In the development of inflammatory bowel disease (IBD), the gut microbiota has been established as a key factor. Recently, metabolomics has become important for understanding the functional relevance of gut microbial changes in disease. Animal models for IBD enable the study of factors involved in disease development. However, results from animal studies may not represent the human situation. The aim of this study was to investigate whether results from metabolomics studies on animal models for IBD were similar to those from studies on IBD patients. Medline and Embase were searched for relevant studies up to May 2017. The Covidence systematic review software was used for study screening, and quality assessment was conducted for all included studies. Data showed a convergence of ~17% for metabolites differentiated between IBD and controls in human and animal studies with amino acids being the most differentiated metabolite subclass. The acute dextran sodium sulfate model appeared as a good model for analysis of systemic metabolites in IBD, but analytical platform, age, and biological sample type did not show clear correlations with any significant metabolites. In conclusion, this systematic review highlights the variation in metabolomics results, and emphasizes the importance of expanding the applied detection methods to ensure greater coverage and convergence between the various different patient phenotypes and animal models of inflammatory bowel disease.
- inflammatory bowel disease
- metabolomics
- animal models
- systematic review
1. Study Characteristics
Fifty-eight studies met our search criteria and were included in this review (Figure 1), of which 32 were human studies, 25 were animal model studies, and one study presented data from both humans and an animal model. The human studies were categorized according to disease (CD, UC, IBD) and age, while the animal model studies were categorized according to model type and age of the animals (Table 1). If animals in a study were grouped spanning more than one age group, the study was characterized according to the older age group. Descriptive characteristics for all studies were extracted, with different tables for the human and animal studies, respectively (Supplementary Tables S1 and S2).
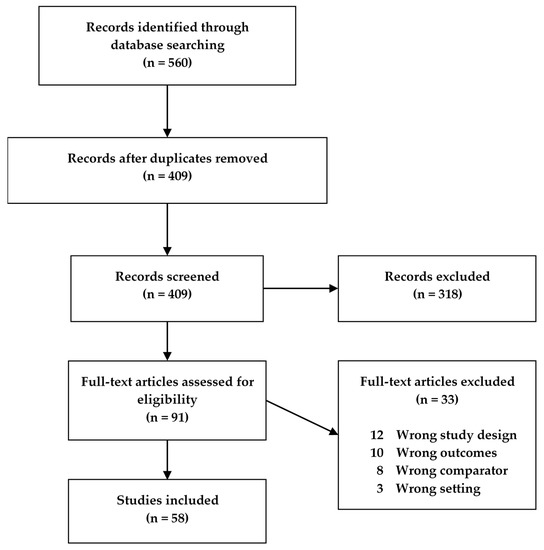
Figure 1. Flowchart of the study screening process for original studies in metabolomics for inflammatory bowel disease (IBD) patients and IBD animal models.
Table 1. Age categories for mouse studies (a) and human studies (b) in the systematic review on metabolomics in inflammatory bowel disease (IBD) patients and IBD animal models.
| Mouse Studies | Human Studies | ||
|---|---|---|---|
| Old | |||
| >24 | Old | 60+ | |
2. Quality Assessment
Two sets of quality criteria were used to assess the quality of the human and animal studies, respectively (Supplementary Tables S3 and S4). Each study was assigned as being of “good”, “medium”, or “poor” quality, based on the amount of quality criteria fulfilled, as presented in Table 2. The majority of studies (75%) were of medium quality, while only 9% of all studies were considered good.
Table 2. Quality assessment of studies included in the systematic review on metabolomics in inflammatory bowel disease (IBD) patients and IBD animal models.
| Level of Quality | % of Criteria Fulfilled | Animal Studies | Human Studies | All Studies |
|---|---|---|---|---|
| Phase of Life | Age in Weeks | Phase of Life | Age (Years) | |
| [ | ||||
| 7 | ||||
| ] | ||||
Table 5. Metabolites significantly decreased in inflammatory bowel disease (IBD) vs healthy controls in both humans and animals in the systematic review.
| Human Studies | Animal Studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite * | Disease | Activity | Sample Type | Age Group | Platform | References | Species | Sample Type | Age (Weeks) | Platform | ||
| Infant | 0–3 | Infant | 0–1 |
| 15% | |||
| 17% |
3. Metabolites Differentiated in Inflammatory Bowel Disease (IBD) Cases Versus Healthy Controls in Both Humans and Animal Models
A total of 200 different metabolites were reported as being increased in IBD across all included human studies, while 218 were decreased (Table 3). The numbers were higher for the animal studies with a total of 280 different metabolites reported as being increased in IBD, while 253 were decreased. Some metabolites were reported as both increased and decreased in each study type, but the majority was exclusively reported as increased or decreased. Results for human and animal model studies, respectively, are presented in separate tables for metabolites that are increased and decreased in each type of study Supplementary Tables S5-S8.
Table 3. Number of differentiated metabolites detected across study types included in the systematic review on metabolomics in inflammatory bowel disease (IBD) patients and IBD animal models.
| Number of Different Metabolites Detected |  |
 |
 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal Studies | Human Studies | Both | |||||||
| Model | References | ||||||||
| Good | ≥70% | 12% | 6% | 9% | |||||
| Increased | 280 | 200 | 48 | 48/280 = 17% | Y, A, O | 1 | H NMR | [40] | Mouse |
| Juvenile | >3–8 | Very early onset and young | >1 and <18 | ||||||
| Medium | 40–70% | ||||||||
| [ | |||||||||
| 11 | |||||||||
| ] | |||||||||
| UC | |||||||||
| AC | |||||||||
| 4-Cresol sulfate | CD | All | Urine | Urine | >8–24 | 1 | H NMR | DSS (A) | [ | 10 | ] |
| 69% | 79% | 75% | |||||||||
| Decreased | 253 | 218 | 41 | 41/253 = 16% | Adult | >8–24 | Adult | 18–60 | |||
| Poor | <40% | 19% | |||||||||
| Exclusively increased | 215 | 135 | 27 | ||||||||
| Exclusively decreased | 190 | 153 | 20 |
To assess the similarities in metabolomics findings between study types, metabolites increased or decreased in IBD in both human and animal studies were identified and are presented in Table 4; Table 5. Forty-eight metabolites were found to be increased in both types of studies, while 41 metabolites were decreased. This corresponds to 17% of metabolites found increased and 16% of metabolites found decreased in IBD in animal studies also being reported as increased and decreased, respectively, in human IBD studies. Of this subgroup of metabolites, 21 were reported as both increased and decreased, respectively, in IBD including several amino acids, and this overlap can largely be explained by the variation in study details. This leaves 27 metabolites exclusively increased, and 20 metabolites exclusively decreased in IBD in both human and animal studies (in bold in Table 4 and Table 5).
Table 4. Metabolites significantly increased in inflammatory bowel disease (IBD) vs healthy controls in both humans and animals in the systematic review.
| Human Studies | Animal Studies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite * | Disease | Activity | Sample Type | Age Group | Platform | References | Species | Sample Type | Age (Weeks) | Platform | Model | References | ||
| 3-Hydroxybutyric | UC, IBD | AC | Serum | A, O | 1 | H NMR | [1][2] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [3] |
| acid | Mouse | Serum | >8–24 | GC-MS | ||||||||||
| Acetic acid | DSS (A) | CD | [ | 4 | ] | |||||||||
| AC | Serum | A, O | 1 | H NMR | [ | 5] | 4-Hydroxyphenyl- | |||||||
4. Metabolites of Special Interest
Several tryptophan metabolites were found to be regulated in human studies, animal studies, or both. Kynurenine and quinolinic acid were increased in UC and CD patients, respectively (Supplementary Tables S5). Kynurenine was also found to be increased in DSS (dextran sodium sulfate) and IL-10-/- mouse models (Supplementary Table S7), while quinolinic acid was decreased in IL-10-/- mice along with kynurenic acid and 5-hydroxyindoleacetic acid (Supplementary Table S8). Additionally, 5-hydroxytryptophan and 3-hydroxykynurenine were also increased in DSS and IL-10-/- mouse models, respectively (Supplementary Table S7). Conflicting observations were made for tryptophan itself, which was reported to be both increased and decreased in human studies as well as the DSS mouse model (see Table 4 and Table 5). SCFAs were reported to be regulated in numerous human IBD studies, although some results were conflicting. Formic acid and acetic acid were thus observed to be both increased and decreased in CD and UC patients, depending on the study (Supplementary Tables S5 and S6). However, propionic acid, butanoic acid, isobutyric acid, and pentanoic acid were all observed to be decreased in CD and UC patients (Supplementary Table S6). Interestingly, only animal studies using the acute DSS mouse model or the TNBS (2,4,6-trinitrobenzenesulfonic acid) rat model reported differentiated levels of SCFAs (Supplementary Tables S7 and S8). Acetic acid was decreased in the DSS model, while butanoic acid was decreased in the TNBS model (Supplementary Table S8). Dong et al. [10] also observed butanoic acid to be decreased, but only on the first day of DSS, after which it was increased throughout the experiment.
