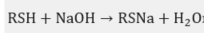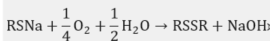The review presented is based on an extensive and updated search and literature review of the research carried out on this topic. The advantages and disadvantages of the different treatment methods are highlighted and some criteria to be taken into account when evaluating the application of each of these treatments are considered.
- sulfides
- phenols
- petroleum
- oxidation processes
Sulfur compounds are removed from petroleum by the addition of sodium hydroxide at a very high concentration. As a result, a residue called spent soda or spent caustic is generated, being extremely aggressive to the environment. In this work, the chemical properties of this residue are described in detail. The sodium hydroxide remains that have not reacted, sulfur compounds, and organic matter are the primary pollutants reported. Additionally, the main characteristics of the methods of treatment used to reduce them are described.
1. Introduction
Petroleum is around 85% carbon and 12% hydrogen, while in the remaining 3%, we find several elements that consist mainly of oxygen, nitrogen, and sulfur. The sulfur compounds limit the direct use of petroleum in any of its forms, such as liquefied petroleum gas (LPG), due to its odorous, corrosive, and environmentally harmful characteristics. Different techniques or methods are necessary for the reduction of these compounds in petroleum. Among these sulfur compounds are mercaptans and sulfides. Mercaptans are thiols containing the functional group formed by a sulfur atom and a hydrogen atom (-SH). This functional group is called the thiol or sulfhydryl group. Examples of these compounds present in LPG are methylmercaptan (H3C-SH) and ethylmercaptan (H5C2-SH).
The technology used globally for mercaptan removal content in the oil industry is the mercaptan oxidation (MEROX) process [1][2]. It is a catalytic conversion process in which mercaptans react to produce disulfides. It is promoted by a catalyst which activates the oxidation at room temperature, using atmospheric oxygen according to the following reactions:
Mercaptan + NaOH to form sodium mercaptan:
Mercaptan oxidation to form disulfides + NaOH:
As sodium hydroxide depletes as it reacts, its ability to remove sulfides and mercaptans also decreases. When the disulfides accumulate to a few milligrams per liter, the OH− content falls below 5%, whereby this depleted soda solution is purged. Other causes of sodas depletion are the accumulation of mercaptans, Na2S, phenolic compounds, emulsified naphthalenes, thiosulfates, carbonates, and Fe+2 precipitates [2]. The H2S and CO2 that are also present in the medium to be oxidized react with the caustic soda according to the following competitive reactions [3]:
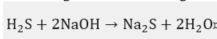 |
(3) |
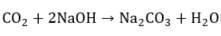 |
(4) |
These side effects can cause several problems, such as the irreversible consumption of caustic soda, low absorbing mercaptans by the presence of sodium salts, and the precipitation of solid due to the accumulation of salt in the caustic solution. That is why a caustic solution purge and a fresh soda replenishment are provided to prevent salt accumulation (Na2S/Na2CO3) from restoring the appropriate NaOH concentration. Spent caustic (SC) is the name of the solution obtained once the mercaptans react with NaOH. Without adequate treatment, SC can cause environmental problems.
The main focus of this comprehensive review is to expose different techniques released and mentioned in the literature, to process the SC. The first approach is a brief description of its characteristics that explain the environmental problems caused by SC. Following, the main body of this paper has a series of processes that help neutralize sodas and allow their subsequent biological treatment. There is no review published before or paper that reports this relevant information that could help engineers to make better decisions in the petroleum industry. The analysis included information and results from reports, theses, and research articles published recently.
2. SC Characteristics
SC is classified into three types [4]: sulfidic (SSC), cresylic (SCC), and naphthenic (SNC). The composition of this type of waste is highly variable as can be seen in Table 1. SC solutions are characterized by high pH (pH > 12) [5] and high sodium concentrations up to 2%–15% (w/w). Conner et al. [6] also found that spent sulfidic caustics contained hydrosulfides (HS−) and sulfides2− greater than 2%–3% (w/w).
