Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 4 by Lindsay Dong.
Black locust (Robinia pseudoacacia L.) is a North American species that was introduced in Romania around the end of the 17th century, and it was first used in large-scale afforestation of degraded lands in the year 1852. Due to its remarkable adaptability, fast growth, and vigorous sprouting capacity, it has become one of the most widely spread exotic species in Romania. Using black locust for afforestation, Romanian foresters successfully reclaimed large areas of abandoned agricultural land degraded by “flying sands” in southwest of the country, thus contributing to mitigation of the aridization phenomena. Driving the extensive use of the species was its durable and versatile wood, much appreciated by rural communities, complemented with major benefits from crop fields and settlement protection against wind/sand deflation, as well as economically viable byproducts. In addition to the immediate improvements to local microclimate, afforestations also contributed to long-term climate change mitigation by sequestering atmospheric CO2 in the carbon pools (living tree biomass, soil organic matter, litter) as well as downstream wood products (e.g., furniture). Nevertheless, in the past decade, awareness has been raised towards its invasive potential in protected areas.
- black locust
- silviculture
- ecology
- risks
1. Black Locust in Romania
Robinia pseudoacacia is a fast-growing species, reaching heights above 30 m and ages in excess of 100 years, while maturing at early ages of 5–7 years old. It regenerates both from stump sprouts and root suckers, and it can be easily propagated by cutting or grafting, although in Romanian forestry natural regeneration from root suckers and plantation of seedlings are almost exclusively used. It is reported to become invasive if inadequately planted in sites with high productivity potential [1], but also on poor sites due to its intolerance to other large tree species, so much that it forms pure stands. Nevertheless, it was also reported in association with shrubs and a few other compatible tree species whenever adequate planting schemes are applied [1][2]. Being a sun loving species, it prefers areas with long summers (mean annual temperatures above 10 °C), and annual rainfall between 400 and 600 mm. According to Ivanschi et al. [3] it grows well on sandy and sandy loam soils, with loose to lightly compaction, deep, with a medium humidity regime; conversely, it does not grow/survive on compact soils with calcium carbonates or soluble salts in top layers of soil or in the case of excess of humidity. Calcium carbonates (CaCO3) in the top layers of soil (<40 cm) inhibit growth. Due to its nutritional particularities, it fixes nitrogen (N) in the soil (in symbiosis with N fixing bacteria) but it also consumes large quantities of soil minerals. It has a hard and durable wood, comparable with that of oak, which makes it very appreciated by rural populations.
Geographically, black locust stands occupy about 5% of the national forested area (250,000 ha), concentrated in the southwest, west, and in the east of the country [4].
The largest areas of compact stands and also the most valuable ones are located in the southwest of the country in the Oltenia region (Figure 1), which was also the location of its first introduction in Romania. Currently, the species is considered as a sub-spontaneous one, its range spreading from the plains to the lower mountain regions [5].
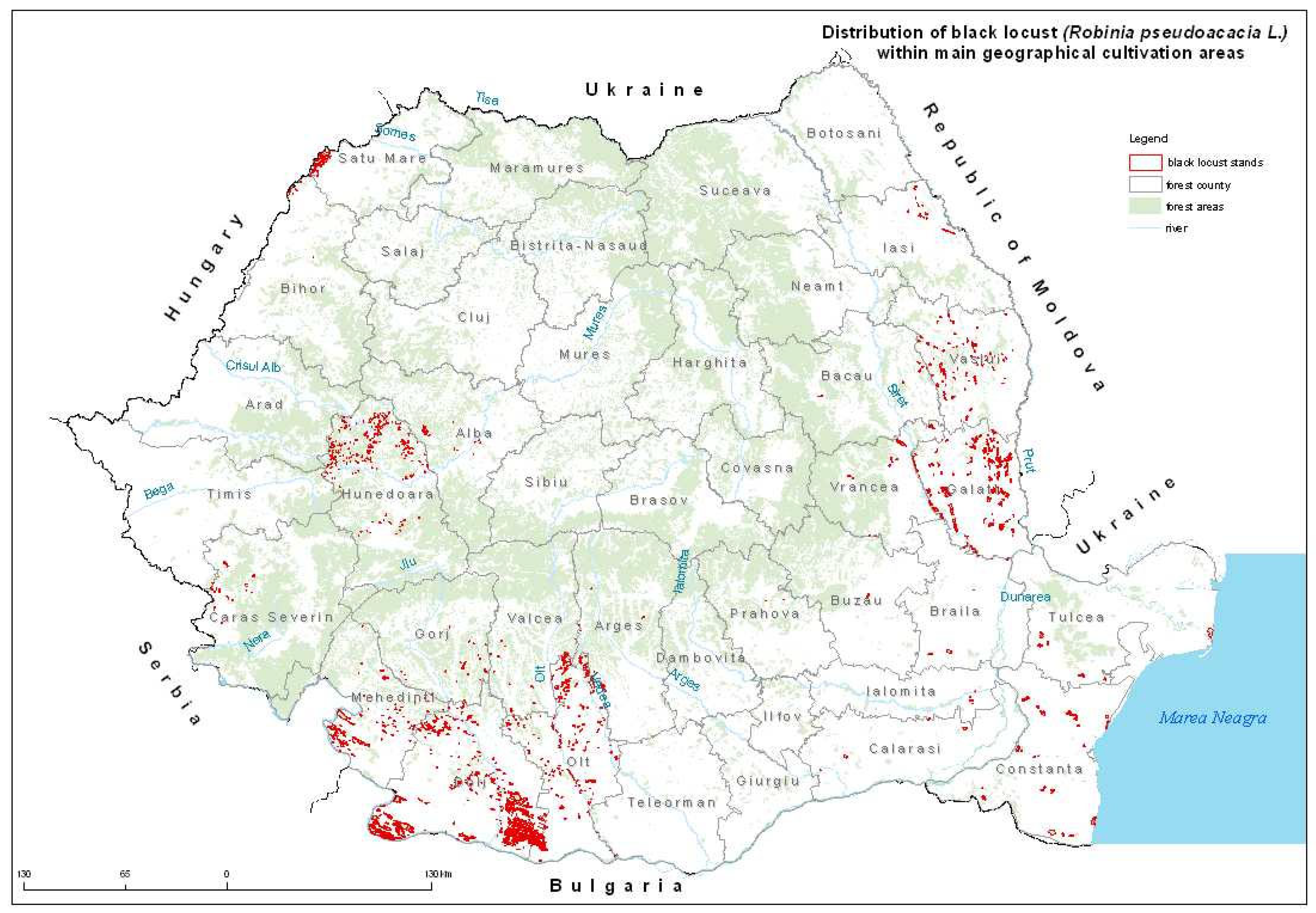
Figure 1. Distribution of black locust stands in the main culture regions of Romania [1].
2. Management Approaches
2.1. Forest Regeneration and Establishment of Plantations
Although black locust produces annually a very large number of seeds, because of their thick tegument, successful natural stand regeneration is missing almost completely [4]. Exceptionally isolated trees can be recognized as being from seeds in very particular sites, such as upper sides of river banks [6] or at pile burning sites where forest residues are burned [7]. Therefore, vegetative regeneration of harvested stands by mechanical stimulation of root sprouting and artificial regeneration by plantation of nursery seedlings in reforestation and afforestation of non-forest lands are preferred and largely practiced across the country [8].