5. Included Studies Are Characterized by Great Variation in the Key Experimental Elements
A metabolomics study consists of several different key experimental elements that can vary between studies. Here, these elements are the experimental subjects (disease subtype for the human studies and species, strain, and type of model for the animal studies), biological sample type, analysis methodology, and age of experimental subjects/study population. Large variations in these elements can make it difficult to compare results across the different studies and thereby difficult to draw any overall assumptions on the topic in question.
To clearly elucidate the large variation between the different studies included in this review, we tallied up the number of studies containing the different variants of each key experimental element in animal studies and human studies, respectively (see Table 6 and Table 7). Looking at Table 6 and Table 7, it becomes immediately clear that there could be a very high degree of variation between studies as a result of the different elements applied in the studies. For the animal studies (Table 6), three different species with a total of 11 different mouse and rat strains were used along with eight different IBD animal models, three main analytical platforms, 13 different sample types, and four different age groups across the 26 studies. The variation in study population and sample type was less for the human studies (Table 7), however seven different analytical platforms were applied, giving rise to a considerable heterogeneity across the human studies.
Table 6. Overview of the variation in key experimental elements in animal model studies and the number of studies containing the different versions of each element.
| Species & Strain * | Model | Analytical Platform | Biological Sample Type | Age Group (Weeks) |
|---|
| Mouse | 22 | DSS (A) | 12 | LC-MS ** | 15 | Colon | 12 | 0–3 | 3 | |||||||||||||||||||||||
| CD | 27 | NMR * | 13 | Feces | 9 | 0–1 | 0 | |||||||||||||||||||||||||
| C57BL/6 | 14 | DSS (C) | 2 | NMR *** | 8 | Plasma | 8 | >3–8 | 15 | |||||||||||||||||||||||
| UC | 24 | GC-MS ** | 11 | Urine | 9 | >1 and <18 | 6 | CD | AC | Urine | A, O | 1 | H NMR | [5] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [4] | ||||||||||||
| BALB/c | 2 | IL10-/- | (C) | 6 | GC-MS | 6 | Urine | 8 | >8–24 | 19 | ||||||||||||||||||||||
| ESI-MS | ||||||||||||||||||||||||||||||||
| IBD | 1 | LC-MS *** | 5 | Colon | 4 | 18–60 | 21 | C57Bl6/N | 1 | TNBS (A) | 3 | Serum | 7 | >24 | 2 | |||||||||||||||||
| Masseter | ||||||||||||||||||||||||||||||||
| SIFT-MS | 3 | Breath | 4 | 60+ | 13 | Winnie | [41] | |||||||||||||||||||||||||
| UC | All | Feces | A | |||||||||||||||||||||||||||||
| Feces | ||||||||||||||||||||||||||||||||
| Y, A, O | ||||||||||||||||||||||||||||||||
| FT-ICR-MS | ||||||||||||||||||||||||||||||||
| [ | ||||||||||||||||||||||||||||||||
| 15 | ||||||||||||||||||||||||||||||||
| ] | ||||||||||||||||||||||||||||||||
| Mouse | ||||||||||||||||||||||||||||||||
| Feces | ||||||||||||||||||||||||||||||||
| >8–24 | ||||||||||||||||||||||||||||||||
| GC-MS | ||||||||||||||||||||||||||||||||
| Winnie | ||||||||||||||||||||||||||||||||
| [ | ||||||||||||||||||||||||||||||||
| 21 | ||||||||||||||||||||||||||||||||
| ] | ||||||||||||||||||||||||||||||||
| Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 7 | ] | ||||||||||||||||||||||||
| CD | All | Urine | A | 1 | H NMR | [33] | Mouse | Plasma | >8–24 | 1 | H NMR | DSS (A) | [10] | acetic acid | CD, UC | All | Urine | Y | 1 | H NMR | [6] | |||||||||||
| CD | Unknown | Feces | Y, A, O | 1 | H NMR | [12] | Acetoacetatic acid | IBD | AC | Serum | A, O | 1 | H NMR | [2] | Mouse | Serum | ||||||||||||||||
| UC | >3–8 | AC | 1 | H NMR | DSS (A) | [ | 5 | ] | ||||||||||||||||||||||||
| Serum | 1 | TNFΔARE/WT | (C) | A, O | 1 | Feces | 4 | Not reported | 2 | IBD | IA | Urine | A, O | 1 | H NMR | [2 | ||||||||||||||||
| ICR | 1 | T-synthase | ] | |||||||||||||||||||||||||||||
| 1 | Liver | 4 | Acetylaspartic acid | UC | All, AC, IA | Serum | Y, A, O | GC-MS | GC-MS | [36][7] | Mouse | Colon (distal), cecum | 0–3 | UPLC/Tof-MS | T-syn deficiency | [8] | ||||||||||||||||
| Acetylcarnitine | ||||||||||||||||||||||||||||||||
| CD1 | 1 | deficiency (C) | Spleen | 2 | CD, UC | |||||||||||||||||||||||||||
| IBD | AC | Urine | A, O | 1 | H NMR | [5] | Mouse | Colon | >8–24 | LC-qTOF-MS | DSS (C) | All | Urine | [ | A, O | NMR | [26] | 9] | ||||||||||||||
| Acylcarnitine | CD | All | Urine | Y | 1 | H NMR | [6] | Mouse | Ileum (distal) | >8–24 | LC-MS | TNFΔARE/WT | ||||||||||||||||||||
| Acetylcarnitine | UC | AC | Serum | A, O | 1 | H NMR | [5] | Mouse | [ | Spleen | >8–24 | 10 | LC-qTOF-MS | ] | ||||||||||||||||||
| DSS (C) | [ | 29 | ] | Alanine | CD | All | Serum | Y, A, O | GC-MS | [11] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [6] | ||||||||||||||||
| Acetylglutamic acid | CD | IA | Feces | Unknown | UPLC-tof-MS | [4] | Mouse | Serum | >24 | UPLC-ESI-TOF-MS | H. hepaticus | [30] | CD | Unknown | Feces | Y, A, O | ||||||||||||||||
| Aconitic acid | CD, UC | All | 1 | H NMR | [12] | Mouse | Plasma | >3–24 | 1 | H NMR | IL10-/- | [ | Urine | Y | 1 | H NMR | [6] | Mouse | Urine | >3–24 | 13 | GC-MS | ] | |||||||||
| IL10 | -/- | [ | 39 | ] | [ | 20 | ] | CD, UC | AC | Feces | A, O | 1 | H NMR | [14] | ||||||||||||||||||
| UC | AC | Serum | Y, A, O | GC-MS | [7] | Arachidonic acid | CD (ICD) | IA | Feces | Y, A, O | FT-ICR-MS | [15] | Mouse | Ileum (distal) | >8–24 | LC-MS | TNF | |||||||||||||||
| IBD | All | Urine | A, O | NMR | [26] | ΔARE/WT | [16] | |||||||||||||||||||||||||
| Mouse | Colon (distal), cecum | 0–3 | UPLC/Tof-MS | T-syn deficiency | [14] | |||||||||||||||||||||||||||
| Acylcarnitine | UC | All | Urine | Y | 1 | H NMR | [6] | Mouse | Ileum (distal) | >3–24 | LC-MS | TNFΔARE/WT | [16] | Arginine | ||||||||||||||||||
| Alanine | CD | AC | Plasma, serum | A, O | 1 | H NMR | [5] | Mouse | Liver | >8–24 | LC-qTOF-MS | DSS (C) | [15] | |||||||||||||||||||
| CD | All | Urine | A | 1 | H NMR | [33] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [7] | UC | AC | Urine | A, O | 1 | H NMR | [5] | Mouse | Plasma | ||||||||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | Mouse | Urine | >3–24 | 1 | H NMR | >8–24 | 1 | H NMR | IL10-/- | [17] | |||||||||||||||||
| Adoptive transfer | [ | 16 | ] | Butanal | CD | All | Breath | A, O | SIFT-MS | [16] | Mouse | Feces | >8–24 | GC-MS | Winnie | [18] | ||||||||||||||||
| CD, UC | AC | Colonic mucosa | Unknown | Proton MRS | [17] | Carnitine | CD, UC | AC | Urine | A, O | 1 | |||||||||||||||||||||