Table 1. Main chemical components present in spent sodas.[5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21]
|
Parameters |
SMC |
Sulfidic |
Cresylic |
Naphtenic |
Reference |
|
pH |
- |
11–12.5 |
- |
- |
[7] |
|
- |
12–13.5 |
- |
- |
[8] |
|
|
- |
13.1–13.5 |
- |
- |
[9] |
|
|
7.5–13 |
- |
- |
- |
[10] |
|
|
- |
11.2 -13 |
- |
- |
[5] |
|
|
- |
12–14 |
12–14 |
12–14 |
[11] |
|
|
- |
13.1–13.5 |
- |
- |
[12] |
|
|
- |
13–14 |
12–14 |
12–14 |
[13] |
|
|
- |
13–14 |
12–14 |
12–14 |
[14] |
|
|
- |
11.6–12.5 |
- |
- |
[15] |
|
|
COD (g O2/L) |
- |
7.5–60 |
- |
- |
[7] |
|
|
60.3–68.1 |
- |
- |
[16] |
|
|
|
100–200 |
- |
- |
[8] |
|
|
|
20–60 |
- |
- |
[17] |
|
|
13.1–98.8 |
- |
- |
- |
[10] |
|
|
- |
62.7 |
- |
- |
[18] |
|
|
49.3 1 |
- |
- |
- |
[18] |
|
|
74 |
- |
- |
- |
[18] |
|
|
261 |
- |
- |
- |
[19] |
|
|
- |
5–90 |
50–100 |
150–240 |
[20] |
|
|
- |
66.7–156.5 |
- |
- |
[15][20] |
|
|
114 |
- |
- |
- |
[21] |
|
|
TOC (g C/L) |
- |
6–20 |
- |
- |
[17] |
|
1.6–23.6 |
- |
- |
- |
[10] |
|
|
92 |
- |
- |
- |
[19] |
|
|
- |
7.6 |
- |
- |
[18][19] |
|
|
- |
1–1.6 |
- |
- |
[12] |
|
|
- |
2–3 |
24–60 |
10–24 |
[20] |
|
|
BOD5 (mg O2 /L) |
- |
5000–10,000 |
- |
- |
[17] |
|
- |
18,100 |
- |
- |
[18] |
|
|
20,100 1 |
- |
- |
- |
[18] |
|
|
Sulfides (mg/L) |
- |
5000–20,000 |
- |
- |
[7] |
|
- |
80–90 |
- |
- |
[8] |
|
|
|
34,500 |
- |
- |
[17] |
|
|
6500–22,500 |
- |
- |
- |
[10] |
|
|
- |
5100–7700 |
- |
- |
[7] |
|
|
- |
17,800 |
- |
- |
[18] |
|
|
8040 |
- |
- |
- |
[18] |
|
|
- |
15,200–17,600 |
- |
- |
[12] |
|
|
- |
2000–52,000 |
0–63 |
<1 |
[20] |
|
|
|
- |
0–1 |
0.1 |
[14] |
|
|
|
30,600–66,800 |
- |
- |
[15] |
|
|
24,000 |
- |
- |
- |
[21] |
|
|
Sulfides (% w/w) |
- |
1–4 |
- |
- |
[17] |
|
- |
0.5–4 |
0–1 |
0–0.1 |
[11] |
|
|
1.4 |
- |
- |
- |
[19] |
|
|
- |
0.5–4 |
0–4 |
0–0.1 |
[4] |
|
|
- |
0.5–4 |
- |
- |
[14] |
|
|
- |
0–5 |
- |
- |
[7] |
|
|
- |
2–300 |
- |
- |
[8] |
|
|
- |
0–2000 |
- |
- |
[17] |
|
|
1.6–20 |
- |
- |
- |
[10] |
|
|
Phenols (mg/L) |
1990 1 |
- |
- |
- |
[18] |
|
6110 |
- |
- |
- |
[18] |
|
|
- |
1.8–33.8 |
- |
- |
[12] |
|
|
540 |
- |
- |
- |
[21] |
|
|
- |
0–30,000 |
- |
- |
[8] |
|
|
Mercaptans (mg/L) (% w/w) a |
- |
0.1–4 a |
- |
- |
[17] |
|
- |
9800 |
- |
- |
[18] |
|
|
1800 |
- |
- |
- |
[18] |
|
|
- |
0–30,000 |
0–5400 |
<30 |
[20] |
|
|
Benzene (mg/L) |
- |
47–780 |
- |
- |
[7] |
|
- |
7.8–63.1 |
- |
- |
[12] |
|
|
- |
600 |
- |
- |
[15] |
|
|
Toluene (mg/L) |
- |
0.2–7.8 |
- |
- |
[12] |
|
- |
360 |
- |
- |
[15] |
|
|
Cresylic acids (% w/w) |
- |
- |
10–25 |
0–3 |
[11] |
|
- |
0–4 |
2–25 |
0–3 |
[4] |
|
|
- |
- |
2–25 |
0–3 |
[14] |
|
|
Napthenic acids (% w/w) (mg/L) b |
- |
- |
- |
2–15 |
[11] |
|
19,700 1,b |
- |
- |
- |
[18] |
|
|
- |
- |
- |
2–15 |
[4] |
|
|
- |
- |
- |
2–15 |
[14] |
|
|
NaOH (% w/w) |
- |
4–5 |
- |
- |
[8] |
|
- |
7.5 |
- |
- |
[17] |
|
|
2–2.9 |
- |
- |
- |
[10] |
|
|
- |
11.1 |
- |
- |
[3] |
|
|
- |
2–10 |
10–15 |
1–4 |
[11] |
|
|
- |
2–10 |
1–15 |
1–4 |
[4] |
|
|
- |
2–10 |
1–15 |
1–4 |
[14] |
|
|
Carbonates (% w/w) |
2–2.9 |
- |
- |
- |
[10] |
|
- |
0–4 |
0–0.5 |
- |
[11] |
|
|
- |
- |
0–0.5 |
- |
[4] |
|
|
- |
0–4 |
0–0.5 |
- |
[14] |
1 napthenic + cresylic.
Often plants do not have the facilities to segregate the soda solutions used in these three types of classifications. Therefore, a spent mixed caustic (SMC) is usually produced. The latter is considered the fourth type of depleted or spent soda.
Processes of fuel gas, liquefied petroleum gas, and gasoline treatment generate sulfidic soda . This depleted soda has a high concentration of sulfide and also a terrible smell. This type of waste can not only be treated but could also be a reactant for the cellulose pulping process. Nevertheless, the cost of transport generally discards this possibility.