2.2. Vegetative Regeneration
While the harvesting technique is always clear cutting, stand regeneration occurs via sprouts on the aboveground part of the stump or suckers by mechanical stimulation of roots by: (a) ploughing of the soil among the stumps at 20 cm depth; or (b) digging out the stump together with the roots around it in a radius of 50 cm combined with additional ploughing. When regeneration targets root-suckers, the sprouts must be removed through early tending operations, so as to not overwhelm the slow growing root suckers [4]. After two cycles of vegetative regeneration from sprouts, the stumps weaken and lose their sprouting capacity (due to fungal impact and soil nutrient depletion); and by the third generation, the stand’s productivity drops to 40% of that of plantations [9]. That is why, after a second generation of sprouts, regeneration must be ensured from root-suckers [10].2.3. Generative Regeneration
The recommended technique for seedling production includes harvesting the seeds directly from trees (late in the winter or early January) or separated from litter and mechanical or chemical ‘forcing’ before sowing next spring. Seedlings are almost without exception ready for transplantation after one season of growth (e.g., root collar diameter of 4 mm). After a second year in the nursery, they generally surpass the planting dimension standards which are associated with substantial risk of not surviving summer drought. Although, for afforestation in such areas, specific standards apply to ensure more robust plants are used—e.g., root collar diameter of 8 mm [10]. The seeds for nursery production of seedlings are often collected from seed orchards and have to meet standard requirements for purity, technical germination, and mass [11].2.4. Afforestation Techniques
According to technical norms in force in Romania [12], afforestation with black locust is exclusively carried out by planting bare-root seedlings in hand-dug holes. This method has proved so successful in practice that there has been no more research on other methods, although associated costs may be prohibitive in an expensive labor market. The literature states that sowing the previously treated seeds can be used to create black locust stands [4]. Planting can be done both in spring as in autumn. Spring planting ensures adequate soil humidity (common in upper altitude regions), while planting in autumn (usually in the southern ad low-altitude regions) ensures root growth over winter and avoids spring flooding and early droughts [13]. The technical norms state that, in the case of sandy soils or degraded lands, optimum planting density for black locust is 5000 seedlings per hectare—with 2.0 m of distance between rows and 1.0 m between seedlings—and the planting pit is 40 cm wide and 40 cm deep. In the case of non-degraded lands, the planting density for black locust is 4000 seedlings per hectare—with 2.0 m of distance between rows and 1.25 m between seedlings—and the planting pit width, length, and depth is 40 cm. In order to ensure the highest survival rate, a key technical intervention after planting seedlings is trimming of their stems 1 cm above the soil before growing season starts [13].2.5. Tending Operations
Being a fast-growing species, canopy closure takes place early (1–3 years following planting), therefore tending operations (especially cleaning and thinning) need to be made at shorter periods of time (3–5 years) depending on the tree origin (seedlings or root-suckers) and density of the stand. Periodicity of tendings varies in function of stand development (size of trees) and influences its productivity (stand density influences the rate of growth) as well as its protective function, in which case higher stand densities are preferable only on degraded lands due to the slenderness coefficient [10].2.6. Stand Management
Forest management planning sets either productive or protective objectives for black locust forests, following strict management indicators: land use, silvicultural regime, stand structure, rotation, and harvesting technique. When dealing with such technical requirements, the forest management plan has to follow ecological, social, and economic local needs. While some stands’ fate is wood production (mainly timber and construction wood), many stands have additional strong protective functions (e.g., degraded land reclamation, protection of soils and infrastructure, etc.).2.7. Silvicultural Regime
In Romanian forestry law and official technical norms [12], the recommended form of management system for black locust stands is the coppice. Two forms can be differentiated: the simple one applied extensively and the selection coppice applied locally and occasionally. The simple coppice consists of clear-cutting of the stand followed by vegetative regeneration or seedling plantations. In the coppice selection system, a part of the sprouts/root-suckers from each stump can be preserved, eliminating only the crooked ones and those that have reached the targeted diameter. It can be applied with experimental purpose in the case of plantations on severely degraded lands or with small privately owned forests.2.8. Stand Composition and Structure
Black locust generally achieves large areas of continuous pure stands. Being a typical exclusivist species, stands are generally pure and even aged, their intraspecific competition being poor [14]. Nevertheless, on degraded lands, it is planted together with other exotic species. Black locust can tolerate other species, most commonly honey locust (Gleditsia triacanthos L.) on sandy soils in the south and east of the country, or with black pine (Pinus nigra L.) on eroded slopes in the east of Romania [14][15]. It behaves as an exclusivist species for ground vegetation, black locust stands have very poor herbaceous diversity (e.g., frequent are Urtica sp., Sambucus ebulus).2.9. Harvesting and Rotation/Cycle Length