| IBD | H NMR | [ | 5 | ] | Mouse | Colon | >8–24 | LC-qTOF-MS | DSS (C) | [ | IA | 15] | ||||||||||||||||||||
| Urine | A, O | 1 | H NMR | [ | 2] | Cholic acid | CD | IA | Feces | Y, Unknown | UPLC/ToFMS | [4 | ||||||||||||||||||||
| IBD | ] | AC | Colonic mucosa | A | 1 | H NMR | [25] | Rat | Plasma | ? | UPLC-ESI-QTOF-MS | TNBS | [19] | |||||||||||||||||||
| Creatine | CD | AC | ||||||||||||||||||||||||||||||
| Aspartic acid | CD | Plasma | A, O | 1 | H NMR | [5] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | IA | [5] | ||||||||||||||||||
| Feces | A, O | 1 | H NMR | [ | 14 | ] | Mouse | Feces | >3–8 | 1 | H NMR | DSS (A) | [22] | UC | AC | Plasma, serum | A, O | 1 | H NMR | [5] | Mouse | Plasma | >3–8 | 1 | H NMR | IL10 | ||||||
| UC | -/- | AC, IA, All | [ | Serum | Y, A, O | GC-MS | [7] | 17 | ] | |||||||||||||||||||||||
| Dimethylamine | IBD | IA | Serum | A, O | 1 | |||||||||||||||||||||||||||
| Betaine | CD, UC | AC | Plasma, urine | A, O | H NMR | [2] | Rat | 1 | Urine | H NMR | ? | UPLC-MS/MS | TNBS | [20] | ||||||||||||||||||
| [ | 5 | ] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 12 | ] | Ethylmalonic acid | UC | |||||||||||||||||||
| 129/SvEv | Rag2-/- | 1 | H. hepaticus (C) | 1 | Ileum | 1 | ||||||||||||||||||||||||||
| 1 | ||||||||||||||||||||||||||||||||
| 1 | Serum | 3 | Not reported | 1 | Mouse | |||||||||||||||||||||||||||
| FT-ICR-MS | 1 | Plasma | 2 | |||||||||||||||||||||||||||||
| Proton MRS | 1 | Ileum | 1 | 129(B6)- | Il10 | Plasma | >8–24 | 1 | H NMR | |||||||||||||||||||||||
| PBMC Macrophages | 1 | tm1Cgn | /J | 1 | Winnie | 1 | Cecum | 1 | ||||||||||||||||||||||||
| 129/SvEv | 1 | (spontaneous) (C) | Small intestine | 1 | ||||||||||||||||||||||||||||
| Rat | 3 | Adoptive | 1 | Red blood cells | 1 | All, AC, IA | Serum | Y, A, O | GC-MS | [7] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [6] | ||||||||||||||||
| Mouse | Colon | >8–24 | NMR (1H, 1C, 1P) | DSS (A) | [23] | Fructose | UC | IA | Serum | |||||||||||||||||||||||
| Butanoic acid | CD, UC | AC | Y, A, O | GC-MS | [ | 7] | Feces | Mouse | A, O | GC-MS | [41] | Feces | >8–24 | Mouse | Urine | >8–24 | GC-MS | Winnie | [21] | |||||||||||||
| 1 | H NMR | DSS (A) | [ | 16 | ] | Fumaric acid | CD, UC | All | Serum | Y, A, O | GC-MS | [11] | Mouse | Urine | >8–24 | |||||||||||||||||
| CD | AC | 1 | Feces | H NMR | A | GC-MS | [37] | Rat | Urine, Feces | ? | UPLC-MS/MS | TNBS | DSS (A) | [26[22] | ||||||||||||||||||
| Mouse | Plasma | >3–8 | 1 | H NMR | IL10-/- | [17] | ||||||||||||||||||||||||||
| ] | ||||||||||||||||||||||||||||||||
| CD | AC | Feces | A, O | 1 | H NMR | [14] | Glucose | UC | AC | Serum | A, O | 1 | H NMR | [1][5] | Mouse | Urine | >8–24 | GC-MS | IL10-/- | [23] | ||||||||||||
| CD | Unknown | Feces | Y, A, O | 1 | H NMR | [12] | UC | All | Feces | A, O | 1 | H NMR | [ | |||||||||||||||||||
| Carnitine | CD, UC | All | 24 | Urine | ] | |||||||||||||||||||||||||||
| Y | 1 | H NMR | [ | 6 | ] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [12] | UC | All, AC, IA | ||||||||||||||||||
| Citric acid | CD, UC | Serum | Y, A, O | GC-MS | AC | Serum | [7] | A, O | 1 | H NMR | [5 | ] | Mouse | |||||||||||||||||||
| Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 12 | ] | UC | IA | Colon | Unknown | Proton MRS | [17] | |||||||||||||||||||
| CD, UC | All | Urine | A | 1 | H NMR | [33 | ] | Mouse | ||||||||||||||||||||||||
| Plasma | >8–24 | UPLC-MS | DSS (A) | [ | 22 | ] | CD, UC | AC | Colon | Unknown | Proton MRS | [17] | ||||||||||||||||||||
| UC | AC | Urine | A, O | 1 | H NMR | [5] | Mouse | Serum | ||||||||||||||||||||||||
| >8–24 | GC-MS | DSS (A) | [ | 4 | ] | IBD | AC | Colon | A | 1 | H NMR | [25] | ||||||||||||||||||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | Mouse | Urine | >8–24 | NMR | IL10-/- | [28] | Glutamic acid | UC | Unknown | Feces | Y, A, O | 1 | H NMR | [12] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [ | |||||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | 6 | ] | ||||||||||||||||||||||||||
| [ | 7 | ] | Mouse | Serum | >8 | UPLC-ESI-TOF-MS | H. hepaticus | [ | 30 | ] | UC | All, AC, IA | Serum | Y, A, O | GC-MS | [7] | ||||||||||||||||
| IBD | AC, IA | Urine | A, O | 1 | H NMR | |||||||||||||||||||||||||||
| [ | 2 | ] | Glycerol | UC | AC | Serum | Y, A, O | GC-MS | [7] | Mouse | Plasma | >8–24 | 1 | H NMR | DSS (A) | [26] | ||||||||||||||||
| IBD | All | Urine | A, O | NMR | [26] | CD | AC | Plasma | A, O | |||||||||||||||||||||||
| Creatine | IBD | 1 | AC | H NMR | [ | 5] | Mouse | Serum | Feces | A, O | 1 | H NMR | [2 | >8–24 | GC-MS | Winnie | [21] | |||||||||||||||
| ] | Mouse | Plasma | >8–24 | 1 | H NMR | IL10 | -/- | |||||||||||||||||||||||||
| Sprague-Dawley | 2 | Transfer (C) | Fischer 344 | 1 | [ | 21 | Glycine | CD | AC | Serum | A, O | 1 | H NMR | [5] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [27] | ||||||||||||
| ] | ||||||||||||||||||||||||||||||||
| IBD | All | Urine | A, O | NMR | [26] | CD | AC, IA | |||||||||||||||||||||||||
| Dimethylglycine | CD | Feces | A, O | 1 | H NMR | [14] | Mouse | Feces | >8–24 | 1 | H NMR | Adoptive | All | [28] | ||||||||||||||||||
| Urine | A | 1 | H NMR | [ | 33 | ] | Mouse | Plasma | 0–3, >8–24 | 1 | H NMR | IL10-/- | [21] | CD, UC | All | Urine | Y | 1 | ||||||||||||||
| Fumaric acid | H NMR | [ | 6 | ] | UC | All | Rectum | Y, A, O | GC-MS | [11] | Mouse | transfer | ||||||||||||||||||||
| Serum | >8–24 | GC-MS | DSS (A) | [ | 4 | ] | CD, UC | All | Serum | Y, A, O | ||||||||||||||||||||||
| UC | GC-MS | AC, IA, all | Serum | Y, A, O | GC-MS | [11] | [ | 7 | ||||||||||||||||||||||||
| ] | Mouse | Liver | >8–24 | 1 | H NMR | DSS (A) | [ | 16 | ] | IBD | AC | Serum | A, O | 1 | H NMR | [2] | ||||||||||||||||
| Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 12 | ] | Hydroxybenzoic acid | UC | All, AC | Serum | Y, A, O | GC-MS | [7] | Mouse | Colon, serum | >8–24 | GC-MS | DSS (A) | ||||||||||||
| [ | 6 | ] | ||||||||||||||||||||||||||||||
| Mouse | Urine | >8–24 | NMR | IL10 | -/- | Inositol | CD | AC | Feces | A | GC-MS | [5] | Mouse | Feces | >8–24 | GC-MS | Winnie | [21] | ||||||||||||||
| Isoleucine | CD | AC | Serum | A | 1 | H NMR | [29] | Mouse | Colon, serum | >8–24 | GC-MS | DSS (A) | [6] | |||||||||||||||||||
| CD | Unknown | Feces | Y, A, O | 1 | H NMR | [12] | Mouse | Plasma | >8–24 | 1 | H NMR | IL10-/- | [17] | |||||||||||||||||||
| CD, UC | AC | Feces | A, O | 1 | H NMR | [14] | Mouse | Feces | >8–24 | 1 | H NMR | Adoptive | [10] | |||||||||||||||||||
| CD, UC | AC | Serum, plasma | A, O | 1 | H NMR | [5] | transfer | |||||||||||||||||||||||||
| IBD | AC | Serum | A, O | 1 | H NMR | [2] | ||||||||||||||||||||||||||
| [ | 28 | ] | ||||||||||||||||||||||||||||||