The washing of the diesel and the fractions of jet fuels generate the exhausted naphthenic soda. It contains very few sulfides, being mainly naphthenic acids. Naphthenes generally do not impart unpleasant odors or toxicity to the caustic sodas, being partially soluble in sodas by increasing the total organic carbon considerably in SC. Naphtenes is a general term used for cyclic alkanes or non-aromatic hydrocarbons called cycloalkanes. Refinery naphthenes typically include cyclopentanes and cyclohexanes as compounds of this group. This type of waste can also be sold if it is kept insufficiently in pure form. However, processing at the same plant is most often the best option.
The exhausted cresylated soda (often called phenolic) is generated from the washing of gasoline fractions and is composed mainly of aromatic, acid oils, cresols, and other organic acids. Cresylates are a form of phenols containing a methyl group (CH3), often called phenolics as a family or group of compounds. In their three forms, ortho, meta, and para cresol, they are not as toxic as phenols. However, the cresylates have relatively high concentrations, between 1% and 35%. Cresylates or cresylic acids, often referred to as acid oils, are solubilized at high pH, but readily separated at low pH. It is not a question of eliminating these compounds from petroleum since they increase the amount of octane in the final fuel; however, these are present in different concentrations in the depleted sodas. The number of phenols in spent soda is the factor to consider it as a reagent for the production of phenolic compounds. Otherwise, the low amount of oily acids drastically increases the costs of transportation and its treatment in the plant is the best option.
Due to the high content of organic matter, in addition to sulfur compounds, phenols, and mercaptans, the depleted sodas are a liquid residue tough to treat , with a series of related problems with their treatment of depleted among those highlights :
- Strong odors of sulfides and mercaptans: the odor traces of these compounds are in the order of parts per billion. As is known, these compounds are highly toxic even at minimal concentrations.
- High phenol concentrations: phenol is a highly inhibiting compound of biological activity. Deficient concentrations of phenol have been shown to inhibit the biological removal of organic matter.
- High concentrations of bio-refractory material: the presence of cresylic and naphthenic acids, which are difficult to biodegrade, is another problem associated with SC. Naphthenic acids facilitate foaming formation.
Shailja Singh and Shikha [22] mentioned the primary pollutants found in the effluent from various processes in refineries. The liquid effluents generated in the oil refineries differ from one industrial plant to another due to the variation in the configuration of the plant and in particular, to the type of petroleum processed. For example, ammonia and hydrogen sulfide are the main substances present in the wastewater from the isomerization process; meanwhile, the alkylation produces hydrofluoric acid. Other processes in this industry such as fluid catalytic cracking, crude desalting, catalytic hydrocracking, coking, distillation, catalytic hydrotreating, lubricating oil manufacture, sulfur removal, catalytic reforming, and thermal cracking are summarized in the same publication.
In general, liquid petrochemical residues contain significant concentrations of suspended solids (SS), chemical oxygen demand (COD), biochemical oxygen demand (BOD), oils and fats, sulfides, ammonium, phenols, hydrocarbons, benzene, toluene, ethylbenzene, xylene, polycyclic aromatic hydrocarbons, and heavy metals [23][24][25][26].
In order to avoid or reduce the environmental problems caused by SC, a series of processes have been proposed that help neutralize sodas and allow their subsequent biological treatment.
3. Treatment Methods of SC
3.1. Initial Considerations
In general terms, the treatment of spent sodas is carried out in two types of processes in series; the first one considers the removal of sulfides, phenols, and other compounds that can inhibit the biological processes. These are later applied in order to remove the organic matter present in this residue. The spent sodas cannot be directly biotreated for different reasons:
- The presence of phenols inhibits, at a specific concentration, the healthy metabolism of the microorganisms that operate in the biological process.
- Spent sodas contain some low biodegradable compounds, such as naphthenic acids.
- The presence of naphthenic acids may result in operational issues in aerobic processes through foam formation.
- The high COD concentration in SC makes impossible their direct treatment.
- They have a high pH, which is not adequate for the development of microorganisms.
3.2. Wet Air Oxidation (WAO)
In practice, this is the most widely used chemical treatment applied to spent sodas. This process has been executed on several occasions as a pilot [27] at a plant [28][29][30][31][32] and laboratory level [33][34], in addition to the diverse industrial plants [35] that have implemented the WAO system. It is basically the oxidation of the soluble and suspended compounds that are present in this residue using oxygen or air as an oxidant . The oxidation is carried out at very high temperatures and pressures, which depend on the strength of the material to be oxidized in the process and the quality required from the final effluent.