Black locust stands have the shortest rotation length in Romanian forestry. Rotation length differs as a function of the forest primary purpose (wood production or protection). If the stand primarily has a production purpose (e.g., timber or construction wood), harvesting age varies between 15 years for coppices classified under the lowest production class to 35 years for the planted stands classified as the highest production class. If classified as having protection function (e.g., steep terrains prone to soil erosion), the harvesting age can reach 40 years in stands [12]. Wood harvesting occurs through clear-cuts in plots with a maximum area of 5 ha.3. Genetics, Selection, and Tree Breeding
An outstanding natural variety—Robinia pseudoacacia var. oltenica—was identified in the stands located in the south-west part of Romania (Oltenia region) by Bârlănescu, Costea, and Stoiculescu in 1966 [16]. Due to its valuable auxological and morphological characteristics [17][18], this variety was the subject of tree selection and breeding by grafting/cutting [19][20] and in vitro micropropagation. Later on, eco-physiological research by Bolea at al. [21] showed that this variety has both higher intensity of photosynthesis and tolerance to droughts, due to larger leaf area index (LAI), compared to common stands. Conservation and expansion of the Oltenica variety was continued in recent years by producing seedlings from cuttings, as maternal lineages of identified plus-trees [4]. Genetics research prior to 1990 focused on tree selection and breeding for enhancement of productivity and wood standing volume [19][22][23], as well as selection of valuable forest and beekeeping genotypes by hybridization. Trials consisting in testing different provenances in comparative cultures, identified “plus” trees and enhanced the number of clones and establishing vegetative propagation methods (e.g., grafting) for the valuable forms [24].4. Vulnerability of Black Locust
Systematic records of biotic and non-biotic factors and damage data in Romanian forests have been collected through the Forest National Survey and Forecast System operations since 1958. Based on such data, the main cryptogamic diseases affecting Robinia pseudoacacia L. were listed as follows [11]: virosis, Fusarium sp. and Cuscuta sp. attacks on seedlings, mildew (Oidium sp.), sooty mold (Coniothyrium sp., Alternaria sp., Cladosporium sp.), leaf spots (Phleospora robiniae) and tar spots on leaves (Ectostroma sp.), twig blight (Pseudovalsa sp.) and Chorostate sp. on young shoots, black spots (Cucurbitaria sp.) on old shoots, and wood-destroying fungi (Hironela sp, Trametes sp., Phellinus sp.).5. Wood Products and Other Uses of Black Locust
5.1. Wood Production
As a result of high percentage of coppice stands [25], the size of the black locust round wood usually does not exceed 20 cm in diameter at thin end, and quality of timber is relatively poor compared to key native species. In most of the Romanian regions, short longevity because of the tree dieback is the main cause for rather small tree dimensions comparative to local broadleaved tree species. Therefore, majority of wood generated by black locust stands is mostly used as firewood or in rural construction (e.g., fences, sheds, poles) or less for props for gardening and vineyards [26]. The good quality wood can also be used for interior and garden furniture, parquet and floors or woodchip boards while more common uses are for fenceposts, poles, railroad crossties, stakes and fuel wood [27][28][29].5.2. Non-Wood Products
Black locust is so appreciated by foresters and farmers alike due to a range of non-wood products that are available already at an early age of stands [30]. The most valued non-wood byproduct is honey, considered of the highest quality [31], which makes it the most expensive on the market. Compared to the traditional melliferous species (linden/lime, black locust, sunflower, rapeseed), black locust is the first to bloom (in May); therefore, beekeepers start the pastoral beekeeping in the black locust forests. Black locust stands have a high melliferous potential in Romania with up to 697,000 tonnes of honey per year. In order to ensure that the full benefit is reached, an application to support the planning of the pastoral beekeeping was developed based on forest maps, as a tool for decision makers at a national level in the planning of pastoral activity of the beekeepers. Black locust flowers show medicinal use in teas or infusions for digestive and pulmonary effects, and they have a calming effect on the nervous system [32][33][34]. A mix of flowers and leaves can be used as a tea drink to treat stomach pains and migraine.6. Landscape Improvement Contribution
In Romania, black locust was very successful in reclaiming degraded lands [35] by exercising its anti-erosion role together with phytoremediation [36], and biomass accumulation in site conditions strongly prohibitive to other species [37]. On industrial or mining dumps, black locust plantations reached volumes of up to 73 m3/ha among 8-year-old stands [38]. It was also successfully used in the ecological remediation of historically heavily polluted lands [39]. In a Kyoto Protocol project of afforestation implemented in Romania, about 2500 ha were afforested with black locust in the south of the country to reclaim degraded and marginal agricultural lands [40]. Ten years after their afforestation, black locust plantations have reached heights of 14 m and basal diameters of 16 cm (Figure 2).
Figure 2. (a) Four-year-old black locust plantation; (b) Ten-year-old plantations of oak mix (left) and black locust (right).