| Mouse | Plasma | 0–3 | 1 | H NMR | IL10-/- | [21] | IL10-/- | [21] | Kynurenine | UC | All, AC, IA | Serum | Y, A, O | GC-MS | [7] | Mouse | Plasma | >8–24 | LC-MS | IL10-/- | [30] | |||||||||||
| Glutamic acid | CD, UC | AC | Colonic mucosa | Unknown | Proton MRS | [17] | Mouse | Feces | >3–8 | 1 | H NMR | DSS (A) | [3] | Mouse | Plasma | >8–24 | UPLC-MS | DSS (A) | [31 | |||||||||||||
| CD | IA | Feces | A, O | ] | ||||||||||||||||||||||||||||
| 1 | H NMR | [ | 14 | ] | Lactic acid | CD | AC | Plasma, urine | A, O | 1 | H NMR | [5] | Mouse | Colon | >8–24 | NMR ( | 1 | |||||||||||||||
| UC | IA, All | Serum | Y, A, O | GC-MS | [7] | H, | 1 | C, | 1 | P) | DSS (A) | [32] | ||||||||||||||||||||
| UC | AC | Urine | A, O | 1 | H NMR | [5] | Mouse | Plasma | >3–24 | 1 | H NMR | IL10-/- | [17] | |||||||||||||||||||
| UC | AC | Feces | A, O | 1 | H NMR | [14] | ||||||||||||||||||||||||||
| UC | All | Urine | Y | 1 | H NMR | [6] | ||||||||||||||||||||||||||
| UC | All, AC, IA | Serum | Y, A, O | GC-MS | [7] | |||||||||||||||||||||||||||
| IBD | AC | Serum | A, O | 1 | H NMR | [2] | ||||||||||||||||||||||||||
| Leucine | CD | Unknown | Feces | Y, A, O | 1 | H NMR | [12] | Mouse | Colon, serum | >8–24 | GC-MS | DSS (A) | [6] | |||||||||||||||||||
| CD | AC, IA | Feces | A, O | 1 | H NMR | [14] | ||||||||||||||||||||||||||
| UC | AC | Feces | A, O | 1 | H NMR | [14] | ||||||||||||||||||||||||||
| Longissimus dorsi | 1 | |||||||||||||||||||||||||||||||
| Piglet | 1 | UC | All | Rectum | Y, A, O | GC-MS | [11] | |||||||||||||||||||||||||
| IBD | AC | Colonic mucosa | A | 1 | H NMR | [25] | ||||||||||||||||||||||||||
| Glutamine | CD | AC | Plasma, urine | A, O | 1 | H NMR | [5] | Mouse | Feces | >3–8 | 1 | H NMR | DSS (A) | [3] | ||||||||||||||||||
| CD | All | Serum | Y, A, O | GC-MS | [11] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [12] | ||||||||||||||||||||
| CD, UC | AC | Colonic mucosa | Unknown | Proton MRS | [17] | Mouse | Colon, serum | >8–24 | GC-MS | DSS (A) | [4] | |||||||||||||||||||||
| UC | All | Serum, rectum | Y, A, O | GC-MS | [11] | Mouse | Liver | >8–24 | 1 | H NMR | DSS (A) | [16] | ||||||||||||||||||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | Mouse | Plasma | >8–24 | 1 | H NMR | IL10-/- | [21] | ||||||||||||||||||||
| UC | AC | Serum | A, O | GC-MS | [4] | Mouse | Feces | >8–24 | 1 | H NMR | Adoptive | [33] | IBD | AC | Serum | A, O | 1 | H NMR | [2] | |||||||||||||
| IBD | AC | Colonic mucosa | ||||||||||||||||||||||||||||||
| A | 1 | H NMR | [ | 9 | ] | transfer | Linoleic acid | CD (ICD) | IA | Feces | Y, A, O | FT-ICR-MS | [15] | Mouse | Colon (distal), cecum | |||||||||||||||||
| Glycero- | >3–8 | UPLC/ToFMS | T-syn deficiency | phosphocholine | [ | 14 | CD, UC | AC | Colonic mucosa | ] | ||||||||||||||||||||||
| Unknown | Proton MRS | [ | 21 | ] | Mouse | Colon | >8–24 | 1 | H NMR | DSS (A) | [ | 16] | Lysine | CD | AC | Plasma | A, O | 1 | H NMR | [5] | Mouse | Colon, plasma, liver | >8–24 | 1 | ||||||||
| UC | IA | H NMR | Colonic mucosa | Unknown | Proton MRS | DSS (A) | [ | 26 | ] | |||||||||||||||||||||||
| [ | 21 | ] | UC | AC | Serum, plasma | A, O | 1 | H NMR | [5] | Mouse | Plasma | >3–8 | ||||||||||||||||||||
| IBD | AC | Colonic mucosa | A | 1 | H NMR | 1 | H NMR | IL10 | -/- | [ | 17] | |||||||||||||||||||||
| [ | 9 | ] | CD, UC | AC | Feces | A, O | 1 | |||||||||||||||||||||||||
| Glycine | H NMR | UC | All | Rectum | [14] | Mouse | Feces | >8–24 | 1 | H NMR | Adoptive | [10] | ||||||||||||||||||||
| Y, A, O | GC-MS | [ | 4 | ] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 12] | CD, UC | Unknown | Feces | Y, A, O | 1 | H NMR | [12 | |||||||||||||
| IBD | IA | Urine | ] | transfer | ||||||||||||||||||||||||||||
| A | 1 | H NMR | [ | 2 | ] | Mouse | Serum | >8–24 | GC-MS | DSS (A) | [4] | Maleic acid | UC | All, AC, IA | Serum | Y, A, O | GC-MS | [7] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | |||||||||
| Mouse | [ | 6 | Feces | >8–24 | GC-MS | ] | ||||||||||||||||||||||||||
| Winnie | [ | 21 | ] | Malic acid | CD | All | Serum | Y, A, O | GC-MS | [11] | Mouse | |||||||||||||||||||||
| Hippuric acid | CD | IA | Urine | A, O | 1 | Colon, serum | >8–24 | GC-MS | DSS (A) | H NMR | [42] | [6][9 | Mouse | Urine | >8–24 | ] | ||||||||||||||||
| 1 | H NMR | DSS (A) | [ | 16 | ] | Mannose | CD, UC | |||||||||||||||||||||||||
| CD, UC | AC | AC | Serum, plasma | A, O | 1 | H NMR | Urine | A, O | 1 | H NMR | [[5] | Mouse | Serum | >3–8 | 5 | 1 | H NMR | DSS (A) | [5] | |||||||||||||
| ] | Mouse | Serum | >24 | UPLC-ESI-TOF-MS | H. hepaticus | [ | 30 | ] | Methionine | CD | ||||||||||||||||||||||
| CD, UC | All | All | Urine | Serum | A | 1 | H NMR | [33] | ||||||||||||||||||||||||
| CD, UC | All | Urine | Y, A, O | 1 | H NMR | [40] | ||||||||||||||||||||||||||
| CD, UC | All | Urine | Y | 1 | H NMR | [6] | ||||||||||||||||||||||||||
| IBD | AC, IA | Urine | A, O | 1 | H NMR | [2] | Phenylacetylglycine | UC | All | Urine | A | 1 | H NMR | [33] | Mouse | Urine | >8–24 | NMR | IL10-/- | [34] | ||||||||||||
| IBD | All | Urine | A, O | NMR | [26] | |||||||||||||||||||||||||||
| Histidine | CD, UC | All | Serum | Mouse | Serum | >24 | UPLC-ESI-TOF-MS | H. hepaticus | [35] | |||||||||||||||||||||||
| Y, A, O | GC-MS | [ | 11 | ] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 12] | ||||||||||||||||||||
| UC | AC, IA, All | Rat | Urine | ? | UPLC-MS/MS, UPLC-ESI-QTOF-MS | TNBS | [36][26] | |||||||||||||||||||||||||
| Serum | Y, A, O | GC-MS | [ | 7 | ] | Phenylalanine | CD | AC | Feces | |||||||||||||||||||||||
| IBD | AC | Serum | A, O | 1 | H NMR | [14] | Mouse | Plasma | >8–24 | 1 | H NMR | A, O | DSS (A) | 1 | [10] | |||||||||||||||||
| H NMR | [ | 2 | ] | UC | AC | Serum | A, O | |||||||||||||||||||||||||
| IBD | All | Urine | A, O | 1 | H NMR | [1] | Mouse | Colon, serum | >8–24 | GC-MS | DSS (A) | [ | NMR | [26]11] | ||||||||||||||||||
| IBD | AC | Serum | A, O | 1 | H NMR | [ | ||||||||||||||||||||||||||
| Hypoxanthine | CD | 2 | AC | Urine | A, O | ] | Mouse | Plasma | 1 | >3–24 | H NMR | 1 | [5] | H NMR | IL10-/- | [21] | ||||||||||||||||
| Mouse | Spleen | >8–24 | 1 | H NMR | DSS (A) | [ | 16 | ] | Mouse | Feces | >8–24 | GC-MS | Winnie | [37] | ||||||||||||||||||
| Inositol | CD, UC | AC | Colonic mucosa | Unknown | Proton MRS | [21] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [13] | Mouse | Feces | >8–24 | 1 | H NMR | Adoptive transfer | [16] | |||||||||||||
| UC | IA | Colonic mucosa | Unknown | Proton MRS | [21] | Proline | CD | AC | Serum | A, O | 1 | H NMR | [5] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [11] | |||||||||||||