In the WAO process, several chemical reactions take place, such as:
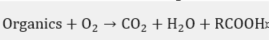 |
(5) |
 |
(6) |
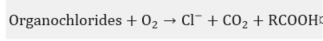 |
(7) |
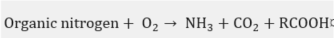 |
(8) |
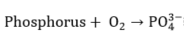 |
(9) |
Three ranges divide the operational conditions: low, medium, and high-temperature systems [36]. Oxidation takes place at temperatures of 200 °C and 27.5 bars in low-temperature systems. This system partially oxidizes sulfides into sulfates and thiosulfates [37]. In medium temperature systems, oxidation takes place in the range of 200 °C and 27.5 bars to 260 °C and 86 bars. The ideal supply for this system is naphthenic caustics sodas. Sulfides react to sulfates, and the mercaptans are also destroyed [38]. The high-temperature systems oxidize the cresylic caustics at a range from 240 °C and 55 bars to 260 °C and 85 bars. Complete oxidation of sulfides, mercaptans, and other organic compounds can be carried out, such as cresylic acids [39]. The typical industrial WAO systems can work with spent sodas flows between 1 and 50 m3/h and with a COD between 10,000 and 100,000 mg/L. However, for COD levels above 50,000 mg/L, dilutions with water or fresh caustic solution are required. Diluted caustic solutions help to reach different goals, among them, avoiding diluted salts concentrations below the solubility levels in order to prevent incrustations. The other reason is to guarantee that alkalinity is not consumed by oxidation when acid effluents could damage the construction materials of the system. Finally, the addition of the caustic solution can help the oxidation of contaminants according to the reactions that are described below .
Sulfide.
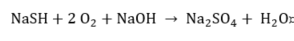 |
(10) |
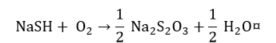 |
(11) |
Thiosulfate.
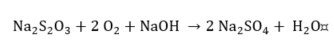 |
(12) |
Mercaptan.
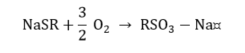 |
(13) |
Cresylic acid.
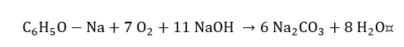 |
(14) |
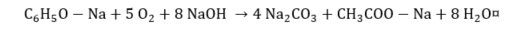 |
(15) |
Naphthenic acid.
 |
(16) |
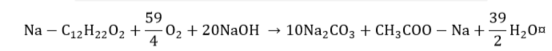 |
(17) |
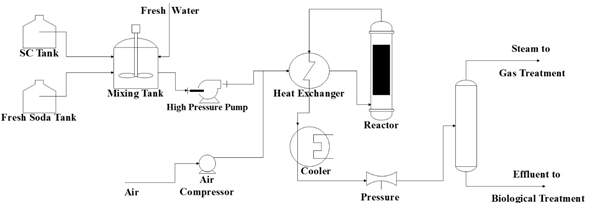
The WAO process can treat liquid currents containing cyanide, several heterocyclic compounds, industrial sludge, and spent coal from adsorption processes [40]. Several industrial residues apply this process, such as paper manufacture, textile sludge, among others. Figure 1 shows an outline of a typical WAO process.
Figure 1. Typical outline of a wet air oxidation (WAO) system.
As can be seen in Figure 1, fresh soda or freshwater dilute the spent soda. The dilution ratio depends on the strength of the spent soda. Then, the diluted spent soda is pumped at 27.5–85 bars through a high-pressure pump. The addition of compressed air to the diluted spent soda is required in order to supply the needed oxygen for the oxidation reaction. A heat exchanger preheated the mixture at 200–260 °C. The reactor must operate for a sufficient residence time that allows high oxidation of the organic matter contained in the spent sodas (COD disposal). Since the reaction is exothermic, the reactor’s effluent has a high temperature, so it can be used to preheat the diluted spent sodas before entering the reactor . Subsequently, the effluent coming out of the heat exchanger is cooled and depressurized before being sent to a gas–liquid separator. Then, the separated liquid goes to biological treatment. The effluent gas has 5%–21% of oxygen and some volatile organic compounds. In Table 2, the values of a sample of the operational conditions of a WAO system from the Oil Refinery of Manguinhos, S. A. in Rio de Janeiro, Brazil, are presented.
Table 2. Operational conditions of the WAO system in Manguinhos, Brazil .
|
Parameters |
Supply |
Effluent |
|
Residence time (h) |
- |
1 |
|
Oxidation temperature (K) |
- |
533 |
|
COD (g/L) |
114 |
≈23 |
|
Sulfides (g/L) |
≈24 |
<0.001 |
|
Total phenols (g/L) |
≈0.45 |
<0.002 |
As can be observed, despite obtaining a removal percentage of 80% of COD, measured from organic matter, it is still very high. This concentration is required to be reduced until reaching the possible values to discharge it into a receiving current or another place for its final disposal. It could be carried out through a biological process that can be done in this case, provided the low levels of sulfides and phenols in the WAO effluent since the two chemical species were reduced in the process by almost 100%. Additionally, most of the remaining COD in the treated effluent comes from aliphatic acids of low molecular weight, which can be biologically oxidized . The oxidation pressure is directly related to the oxidation temperature. The oxidation pressure is responsible for keeping the reaction in the liquid phase. In this way, as the pressure increases, the oxidation pressure rises to keep the reaction in the liquid phase. The WAO processes typically operate with hydraulic residence times of 45–120 min, the oxidation degree that takes place within the reactor affecting this parameter. Each WAO system uses different hydraulic residence times to reach the degree of COD reduction required. The selection of the construction material is critical in the WAO system, considering that the operation takes place at a high temperature and pressure. Thus, the materials used must be appropriate for these conditions. The WAO system presents some disadvantages and difficulties, among which the initial investment cost stands out. This cost depends mainly on the operating conditions, which, at the same time, are related to the strength of the residue to be treated. As this strength increases, more severe operating conditions are required, which increases the cost of the plant.
