of Aortas Associated With Bicuspid and Tricuspid Aortic Valves. J. Am. Heart Assoc. 2020, 9, e016715.
- Tang, M.; Eliathamby, D.; Ouzounian, M.; Simmons, C.A.; Chung, J.C. Dependency of energy loss on strain rate, strain mag-nitude and preload: Towards development of a novel biomarker for aortic aneurysm dissection risk. J. Mech. Behav. Biomed. Mater. 2021, 124, 104736.
- Salmasi, M.Y.; Pirola, S.; Mahuttanatan, S.; Fisichella, S.M.; Sengupta, S.; Jarral, O.A.; Oo, A.; O’Regan, D.; Xu, X.Y.; Athanasiou, T. Geometry and flow in ascending aortic aneurysms are influenced by left ventricular outflow tract orientation: Detecting increased wall shear stress on the outer curve of proximal aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2021, S0022-5223(21)00913-2, Online ahead of print.
- Bissell, M.M.; Dall’Armellina, E.; Choudhury, R.P. Flow vortices in the aortic root: In Vivo 4D-MRI confirms predictions of Leonardo da Vinci. Eur. Heart J. 2014, 35, 1344.
- Manchester, E.L.; Pirola, S.; Salmasi, M.Y.; O’Regan, D.P.; Athanasiou, T.; Xu, X.Y. Analysis of Turbulence Effects in a Pa-tient-Specific Aorta with Aortic Valve Stenosis. Cardiovasc. Eng. Technol. 2021, 12, 438–453.
- van Ooij, P.; Farag, E.S.; Blanken, C.P.S.; Nederveen, A.J.; Groenink, M.; Planken, R.N.; Boekholdt, S.M. Fully quantitative mapping of abnormal aortic velocity and wall shear stress direction in patients with bicuspid aortic valves and repaired coarctation using 4D flow cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reason. 2021, 23, 9.
- De Nisco, G.; Tasso, P.; Calò, K.; Mazzi, V.; Gallo, D.; Condemi, F.; Farzaneh, S.; Avril, S.; Morbiducci, U. Deciphering ascending thoracic aortic aneurysm hemodynamics in relation to biomechanical properties. Med. Eng. Phys. 2020, 82, 119–129.
- Emerel, L.; Thunes, J.; Kickliter, T.; Billaud, M.; Phillippi, J.A.; Vorp, D.A.; Maiti, S.; Gleason, T.G. Predissection-derived geometric and distensibility indices reveal increased peak longitudinal stress and stiffness in patients sustaining acute type A aortic dissection: Implications for predicting dissection. J. Thorac. Cardiovasc. Surg. 2019, 158, 355–363.
- Suwa, K.; Rahman, O.A.; Bollache, E.; Rose, M.J.; Rahsepar, A.A.; Carr, J.C.; Collins, J.D.; Barker, A.J.; Markl, M. Effect of aortic valve disease on 3D hemo-dynamics in patients with aortic dilation and trileaflet aortic valve morphology. J. Magn. Reason. Imaging 2020, 51, 481–491.
- Catalano, C.; Agnese, V.; Gentile, G.; Raffa, G.M.; Pilato, M.; Pasta, S. Atlas-Based Evaluation of Hemodynamic in Ascending Thoracic Aortic Aneurysms. Appl. Sci. 2022, 12, 394.
- Jayendiran, R.; Campisi, S.; Viallon, M.; Croisille, P.; Avril, S. Hemodynamics alteration in patient-specific dilated ascending thoracic aortas with tricuspid and bicuspid aortic valves. J. Biomech. 2020, 110, 109954.
- Wisneski, A.D.; Mookhoek, A.; Chitsaz, S.; Hope, M.D.; Guccione, J.M.; Ge, L.; Tseng, E.E. Bicuspid aortic valve-associated ascending thoracic aortic aneurysm: Patient-specific finite element analysis. J. Heart Valve Dis. 2015, 24, 714–721.
- Gauer, S.; Balint, B.; Kollmann, C.; Federspiel, J.M.; Henn, D.; Bandner-Risch, D.; Schmied, W.; Schäfers, H.J. Dysregulation of endothelial nitric oxide synthase does not depend on hemodynamic alterations in bicuspid aortic valve aortopathy. J. Am. Heart Assoc. 2020, 9, e016471.
- Sundström, E.; Jonnagiri, R.; Gutmark-Little, I.; Gutmark, E.; Critser, P.; Taylor, M.D.; Tretter, J.T. Effects of normal variation in the rotational position of the aortic root on hemodynamics and tissue biomechanics of the thoracic aorta. Cardiovasc. Eng. Technol. 2020, 11, 47–58.
- Goto, T.; Fukuda, I.; Inamura, T.; Shirota, M.; Minakawa, M. Flow analysis during mock circulation in normal and aortic arch aneurysm models through an aortic cannula toward the aortic arch and root. J. Artif. Organs. 2021, 24, 442–449.
- Numata, S.; Itatani, K.; Kanda, K.; Doi, K.; Yamazaki, S.; Morimoto, K.; Manabe, K.; Ikemoto, K.; Yaku, H. Blood flow analysis of the aortic arch using computational fluid dynamics. Eur. J. Cardiothorac. Surg. 2016, 49, 1578–1585.
- Ong, C.W.; Wee, I.; Syn, N.; Ng, S.; Leo, H.L.; Richards, A.M.; Choong, A.M.T.L. Computational fluid dynamics modeling of hemodynamic parameters in the human diseased aorta: A systematic review. Ann. Vasc. Surg. 2020, 63, 336–381.
More