| CD | ||||||||||||||||||||||||||||||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | All | ||||||||||||||||||||||||||
| IBD | Serum | Y, A, O | GC-MS | AC | Colonic mucosa | A | 1 | H NMR | [11] | [9 | ] | |||||||||||||||||||||
| Prostaglandin E2 | CD | Unknown | Urine | A | LC-MS | |||||||||||||||||||||||||||
| Isocitric acid | [ | 21 | ] | Rat | Colon | >8–24 | LC-MS | DSS (C) | [ | 38 | ] | |||||||||||||||||||||
| UC | All | Rectum | Y, A, O | GC-MS | [ | 11] | Mouse | Serum | >8–24 | GC-MS | DSS (A) | [4] | Pyruvic acid | UC | AC | Serum, urine | A, O | 1 | H NMR | [5] | ||||||||||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | Mouse | Plasma | >3–24 | Mouse | 1 | H NMR | IL10-/- | Urine | [21] | ||||||||||||||||||
| >3–24 | GC-MS | IL10 | -/- | [ | 6 | ] | [ | 26 | ] | Mouse | Feces | >8–24 | GC-MS | Winnie | [ | |||||||||||||||||
| Isoleucine | CD, UC | 37 | ] | |||||||||||||||||||||||||||||
| AC | Colonic mucosa | Unknown | Proton MRS | [ | 21 | ] | Mouse | Feces | >8–24 | Succinic acid | CD | All | Serum | Y, A, O | GC-MS | [11] | Mouse | Urine | >3–24 | GC-MS | IL10-/- | [20] | ||||||||||
| Mouse | Colon | >8–24 | GC-MS | DSS (A) | [11] | |||||||||||||||||||||||||||
| Mouse | Plasma | >3–8 | 1 | H NMR | IL10-/- | [21] | ||||||||||||||||||||||||||
| Rat | Urine | ? | UPLC-MS/MS | TNBS | ||||||||||||||||||||||||||||
| Glucose | CD | AC | Plasma | A, O | 1 | H NMR | [5] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [12] | ||||||||||||||||||
| Mouse | Plasma, liver | >8–24 | 1 | H NMR | DSS (A) | [16] | ||||||||||||||||||||||||||
| Mouse | Serum | >8–24 | GC-MS | DSS (A) | [13] | |||||||||||||||||||||||||||
| Mouse | Urine | >3–24 | GC-MS | IL10 | Y, A, O | GC-MS | [11] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [6] | |||||||||||||||||||
| UC | AC | Serum | A, O | 1 | H NMR | [5] | Mouse | Plasma | >3–8 | 1 | H NMR | IL10-/- | [17] | |||||||||||||||||||
| -/- | Mouse | Feces | >8–24 | GC-MS | Winnie | [26] | ||||||||||||||||||||||||||
| [ | [ | 21 | ] | |||||||||||||||||||||||||||||
| GC-MS | Winnie | [ | 21 | ] | ||||||||||||||||||||||||||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | |||||||||||||||||||||||||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | |||||||||||||||||||||||||||
| Lactic acid | CD | AC | Colonic mucosa | Unknown | Proton MRS | [21] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [12] | |||||||||||||||||||
| UC | AC, IA | Colonic mucosa | Unknown | Proton MRS | [21] | Taurocholic acid | CD (ICD) | IA | Feces | Y, A, O | FT-ICR-MS | [15] | Mouse | Colon (distal), cecum | >3–8 | UPLC/ToFMS | T-syn deficiency | [25] | ||||||||||||||
| IBD | AC | Colonic mucosa | A | NMR | [9] | Threonine | CD, UC | |||||||||||||||||||||||||
| Leucine | CD, UC | AC | Urine | A, O | 1 | H NMR | [5] | Mouse | Colon, serum | >8–24 | GC-MS | DSS (A) | [11] | |||||||||||||||||||
| AC | Colonic mucosa | Unknown | Proton MRS | [ | 21 | ] | Mouse | Plasma | >8–24 | 1 | H NMR | IL10-/- | [21] | Adoptive transfer | [16] | |||||||||||||||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | UC | All | Urine | Y | 1 | ||||||||||||||||||||||
| UC | H NMR | [ | 6 | ] | AC | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [7] | ||||||||||||||||||||
| Plasma | A, O | 1 | H NMR | [ | 5] | Mouse | Liver | >8–24 | LC-qTOF-MS | DSS (C) | [29] | |||||||||||||||||||||
| Lysine | UC | All | Rectum | Y, A, O | GC-MS | Tyrosine | CD | AC | Feces | A, O | 1 | H NMR | [14] | Mouse | Colon | >8–24 | GC-MS | DSS (A) | [11] | |||||||||||||
| 1 | H NMR | [ | 5 | ] | Feces | |||||||||||||||||||||||||||
| UC | AC | Feces | A, O | GC-MS | 6 | ] | [ | 26 | ] | Oleic acid | CD (ICD) | IA | Tryptophan | UC | AC | Urine | A, O | 1 | H NMR | [5] | Mouse | Feces | >8–24 | 1 | [11] | Mouse | Feces | >3–8 | 1 | H NMR | DSS (A) | [22] |
| UC | All, IA | Serum | Y, A, O | GC-MS | [7] | CD (ICD) | IA | Feces | Y, A, O | FT-ICR-MS | [15] | Mouse | Plasma | >3–8 | 1 | H NMR | IL10-/- | [21] | ||||||||||||||
| Mouse | ||||||||||||||||||||||||||||||||
| IBD | All | Urine | A, O | NMR | [26] | Feces | >8–24 | 1 | H NMR | Adoptive transfer | ||||||||||||||||||||||
| Malic acid | UC | AC, IA, All | Serum | Y, A, O | [ | 16 | GC-MS | [7] | ] | |||||||||||||||||||||||
| Mouse | Serum | >8–24 | GC-MS | DSS (A) | [ | 4 | ] | Uracil | UC | All, AC, IA | Serum | Y, A, O | GC-MS | [7] | Mouse | Urine | >3–24 | GC-MS, NMR | IL10-/- | [39][20][28] | ||||||||||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | |||||||||||||||||||||||||||
| Methionine | UC | AC, IA, All | Serum | Y, A, O | Mouse | Colon, serum | >8–24 | GC-MS | DSS (A) | [11] | ||||||||||||||||||||||
| H NMR | GC-MS | [ | 7 | ] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 12] | Urea | UC | All, AC, IA | Serum | Y, A, O | GC-MS | [7] | Mouse | Serum | >8–24 | GC-MS | DSS (A) | [15] | |||||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | Mouse | Plasma | >8–24 | 1 | H NMR | IL10-/- | [21] | Valine | CD | Unknown | Feces | Y, A, O | 1 | H NMR | |||||||||||||
| Methylamine | CD, UC | Unknown | Feces | Y, A, O | [ | 12] | Mouse | Plasma | >8–24 | 1 | H NMR | [12] | 1 | H NMR | DSS (A) | [10] | ||||||||||||||||
| Mouse | Urine | >8–24 | 1 | H NMR | DSS (A) | [ | 16 | ] | CD | |||||||||||||||||||||||
| IBD | AC, IA | Feces | A, O | 1 | H NMR | [ | All | Urine | A, O | 14] | Mouse | Colon, serum | >8–24 | GC-MS | NMR | [26] | DSS (A) | A, O | 1 | H NMR | [14] | |||||||||||
| Proline | UC | AC, All | Serum | Y, A, O | GC-MS | [7] | Mouse | |||||||||||||||||||||||||
| Serum | >3–8 | 1 | H NMR | DSS (A) | [ | 12 | ] | Xylose | CD | AC | ||||||||||||||||||||||
| UC | All | Urine | A, O | 1 | H NMR | [5] | Mouse | Feces | >8–24 | GC-MS | Winnie | [37] | ||||||||||||||||||||
| Rectum | Y, A, O | GC-MS | [ | 11 | ] | Mouse | Urine | >8-24 | 1 | H NMR | Adoptive transfer | [33] | UC | AC | Serum | Y, A, O | GC-MS | |||||||||||||||
| Sebacic acid | UC | IA | Serum | Y, A, O | GC-MS | [7] | Mouse | Feces | >8–24 | GC-MS | Winnie | [21] | ||||||||||||||||||||
| Succinic acid | CD | AC | Plasma, urine | A, O | 1 | H NMR | [5] | Mouse | Serum | >8–24 | GC-MS | DSS (A) | [4] | |||||||||||||||||||
| CD, UC | AC | Colonic mucosa | Unknown | Proton MRS | [21] | Mouse | Urine | >8–24 | NMR | IL10-/- | [28] | |||||||||||||||||||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | Mouse | Urine | >8–24 | 1 | H NMR | Adoptive transfer | [33] | ||||||||||||||||||||
| UC | AC | Urine | A, O | 1 | H NMR | [5] |
Table 7. Overview of the variation in key experimental elements in human studies and the number of studies containing the different versions of each element in the systematic review on metabolomics in inflammatory bowel disease (IBD) patients and IBD animal models.