Tests and research are still carried out in order to improve this system, as well as combining this process with others. A way of reducing the severe operating conditions is the use of solid catalysts in the WAO to accelerate the reactions, and there are reports of cases where catalysts have been used in this process [41]. This innovative technique is referred to as catalytic wet air oxidation (CWAO). In recent years it has been studied in order to increase the efficiency to treat pollutants in industrial effluents [42]. The studies have been developed to remove individual toxic substances and to expose the best selection of metal catalyst-based support at lab scale. In general, the catalytic activity of transition metal such as Vanadium, Ferrum, Nickel, Copper and others have been chosen as the most common option.
Otherwise, researchers are still looking for better conditions of the process to treat most pollutants in spent caustic using this technology.
Nunez et al. [43] added silver to Al2O3-ZrO2 mixed oxide to reduce p-cresol at 160 °C and 15 bar of O2 pressure, showing faster oxidation associated with more selectivity of this metal compared to the test without it. Additionally, it reported that the highest concentration (20%) of ZrO2 in the catalyst removed 50% and 96% for TOC and p-cresol, respectively.
Jagushte and Mahajani studied the effect of the heterogeneous copper catalyst in the kinetics of Equation (12) mentioned above at the lab-scale. As a result, the catalyst reduced the temperature and time process to 30 °C from 150 °C and 4 min from 12 min, respectively, in a conversion of 99% of the pollutant in alkaline conditions. It is remarkable to say that the batch experiments were carried out at low temperatures and 0.69 MPa of oxygen partial pressure, showing first and 0.5 kinetic order concerning thiosulfate and oxygen, respectively. Additionally, this study explained a strategy to treat spent caustic under its results associated with the enhanced effect of phenols in the oxidation of thiosulfate.
A similar study was reported by Zermeño-Montante et al. [44]. In this case, copper catalyst in a silica support material achieved the complete sulfide oxidation at 70 °C in 20 min. It showed better results than a similar experiment with Vanadium/Clinoptilolite just for 6 min latter. Both cases enhanced the WAO process for this pollutant. This report explained some of its results from Jagushte and Mahajani ,
Recently, Barge and Vaidya [45] experimented with ferrous sulfate to eliminate sodium sulfide. That study innovated using a cheap and abundant catalyst, destroying 94% of COD at 100 °C and 0.69 MPa of oxygen partial pressure within 1 h. It is remarkable to say those authors exposed the Langmuir–Hinshelwood as the kinetic model to oxidize the reactant. These same authors, Barge and Vaidya [46] used Graphene oxide (GO) and Ruthenium (Ru) as a catalyst in WAO for the treatment of the cresylic spent caustic, obtaining the best results at 175 °C and 0.60 MPa of O2 pressure, with removals of 54.9%, 48.9%, and 61.2% as TOC, for 0-cresol, m-cresol, and p-cresol, respectively, in the typical WAO. Meanwhile, when the Ru/GO catalyst was used for the same operating conditions, the removals obtained were 66.4%, 53.4%, and 73.9%, as TOC, for 0-cresol, m-cresol, and p-cresol, respectively.
3.3. Acid Neutralization
In this type of process, alkaline caustic solution traps the acid components released from acidified spent sodas . This action results in sulfides and mercaptans being released as acid gases, and naphthenic acids come to form some oil layer. One of the most significant differences between acid neutralization and the WAO process is that the acid components of spent sodas are removed but not destroyed, for example, phenols. In some cases, this entails the requirement of additional treatments for the acidification effluents, for example, gas ignition or methods for sulfur recovery. In other cases, this allows for any component present in spent sodas to be captured and reused.
As phenols are highly soluble in water, they are complicated to acidify. However, acidification eliminates the trend of the formation of foam, similar to the one obtained in the WAO process at high temperatures. The cause of this is mainly the fact that the naphthenic compounds that tend to form foam move to an insoluble phase and, even though they are not eliminated, they reduce their trend to form foam. Additionally, some alkaline compounds that could form foam are acidified, reducing this trend.
In some cases, acidification as a pretreatment for oxidation processes has been studied. Sheu and Weng [47] treated the spent sodas from olefin plants containing a considerable amount of H2S and some mercaptans, phenols, and crude oils undergo a process of acid neutralization before a process with Fenton reagent. Using sulfuric acid with a pH of up to 5, and 70 °C, a little more than 90% of the dissolved H2S changes to the gas phase. From this pH, the conversion to dissolved sulfide (S2−) is minimal [48]. These authors were the first to report this pretreatment process.
Nuñez et al. [49] showed a reduction of 71% and 82% of COD in two batch experiments using this procedure, under conditions of pH below 4. In this context, only the sulfide content was reduced by pH reduction, the phenol concentration not being affected. The removal percentages were lower, considering the analysis made by Sheu and Weng and it was attributed to the use of synthetic and real samples used. The last type has other substances that could inhibit the pH reduction to oxidize the reactants.
In summary, acid neutralization or acidification reduces most of the COD and the trend to form foam removing the naphthenic acids. Sulfuric acid is the chemical agent used in most cases in these processes, due to its strength and lower price in comparison with other acids [50].