| IBD/IBD Subtype | Analytical Platform | Biological Sample Type | Age Group (Years) |
|---|
| UC | |||||||||||||||||
| All | |||||||||||||||||
| Urine | |||||||||||||||||
| Y | |||||||||||||||||
| 1 | |||||||||||||||||
| H NMR | |||||||||||||||||
| [ | |||||||||||||||||
| 6 | |||||||||||||||||
| ] | |||||||||||||||||
| UC | |||||||||||||||||
| All | |||||||||||||||||
| Rectum tissue | |||||||||||||||||
| Y, A, O | |||||||||||||||||
| GC-MS | |||||||||||||||||
| [ | |||||||||||||||||
| 11 | |||||||||||||||||
| ] | |||||||||||||||||
| IBD | |||||||||||||||||
| AC, IA | |||||||||||||||||
| Urine | |||||||||||||||||
| A, O | |||||||||||||||||
| 1 | |||||||||||||||||
| H NMR | |||||||||||||||||
| [ | |||||||||||||||||
| 2 | |||||||||||||||||
| ] | |||||||||||||||||
| IBD | |||||||||||||||||
| All | |||||||||||||||||
| Urine | |||||||||||||||||
| A, O | |||||||||||||||||
| NMR | |||||||||||||||||
| [ | |||||||||||||||||
| 26 | |||||||||||||||||
| ] | |||||||||||||||||
| Taurine | CD, UC | All | Urine | Y | 1 | H NMR | [6] | Mouse | Colon, spleen | >8–24 | 1 | H NMR | DSS (A) | [16] | [ | 13 | ] |
| CD | AC | Urine | A, O | 1 | H NMR | [5] | |||||||||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | ||||||||||||
| IBD | AC, IA | Urine | A, O | 1 | H NMR | [2] | |||||||||||
| IBD | All | Urine | A, O | NMR | [26] | ||||||||||||
| Threonine | UC | IA, All | Serum | Y, A, O | GC-MS | [7] | Mouse | Feces | >3–8 | 1 | H NMR | DSS (A) | [22] | ||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | ||||||||||||
| Triglyceride | UC | All | Plasma | A | LC-MS/MS | [43] | Mouse | Colon (proximal), ileum (distal) | >8–24 | 1 | H NMR | TNFΔARE/WT | [10] | ||||
| Mouse | Liver | >8–24 | 1 | H NMR | Adoptive transfer | [33] | |||||||||||
| Trimethylamine | CD, UC | Unknown | Feces | Y, A, O | 1 | H NMR | [12] | Mouse | Plasma | >8–24 | 1 | H NMR | IL10-/- | [21] | |||
| Tryptophan | CD, UC | All | Serum | Y, A, O | GC-MS | [11] | Mouse | Plasma | >8–24 | UPLC-MS | DSS (A) | [22] | |||||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | Mouse | Serum | >8–24 | GC-MS | DSS (A) | [4] | ||||||
| Mouse | Plasma | >8–24 | LC-MS | IL10-/- | [26] | ||||||||||||
| Tyrosine | CD | AC | Plasma | A, O | 1 | H NMR | [5] | Mouse | Serum | >3–8 | 1 | H NMR | DSS (A) | [12] | |||
| UC | AC, IA, All | Serum | Y, A, O | GC-MS | [7] | Mouse | Serum | >8–24 | GC-MS | DSS (A) | [4] | ||||||
| UC | AC | Serum, plasma | A, O | 1 | H NMR | [5] | Mouse | Plasma | >8–24 | UPLC-MS | DSS (A) | [22] | |||||
| UC | All | Rectum | Y, A, O | GC-MS | [11] | Mouse | Plasma | >8–24 | 1 | H NMR | IL10-/- | [21] | |||||
| Mouse | Feces | >8–24 | GC-MS | Winnie | [21] |
A few studies did, however, share a high degree of similarity in experimental factors. Animal studies by Shiomi et al., Gu et al., and Wang et al. all used C57BL/6J mice from the same age group for a 3% DSS model as well as using gas chromatography-mass spectrometry (GC-MS) to detect metabolites in serum and colon samples (see Supplementary Table S2) [4][29][44], although it is worth noting that Gu et al. and Wang et al. belong to the same department at Kobe University, Japan. Equally, two studies by the same first author also shared a similar degree of similarity using an IL10-/- model [34][35]. For the human studies, two studies used proton nuclear magnetic resonance (1H-NMR) to detect metabolites in serum samples from CD and UC patients of 18-60+ years of age [2][5], while two other studies detected metabolites in serum samples from CD and UC patients in the >1–60+ age groups using GC-MS [8][38]. The authors of the latter two studies are also from the same department and even co-authors the other study, again underlining the difficulties at present comparing studies from different research groups.
6. Differentiation of Metabolites According to Key Experimental Elements
We found that in both human and animal studies, the vast majority of the metabolites were detected by more than one analytical platform (Supplementary Table S9). The study subjects in most of the human studies spanned all age groups from very early onset and young to old, making it difficult to differentiate metabolite detection between age groups in the human studies. However, most metabolites were generally detected in more than one age group in the animal studies, suggesting that age is not a deciding factor when it comes to the metabolome. Nevertheless the amino acid isoleucine stood out, as it was increased only in human subjects above 18 years of age and in mice of >8–24 weeks. One of the animal studies that reported increased levels of isoleucine also included animals of 1 week, but the amino acid was not significantly altered in this group [37].
The subgroup of metabolites differentiated in both study types was sorted according to the biological sample types in which they were detected (Supplementary Table S9). This allowed us to examine any parallels between human and animal studies. Many metabolites were found in several different sample types in both humans and animals, but not necessarily the same. For example, alanine was increased in serum [38] and feces [14][15] from humans and in colon [4] and plasma [37] from mice, but it was decreased in urine [5] and colon [11][25] in humans and serum [12] and urine [33] in animals, illustrating the differences observed for many metabolites (Supplementary Table S9). The highest similarity to human studies was observed with the acute DSS mouse model (Supplementary Table S9). Since this model was used in almost half of the included animal studies, this finding is not surprising. However, only five of the acute DSS mouse model studies analyzed serum samples, but still 11 of the increased and 11 of the decreased metabolites were detected in serum samples from both humans and the DSS mouse model. A total of 34 and 29 different metabolites were reported as increased and decreased in IBD, respectively, in serum samples from the acute DSS mouse model. This means that 32% of the increased metabolites and 38% of the decreased metabolites in serum samples from the acute DSS mouse model were reported to be correspondingly differentiated in the human studies. Conversely, the acute DSS mouse model could account for 16% (22 out of 136 metabolites) of the overall metabolite changes observed in serum of IBD patients. This could suggest serum samples from the acute DSS mouse model as having good translational potential when analyzing systemic metabolites in IBD.
7. Correlation between Animal Models and IBD Subtypes
For all the metabolites significantly differing in both human and animal studies, it was investigated if some animal models were specifically good models for CD or UC when it comes to metabolomics (Supplementary Table S9). Most of the models had similarities with both CD and UC. For instance, regarding metabolites decreased in the IL10−/– mouse model, glucose was also decreased in CD, while leucine was decreased in UC, and trimethylamine in both CD and UC. The TNFΔARE/WT model only had similarities with UC, but this could easily be due to the fact that only one study with this model was included. Overall, this indicates that the metabolomes of the animal models included in this review are not correlated specifically to CD or UC.
8. Metabolite Classifications
All metabolites differentiated between IBD cases and controls in either humans or animals were sorted into metabolite subclasses according to the classification system used in The Human Metabolome Database (www.hmdb.ca) (Supplementary Tables S10 and S11). The most differentiated subclass was “amino acids, peptides, and analogues” in both human and animal studies, representing approximately 16% of all differentiated metabolites reported. “Fatty acids and conjugates” as well as “carbohydrates and carbohydrate conjugates” were also among the most differentiated in both human and animal study types. “Glycerophosphocholines” were also differentiated in both, but to a much larger extent in animal studies. In general, different kinds of lipids were reported more frequently as differentiated in IBD in animal studies compared to human studies. Metabolites from 142 different subclasses were reported as differentiated between IBD and controls overall. Of these, 47 were differentiated in both human and animal studies, while 48 and 47 differentiated subclasses were unique to human and animal studies, respectively. This shows a large gap between the type of metabolites that are investigated and detected in the two study types, as only a third of the total amount of differentiated subclasses are reported in both.
When focusing on the metabolites differentiated in IBD in both human and animal studies, they represented a total of 25 subclasses overall. Metabolites from nine different subclasses were present among both the increased and decreased metabolites, while eight subclasses were exclusively increased and decreased, respectively.