3.4. Advanced Oxidation Process (AOP)
These processes are defined as the processes that involve the formation of radical hydroxyls (OH) that oxidize both organic and inorganic contaminants present in water and wastewater [51]. These radicals are the second most potent oxidants after fluorides [52]. Unlike the physical process such as adsorption, filtration, and air stripping, the AOPs can destroy the contaminants rather than transferring them from one medium into another[53]. The AOPs can be used to treat wastewater with high COD and low biodegradability [54]. With these processes, complete oxidation of residues can be reached, converting contaminants into water, CO2, and innocuous inorganic products. However, it is expensive and impractical to use these processes for the complete mineralization of compounds since there are intermediate products that are resistant to chemical oxidation. A practical solution is to use these processes as a pretreatment for biological treatments. The partial mineralization produced by the AOPs generates intermediates with higher biodegradability and lower toxicity, which makes them available for biological oxidation. It is very important to determine the quality of the residue before selecting any AOP process, since this factor will affect the efficiency of the process, for example, residues with high alkalinity have an excess of carbonate and bicarbonate compounds, which may interfere with the oxidation reactions of hydroxyl radicals. These radicals react with those compounds forming weaker radicals resulting in lower efficiencies of the minor oxidation processes [55]. This issue can be solved by reducing the alkalinity to make sure there is no excess of these compounds in the residues, which can be achieved in a relatively simple way by lowering the pH. Nitrates and nitrites can also affect the efficiency of the AOP processes, which use UV light to generate hydroxyl radicals. These compounds can absorb UV light and, thus, reduce the generation rate of free OH radicals. The turbidity has the same effect over the efficiency of the AOP processes as nitrates and nitrites. Turbidity reduces the production of hydroxyl radicals, since it acts as a barrier against UV light, which cannot penetrate through the residue to be treated.
The AOP reaction systems consist of a catalyst and an oxidant. The catalyst task is to form hydroxyl radicals from oxidants. There are several means to form these radicals, which can be photochemical, photo-catalyzed, ultrasonic, and chemical oxidations.
The photochemical processes include UV radiation. Some studies mention the use of this technology individually. Gurol and Vatistas [56] irradiated by UV a mixture of various phenolic compounds, using 16 low-pressure mercury lamps, each of which emitted 2.2 Watts of radiation at 254 nm. The results showed a faster initial rate remotion of phenol at pH 2.5 than p-cresol and xylenol at the same condition. The duration of the experiments was 100 min, starting in 50 mg/L of the three substances and achieving remotions in the range of 20%–30%. Neutral and basic (pH 9) conditions did not exhibit significant changes in the initial rate remotion, observing the same removal efficiency for those compounds at the same time. The authors concluded that the understanding of the reaction mechanism at low-pH irradiation requires further research.
Spent caustic from the ethene plant were irradiated with UV by Yu et al. [57]. The results showed low-efficiency COD removal (under 10%) in 180 min using an ultraviolet light source of 254 nm wavelength. The high content of several persistent pollutants in spent caustic is not affected when this technology is used by itself. That is why the UV radiation has not attracted industrial applications and also lab-scale studies, and this is the evidence of a few reports about it.
Other photochemical technologies like ozone systems (UV/O3) and (UV/O2) systems have been reported. Photo-catalysis includes photo-Fenton, while chemical oxidation includes O3/H2O2 and H2O2/Fe3+.
References
- Abolghasem, K.; Kharaji, A.G.; Mehrabani-Zeinabad, A.; Faizi, V.; Kazemi, J.; Shariati, A. Synergy between two natural gas sweetening processes. J. Unconv. Oil Gas Resour. 2016, 14, 6–11.
- Heidarinasab, A.; Hashemi, S.R. A study of biological treatment of spent sulfidic caustic. In Proceedings of the International Conference on Chemical, Ecology and Environmental Sciences (ICCEES´2011), Pattaya, Thailand, 17–18 December 2011.
- Hashemi, S.R.; Heidarinasab, A. Spent Caustic Bioregeneration by using Thiobacillus denitrificans Bacteria. World Acad. Sci. Eng. Technol. 2012, 67, 417–419.
- Hawari, A.; Ramadan, H.; Abu-Reesh, I.; Ouederni, M. A comparative study of the treatment of ethylene plant spent caustic by neutralization and classical and advanced oxidation. J. Environ. Manag. 2015, 151, 105–112.
- De Graaff, M.; Klok, J.B.M.; Bijmans, M.F.M.; Muyzer, G.; Janssen, A.J.H. Application of a 2-step process for the biological treatment of sulfidic spent caustics. Water Res. 2012, 46, 723–730.
- Conner, J.A.; Beitle, R.R.; Duncan, K.; Kolhatkar, R.; Sublette, K.L. Biotreatment of Refinery Spent-Sulfidic Caustic Using an Enrichment Culture Immobilized in a Novel Support Matrix. Appl. Biochem. Biotechnol. 2003, 84, 707–719.
- Nasr Esfahani, K.; Farhadian, M.; Solaimany Nazar, A.R. Interaction effects of various reaction parameters on the treatment of sulfidic spent caustic through electro-photo-Fenton. Int. J. Environ. Sci. Technol. 2019, 16, 7165–7174.