References
- Ying Zhang; Lianjie Lin; Yanbin Xu; Yan Lin; Yu Jin; Changqing Zheng; 1H NMR-based spectroscopy detects metabolic alterations in serum of patients with early-stage ulcerative colitis. Biochemical and Biophysical Research Communications 2013, 433, 547-551, 10.1016/j.bbrc.2013.03.012.
- Tomasz Dawiskiba; Stanisław Deja; Agata Mulak; Adam Ząbek; Ewa Jawień; Dorota Pawełka; Mirosław Banasik; Agnieszka Mastalerz-Migas; Waldemar Balcerzak; Krzysztof Kaliszewski; et al.Jan SkóraPiotr BarćKrzysztof KortaKornel PormańczukPrzemyslaw SzyberAdam LitarskiPiotr Młynarz Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World Journal of Gastroenterology 2014, 20, 163-174, 10.3748/wjg.v20.i1.163.
- Natasha S. Stephens; Jesse Siffledeen; Xiaorong Su; Travis B. Murdoch; R. Fedorak; Carolyn M. Slupsky; Urinary NMR metabolomic profiles discriminate inflammatory bowel disease from healthy. Journal of Crohn's and Colitis 2013, 7, e42-e48, 10.1016/j.crohns.2012.04.019.
- Yuuki Shiomi; Shin Nishiumi; Makoto Ooi; Naoya Hatano; Masakazu Shinohara; Tomoo Yoshie; Yasuyuki Kondo; Keisuke Furumatsu; Hideyuki Shiomi; Hiromu Kutsumi; et al.Takeshi AzumaMasaru Yoshida GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflammatory Bowel Diseases 2011, 17, 2261-2274, 10.1002/ibd.21616.
- Arnald Alonso; Antonio Julià; Maria Vinaixa; Eugeni Domènech; Antonio Fernández-Nebro; Juan D. Cañete; Carlos Ferrándiz; Jesús Tornero; Javier P. Gisbert; Pilar Nos; et al.Ana Gutiérrez CasbasLluís PuigIsidoro González-ÁlvaroJosé A. Pinto-TasendeRicardo BlancoMiguel A. RodríguezAntoni BeltranXavier CorreigSara MarsalIMID Consortiumfor the IMID ConsortiumEmilia Fernández Urine metabolome profiling of immune-mediated inflammatory diseases.. BMC Medicine 2016, 14, 133, 10.1186/s12916-016-0681-8.
- J. Chad Johnson; Carl R. Schmidt; Martha J Shrubsole; D. Dean Billheimer; Prashant R. Joshi; J D Morrow; Martin J. Heslin; M. Kay Washington; Reid M. Ness; Wei Zheng; et al.David A. SchwartzRobert J. CoffeyR. Daniel BeauchampNipun B Merchant Urine PGE-M: A Metabolite of Prostaglandin E2 as a Potential Biomarker of Advanced Colorectal Neoplasia. Clinical Gastroenterology and Hepatology 2006, 4, 1358-1365, 10.1016/j.cgh.2006.07.015.
- Gwénaëlle Le Gall; Samah O. Noor; Karyn Ridgway; Louise Scovell; Crawford Jamieson; Ian Johnson; Ian J. Colquhoun; E. Kate Kemsley; Arjan Narbad; Metabolomics of Fecal Extracts Detects Altered Metabolic Activity of Gut Microbiota in Ulcerative Colitis and Irritable Bowel Syndrome. Journal of Proteome Research 2011, 10, 4208-4218, 10.1021/pr2003598.
- Michitaka Kohashi; Shin Nishiumi; Makoto Ooi; Tomoo Yoshie; Atsuki Matsubara; Makoto Suzuki; Namiko Hoshi; Koji Kamikozuru; Yoko Yokoyama; Ken Fukunaga; et al.Shiro NakamuraTakeshi AzumaMasaru Yoshida A novel gas chromatography mass spectrometry-based serum diagnostic and assessment approach to ulcerative colitis. Journal of Crohn's and Colitis 2014, 8, 1010-1021, 10.1016/j.crohns.2014.01.024.
- Jonathan P. Jacobs; Lin Lin; Maryam Goudarzi; Paul Ruegger; Dermot P. B. McGovern; Albert J. Fornace; James Borneman; Lijun Xia; Jonathan Braun; Microbial, metabolomic, and immunologic dynamics in a relapsing genetic mouse model of colitis induced by T-synthase deficiency. Gut Microbes 2016, 8, 1-16, 10.1080/19490976.2016.1257469.
- Douglas J. Kominsky; Simon Keely; Christopher F. MacManus; Louise E. Glover; Melanie Scully; Colm B. Collins; Brittelle E. Bowers; Eric Campbell; Sean P. Colgan; An endogenously anti-inflammatory role for methylation in mucosal inflammation identified through metabolite profiling.. The Journal of Immunology 2011, 186, 6505-14, 10.4049/jimmunol.1002805.
- Krithika Balasubramanian; Sandeep Kumar; Rajeev R. Singh; Uma Sharma; Vineet Ahuja; Govind K. Makharia; Naranamangalam R. Jagannathan; Metabolism of the colonic mucosa in patients with inflammatory bowel diseases: an in vitro proton magnetic resonance spectroscopy study. Magnetic Resonance Imaging 2009, 27, 79-86, 10.1016/j.mri.2008.05.014.
- Rudolf Schicho; Alsu Nazyrova; Rustem Shaykhutdinov; Gavin Duggan; Hans J. Vogel; Martin Storr; Quantitative Metabolomic Profiling of Serum and Urine in DSS-Induced Ulcerative Colitis of Mice by1H NMR Spectroscopy. Journal of Proteome Research 2010, 9, 6265-6273, 10.1021/pr100547y.
- Lucy C. Hicks; Juzheng Huang; Sacheen Kumar; Sam T. Powles; Timothy R. Orchard; George B. Hanna; H.R.T. Williams; Analysis of Exhaled Breath Volatile Organic Compounds in Inflammatory Bowel Disease: A Pilot Study. Journal of Crohn's and Colitis 2015, 9, 731-737, 10.1093/ecco-jcc/jjv102.
- Julian R Marchesi; Elaine Holmes; Fatima Khan; Sunil Kochhar; Pauline D. Scanlan; Fergus Shanahan; Ian D Wilson; Yulan Wang; Rapid and Noninvasive Metabonomic Characterization of Inflammatory Bowel Disease. Journal of Proteome Research 2007, 6, 546-551, 10.1021/pr060470d.
- Jacob Tveiten Bjerrum; Yulan Wang; Fuhua Hao; Mehmet Coskun; Christian Ludwig; Ulrich Günther; Ole Haagen Nielsen; Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics 2014, 11, 122-133, 10.1007/s11306-014-0677-3.
- Fangcong Dong; Lulu Zhang; Fuhua Hao; Huiru Tang; Yulan Wang; Systemic Responses of Mice to Dextran Sulfate Sodium-Induced Acute Ulcerative Colitis Using1H NMR Spectroscopy. Journal of Proteome Research 2013, 12, 2958-2966, 10.1021/pr4002383.
- H.R.T. Williams; I. Jane Cox; David G Walker; Bernard V North; Venisha M Patel; Sara E Marshall; Derek P Jewell; Subrata Ghosh; Huw J W Thomas; Julian Teare; et al.Simon JakobovitsSebastian ZekiKenneth I WelshSimon D Taylor-RobinsonTimothy R Orchard Characterization of Inflammatory Bowel Disease With Urinary Metabolic Profiling. American Journal of Gastroenterology 2009, 104, 1435-1444, 10.1038/ajg.2009.175.
- Travis B. Murdoch; Hao Fu; Sarah Macfarlane; Beate C. Sydora; R. Fedorak; Carolyn M. Slupsky; Urinary Metabolic Profiles of Inflammatory Bowel Disease in Interleukin-10 Gene-Deficient Mice. Analytical Chemistry 2008, 80, 5524-5531, 10.1021/ac8005236.
- Jing Liu; Hai-Tao Xiao; Hong-Sheng Wang; Huai-Xue Mu; Ling Zhao; Jun Du; Depo Yang; Dongmei Wang; Zhaoxiang Bian; Shu-Hai Lin; et al. Halofuginone reduces the inflammatory responses of DSS-induced colitis through metabolic reprogramming. Molecular BioSystems 2016, 12, 2296-2303, 10.1039/C6MB00154H.
- Kun Lu; Charles G. Knutson; John Wishnok; James G. Fox; Steven R. Tannenbaum; Serum Metabolomics in aHelicobacter hepaticusMouse Model of Inflammatory Bowel Disease Reveal Important Changes in the Microbiome, Serum Peptides, and Intermediary Metabolism. Journal of Proteome Research 2012, 11, 4916-4926, 10.1021/pr300429x.
- Fariba Fathi; Laleh Majari-Kasmaee; Ahmad Mani-Varnosfaderani; Anahita Kyani; Mohammad Rostami Nejad; Kaveh Sohrabzadeh; Nosratollah Naderi; Mohammad Reza Zali; Mostafa Rezaei Tavirani; Mohsen Tafazzoli; et al.Afsaneh Arefi Oskouie 1H NMR based metabolic profiling in Crohn's disease by random forest methodology. Magnetic Resonance in Chemistry 2014, 52, 370-376, 10.1002/mrc.4074.