- Karimi, A.; Fatehifar, E.; Alizadeh, R.; Ahadzadeh, I. Regeneration and treatment of sulfidic spent caustic using analytic hierarchy process. Environ. Heal. Eng. Manag. 2016, 3, 203–208.
- Lee, J.H.; Park, J.J.; Seo, K.S.; Choi, G.C.; Lee, T.H. Simultaneous autotrophic & heterotrophic denitrification by the injection of reformed spent sulfidic caustic (SSC) in a pilot-scale sewage treatment plant. Korean J. Chem. Eng. 2013, 30, 139–144.
- Al Jabari, M. Spent Caustic Treatment Using Advanced Oxidation Processes. Master’s Thesis, University of Sharjah, Sharjah, UAE, 2012.
- Veerabhadraiah, G.; Mallika, N.; Jindal, S. Spent caustic management: Remediation review. Hydrocarb. Process. 2011, 90, 41–46.
- Park, J.J.; Byun, I.G.; Park, S.R.; Lee, J.H.; Park, S.H.; Park, T.J.; Lee, T.H. Use of spent sulfidic caustic for autotrophic denitrification in the biological nitrogen removal processes: Lab-scale and pilot-scale experiments. J. Ind. Eng. Chem. 2009, 15, 316–322.
- Alnaizy, R. Economic analysis for wet oxidation processes for the treatment of mixed refinery spent caustic. Environ. Prog. 2008, 27, 295–301.
- Duesel, B.F.; Gibbons, J.P.; Rutsch, M.J. Treatment of Spent Caustic Refinery Effluents. U.S. Patent 7,214,290, 8 May 2007.
- Sipma, J.; Svitelskaya, A.; Van Der Mark, B.; Hulshoff Pol, L.W.; Lettinga, G.; Buisman, C.J.N.; Janssen, A.J.H. Potentials of biological oxidation processes for the treatment of spent sulfidic caustics containing thiols. Water Res. 2004, 38, 4331–4340.
- Sabri, M.A.; Ibrahim, T.H.; Khamis, M.I.; Nancarrow, P.; Hassan, M.F. Spent caustic treatment using hydrophobic room temperatures ionic liquids. J. Ind. Eng. Chem. 2018, 65, 325–333.
- Ben Hariz, I.; Halleb, A.; Adhoum, N.; Monser, L. Treatment of petroleum refinery sulfidic spent caustic wastes by electrocoagulation. Sep. Purif. Technol. 2013, 107, 150–157.
- Kumfer, B.; Felch, C.; Maugans, C. Wet air oxidation treatment of spent caustic in petroleum refineries. In Proceedings of the National Petroleum Refiners Association Conference, Phoenix, AZ, USA, 21–23 March 2010; Volume 23, pp. 49–67.
- Ahmad, W. Neutralization of Spent Caustic from LPG Plant at Preem AB Göteborg. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2010.
- Huaman, F.; Davila Villar, N. Disposal of Spent Caustic at the Repsol YPF Refinery in La Pampilla, Peru. In Proceedings of the Environmental Conference, Austin, TX, USA, 24–25 September 2007.
- Ellis, C.E. Wet air oxidation of refinery spent caustic. Environ. Prog. 1998, 17, 28–30.
- Singh, S.; Shikha Treatment and Recycling of Wastewater from Oil Refinery/Petroleum Industry. In Advances in Biological Treatment of Industrial Waste Water and their Recycling for a Sustainable Future; Springer: Singapore, 2019; pp. 303–332.
- Tobiszewski, M.; Tsakovski, S.; Simeonov, V.; Namieśnik, J. Chlorinated solvents in a petrochemical wastewater treatment plant: An assessment of their removal using self-organising maps. Chemosphere 2012, 87, 962–968.
- Hasan, D.B.; Abdul Aziz, A.R.; Daud, W.M.A.W. Oxidative mineralisation of petroleum refinery effluent using Fenton-like process. Chem. Eng. Res. Des. 2012, 90, 298–307.
- Park, S.; Seon, J.; Byun, I.; Cho, S.; Park, T.; Lee, T. Comparison of nitrogen removal and microbial distribution in wastewater treatment process under different electron donor conditions. Bioresour. Technol. 2010, 101, 2988–2995.
- Al Zarooni, M.; Elshorbagy, W. Characterization and assessment of Al Ruwais refinery wastewater. J. Hazard. Mater. 2006, 136, 398–405.
- Copa, W.M.; Momont, J.A. Wet air oxidation of energetics and chemical agent surrogates. J. Energy Mater. 1995, 13, 235–258.
- DeAngelo, D.J.; Wilhelmi, A.R. Wet air oxidation of spent caustic liquors. Chem. Eng. Prog 1983, 79, 3.
- Copa, W. Wet air oxidation of spent caustics. Natl. Environ. J. 1994, 4, 16–19.
- Matthews, R. Performance update: Low pressure wet air oxidation unit at grangemouth, Scotland. Environ. Prog. 1997, 16, 9–12.
- Ellis, C.; Maugans, C.B. WAO treats spent caustic liquor in Asia and Brazil. Water Wastewater Int. 2004, 19, 35–36.
- Huaman, F.D.; Villar, N.; Felch, C.; Maugans, C.; Olsen, S. A highly refined effort. Water Wastewater Int. 2009, 24, 28–32.
- Chang, C.J.; Lin, J. ‐C.; Chen, C. ‐K. Effects of temperature and Cu2+ catalyst on liquid‐phase oxidation of industrial wastewaters. J. Chem. Technol. Biotechnol. 1993, 57, 355–361.
- Jagushte, M.V.; Mahajani, V.V. Insight into spent caustic treatment: On wet oxidation of thiosulfate to sulfate. J. Chem. Technol. Biotechnol. 1999, 74, 437–444.
- Debellefontaine, H.; Foussard, J.N. Wet air oxidation for the treatment of industrial wastes. Chemical aspects, reactor design and industrial applications in Europe. Waste Manag. 2000, 20, 15–25.
- Seyedin, S.; Hassanzadeganroudsari, M. Evaluation of the Different Methods of Spent Caustic Treatment. Int. J. Adv. Res. Sci. Eng. Technol. 2018, 5, 5275-5283.
- De Haan, S.; Howdeshell, M.; Maugans, C. Update: Spent caustic treatment. Hydrocarb. Process. 2010, 9, 61–61.
- Kim, K.H.; Ihm, S.K. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34.
- Zhu, W.; Bin, Y.; Li, Z.; Jiang, Z.; Yin, T. Application of catalytic wet air oxidation for the treatment of H-acid manufacturing process wastewater. Water Res. 2002, 36, 1947–1954.
- Janssen, A.J.; Lettinga, G.; Bontsema, J.; Van Straten, G.; Kuenen, J.G.; De Zwart, J.M.M. Biological Treatment of Spent Caustic. U.S. Patent 6,045,695, 4 April 2000.
- Oliviero, L.; Barbier, J.; Duprez, D.; Guerrero-Ruiz, A.; Bachiller-Baeza, B.; Rodríguez-Ramos, I. Catalytic wet air oxidation of phenol and acrylic acid over Ru/C and Ru-CeO 2 /C catalysts. Appl. Catal. B Environ. 2000, 25, 267–275.
- Lei, L.; Hu, X.; Chu, H.P.; Chen, G.; Yue, P.L. Catalytic wet air oxidation of dyeing and printing wastewater. Water Sci. Technol. 1997, 35, 311–319.
- Núñez, F.; Del Angel, G.; Tzompantzi, F.; Navarrete, J. Catalytic Wet-Air Oxidation of p-Cresol on Ag/Al2O3−ZrO2 Catalysts. Ind. Eng. Chem. Res. 2011, 50, 2495–2500.
- Zermeño-Montante, I.; Nieto-Delgado, C.; Sagredo-Puente, R.D.; Cárdenas-Galindo, M.G.; Handy, B.E. Catalytic Wet Air Oxidation of Sodium Sulfide Solutions. Effect of the Metal-Support and Acidity of the Catalysts. Top. Catal. 2011, 54, 579–586.
- Barge, A.S.; Vaidya, P.D. Kinetics of wet air oxidation of sodium sulfide over heterogeneous iron catalyst. Int. J. Chem. Kinet. 2019, 52, 92–98.
- Barge, A.S.; Vaidya, P.D. Wet air oxidation of cresylic spent caustic—A model compound study over graphene oxide (GO) and ruthenium/GO catalysts. J. Environ. Manag. 2018, 212, 479–489.
- Sheu, S.H.; Weng, H.S. Treatment of olefin plant spent caustic by combination of neutralization and fenton reaction. Water Res. 2001, 35, 2017–2021.
- Wang, S.; Liu, X.; Zhang, M. Reduction of Ammineruthenium(III) by Sulfide Enables In Vivo Electrochemical Monitoring of Free Endogenous Hydrogen Sulfide. Anal. Chem. 2017, 89, 5382–5388.
- Nuñez, P.; Hansen, H.K.; Rodriguez, N.; Guzman, J.; Gutierrez, C. Electrochemical Generation of Fenton’s Reagent to Treat Spent Caustic Wastewater. Sep. Sci. Technol. 2009, 44, 2223–2233.
- Oprea, F.; Fendu, E.-M.; Nicolae, M.; Pantea, O.; Dunka, M. Experimental studies concerning treatment of the spent caustic solutions from refineries MEROX and EXOMER units. Rev. Chim. 2010, 61, 608–661.
- Poyatos, J.M.; Muñio, M.M.; Almecija, M.C.; Torres, J.C.; Hontoria, E.; Osorio, F. Advanced oxidation processes for wastewater treatment: State of the art. Water. Air. Soil Pollut. 2010, 205, 187–204.
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-A review. Sci. Total Environ. 2011, 409, 4141–4166.
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent organic matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374.
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59.
- Mabury, S. Hydroxyl Radical in Natural Waters. Ph.D. Thesis, University of California, Davis, CA, USA, 1993.
- Gurol, M.D.; Vatistas, R. Oxidation of phenolic compounds by ozone and ozone + u.v. radiation: A comparative study. Water Res. 1987, 21, 895–900.
- Yu, Z.-Z.; Sun, D.-Z.; Li, C.-H.; Shi, P.-F.; Duan, X.-D.; Sun, G.-R.; Liu, J.-X. UV-catalytic treatment of spent caustic from ethene plant with hydrogen peroxide and ozone oxidation. J. Environ. Sci. 2004, 16, 272–275.