- Ina Willenberg; Annika I. Ostermann; Samoa Giovannini; Olivia Kershaw; Anne Von Keutz; Pablo Steinberg; Nils Helge Schebb; Effect of acute and chronic DSS induced colitis on plasma eicosanoid and oxylipin levels in the rat. Prostaglandins & Other Lipid Mediators 2015, 120, 155-160, 10.1016/j.prostaglandins.2015.04.002.
- Jonathan P. Jacobs; Maryam Goudarzi; Namita Singh; Maomeng Tong; Ian H. McHardy; Paul Ruegger; Miro Asadourian; Bo-Hyun Moon; Allyson Ayson; James Borneman; et al.Dermot P.B. McGovernAlbert J. FornaceJonathan BraunMarla Dubinsky A Disease-Associated Microbial and Metabolomics State in Relatives of Pediatric Inflammatory Bowel Disease Patients. Cellular and Molecular Gastroenterology and Hepatology 2016, 2, 750-766, 10.1016/j.jcmgh.2016.06.004.
- Rudolf Schicho; Rustem Shaykhutdinov; Jennifer Ngo; Alsu Nazyrova; Christopher Schneider; Remo Panaccione; Gilaad G Kaplan; Hans J. Vogel; Martin Storr; Quantitative Metabolomic Profiling of Serum, Plasma, and Urine by1H NMR Spectroscopy Discriminates between Patients with Inflammatory Bowel Disease and Healthy Individuals. Journal of Proteome Research 2012, 11, 3344-3357, 10.1021/pr300139q.
- Uma Sharma; Rajiv R. Singh; Vineet Ahuja; Govind K. Makharia; Naranamangalam R. Jagannathan; Similarity in the metabolic profile in macroscopically involved and un-involved colonic mucosa in patients with inflammatory bowel disease: an in vitro proton (1H) MR spectroscopy study. Magnetic Resonance Imaging 2010, 28, 1022-1029, 10.1016/j.mri.2010.03.039.
- Jonathan Kaunitz; Machiels K; Joossens M; Sabino J; De Preter V; Arijs I; Eeckhaut V; Ballet V; Claes K; Van Immerseel F; et al.Verbeke KFerrante MVerhaegen JRutgeerts PVermeire S Faculty Opinions recommendation of A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2015, 63, 1275–1283, 10.3410/f.718105672.793509782.
- Baur, P.; Martin, F.P.; Gruber, L.; Bosco, N.; Brahmbhatt, V.; Collino, S.; Guy, P.; Montoliu, I.; Rozman, J.; Klingenspor, M.; et al.et al Metabolic phenotyping of the Crohn’s disease-like IBD etiopathology in the TNFDELTAARE/WT mouse model. J. Proteome Res. 2011, 10, 5523–5535.
- Zhang, X.; Choi, F.F.; Zhou, Y.; Leung, F.P.; Tan, S.; Lin, S.; Xu, H.; Jia, W.; Sung, J.J.; Cai, Z.; et al.et al Metabolite profiling of plasma and urine from rats with TNBS-induced acute colitis using UPLC-ESI-QTOF-MS-based metabonomics--a pilot study. FEBS J. 2012, 279, 2322–2338.
- Gu, X.; Song, Y.; Chai, Y.; Lu, F.; Gonzalez, F.J.; Fan, G.; Qi, Y; GC-MS metabolomics on PPARalpha-dependent exacerbation of colitis. Mol. Biosyst. 2015, 11, 1329–1337.
- Hou, W.; Zhong, D.; Zhang, P.; Li, Y.; Lin, M.; Liu, G.; Yao, M.; Liao, Q.; Xie, Z; A strategy for the targeted metabolomics analysis of 11 gut microbiota-host co-metabolites in rat serum, urine and feces by ultra high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 207–217.
- Chang Qu; Zhong-Wen Yuan; Xiu-Ting Yu; Yan-Feng Huang; Guang-Hua Yang; Jian-Nan Chen; Xiao-Ping Lai; Zi-Ren Su; Hui-Fang Zeng; Ying Xie; et al.Huang Song Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacological Research 2017, 121, 70-82, 10.1016/j.phrs.2017.04.017.
- Robinson, A.M.; Gondalia, S.V.; Karpe, A.V.; Eri, R.; Beale, D.J.; Morrison, P.D.; Palombo, E.A.; Nurgali, K; Fecal microbiota and metabolome in a mouse model of spontaneous chronic colitis: Relevance to human inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 2767–2787.
- Martin, F.P.J.; Lichti, P.; Bosco, N.; Brahmbhatt, V.; Oliveira, M.; Haller, D.; Benyacoub, J; Metabolic phenotyping of an adoptive transfer mouse model of experimental colitis and impact of dietary fish oil intake. J. Proteome Res. 2015, 14, 1911–1919.
- Lin, H.M.; Edmunds, S.J.; Helsby, N.A.; Ferguson, L.R.; Rowan, D.D; Nontargeted urinary metabolite profiling of a mouse model of crohn’s disease. J. Proteome Res. 2009, 8, 2045–2057.
- Hui-Ming Lin; Matthew P. G. Barnett; Nicole Roy; Nigel I. Joyce; Shuotun Zhu; Kelly Armstrong; Nuala Helsby; Lynnette R. Ferguson; Daryl Rowan; Metabolomic Analysis Identifies Inflammatory and Noninflammatory Metabolic Effects of Genetic Modification in a Mouse Model of Crohn’s Disease. Journal of Proteome Research 2010, 9, 1965-1975, 10.1021/pr901130s.
- Janet K. Jansson; Ben Willing; Marianna Lucio; Ages Fekete; Johan Dicksved; Jonas Halfvarson; Curt Tysk; Philippe Schmitt-Kopplin; Metabolomics Reveals Metabolic Biomarkers of Crohn's Disease. PLOS ONE 2009, 4, e6386, 10.1371/journal.pone.0006386.
- Francois-Pierre Martin; Serge Rezzi; David Philippe; Lionel Tornier; Anja Messlik; Gabriele Hölzlwimmer; Pia Baur; Leticia Quintanilla-Fend; Gunnar Loh; Michael Blaut; et al.Stéphanie BlumSunil KochharDirk Haller Metabolic Assessment of Gradual Development of Moderate Experimental Colitis in IL-10 Deficient Mice. Journal of Proteome Research 2009, 8, 2376-2387, 10.1021/pr801006e.
- Makoto Ooi; Shin Nishiumi; Tomoo Yoshie; Yuuki Shiomi; Michitaka Kohashi; Ken Fukunaga; Shiro Nakamura; Takayuki Matsumoto; Naoya Hatano; Masakazu Shinohara; et al.Yasuhiro IrinoTadaomi TakenawaTakeshi AzumaMasaru Yoshida GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflammation Research 2011, 60, 831-840, 10.1007/s00011-011-0340-7.
- Vicky De Preter; Marie Joossens; Vera Ballet; Ziv Shkedy; Paul Rutgeerts; Séverine Vermeire; Kristin Verbeke; Metabolic Profiling of the Impact of Oligofructose-Enriched Inulin in Crohnʼs Disease Patients: A Double-Blinded Randomized Controlled Trial. Clinical and Translational Gastroenterology 2013, 4, e30-e30, 10.1038/ctg.2012.24.
- I. Ahmed; R. Greenwood; B. Costello; Norman Ratcliffe; C. S. Probert; Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2016, 43, 596-611, 10.1111/apt.13522.
- Francois-Pierre Martin; Jessica Ezri; Ornella Cominetti; Laeticia Da Silva; Martin Kussmann; Jean-Philippe Godin; Andreas Nydegger; Urinary Metabolic Phenotyping Reveals Differences in the Metabolic Status of Healthy and Inflammatory Bowel Disease (IBD) Children in Relation to Growth and Disease Activity. International Journal of Molecular Sciences 2016, 17, 1310, 10.3390/ijms17081310.
- Williams, H.R.; Cox, I.J.; Walker, D.G.; Cobbold, J.F.; Taylor-Robinson, S.D.; Marshall, S.E.; Orchard, T; Differences in gut microbial metabolism are responsible for reduced hippurate synthesis in Crohn's disease. Gastroenterology 2010, 138, S579.
- Yunki Yau; Rupert W Leong; Sean Shin; Sonia Bustamante; Russell Pickford; Leila Hejazi; Beth Campbell; Valerie C. Wasinger; Bimodal plasma metabolomics strategy identifies novel inflammatory metabolites in inflammatory bowel diseases. Discovery medicine 2014, 18, 113–124.
- Renping Wang; Xueqin Gu; Weiquan Dai; Jun Ye; Feng Lu; Yifeng Chai; Guorong Fan; Frank J. Gonzalez; Geng-Li Duan; Yunpeng Qi; et al. A lipidomics investigation into the intervention of celastrol in experimental colitis. Molecular BioSystems 2016, 12, 1436-1444, 10.1039/c5mb00864f.