7. Ecosystem Services
When used on degraded lands, social benefits include improving local microclimate and mitigating the negative effects of climate change [41][42]. Shortly after planting, the stands start ensuring improvement of local biodiversity by offering shelter and food sources for birds, mammals, and other species. The economic and ecological role of black locust in agroforestry is represented by the forest shelterbelts to protect crop fields in the south of Romania [43][44][45][46], showing that the presence of shelterbelts in the Oltenia region led to an increase in different crop productions (e.g., wheat) of up to 130%, as opposed to unprotected fields. The shelterbelts are also a source of wood, honey, and they offer shelter for game and bird species. As one of the main species used in degraded land restoration, black locust plays an important role at both local and regional levels [4] in the effort to adapt and mitigate climate change by diversifying local supply for wood and feedstock or revenues from improved land use. Under recent more advanced ecosystem services payments, afforestation of degraded lands allowed further economic benefits, by trading greenhouse gas emissions reductions generated by tree plantations as financial instruments provided by the Kyoto Protocol [47]. Black locust is also appreciated in landscaping or gardening, for its decorative grape-like—sweet perfumed—white flowers, and also for its robustness and adaptability [48].
8. Invasiveness and Control of Black Locust
In some European countries, the species is considered invasive [49]. Caused in general by inappropriate silvicultural decision-making and practices, the invasive character of black locust manifests itself as a result of the species great adaptability, highly developed vegetative regeneration (especially sprouting), soil condition alteration, and fast rate of growth compared to native species. When planted in close mixtures with slower growing species (e.g., oak) it overwhelmed them, after which it was extremely difficult and expensive to substitute it [50]. In this respect, Romanian forestry law and technical norms provide for the use of black locust only on degraded lands that prohibit the use of native species (severe site conditions). Its invasion on non-forest lands—e.g., orchards and vineyards especially—has occurred on lands abandoned after 1990. While, in other cases, it expands especially because of soil disturbance and stimulation of root-sucker growth.
References
- Ciuvăţ, A.L.; Abrudan, I.V.; Blujdea, V.; Enescu, M.; Marcu, C.; Dinu, C. Distribution and Particularities of Black Locust in Romania. Rev. Silvic. Şi Cinegetică Anul XVIII 2013, 32, 76–85.
- Vlad, R.; Constandache, C.; Dincă, L.; Tudose, N.C.; Sidor, C.G.; Popovici, L.; Ispravnic, A. Influence of climatic, site and stand characteristics on some structural parameters of scots pine (Pinus sylvestris) forests situated on degraded lands from east Romania. Range Manag. Agrofor. 2019, 40, 40–48.
- Ivanschi, T.; Costea, A.; Bîrlănescu, E.; Mărcoiu, A.; Nonuţe, I. Cercetări privind stabilirea staţiunilor apte pentru cultura salcâmului. In Cercetări Privind Cultura Salcâmului; Costea, A., Ed.; Editura Agrosilvică Bucureşti: Bucureşti, Romania, 1969; pp. 11–55.
- Nicolescu, V.-N.; Buzatu-Goanta, C.; Bartlett, D.; Iacob, N. Regeneration and Early Tending of Black Locust (Robinia pseudoacacia L.) Stands in the North-West of Romania. South-East Eur. 2019, 10, 97–105.
- Şofletea, N.; Curtu, L. Dendrologie; Editura Universităţii Transylvania: Bucureşti, Romania, 2007; 540p.
- Dincă, L.; Timiș-Gânsac, V.; Breabăn, I.G. Forest stands from direct accumulation and natural lakes slopes from the Southern Carpathians. Present Environ. Sustain. Dev. 2020, 14, 211–218.
- Oneț, A.; Dincă, L.; Teușdea, A.; Crișan, V.; Bragă, C.; Enescu, R.; Oneț, C. The influence of fires on the biological activity of forest soils in Vrancea, Romania. Environ. Eng. Manag. J. 2019, 18, 2643–2654.
- Costea, A.; Lăzărescu, C.; Bârlănescu, E.; Ivanschi, T.; Spârchez, Z. Studii asupra tipurilor de culturi de salcâm. In Cercetări Privind Cultura Salcâmului; Costea, A., Ed.; Editura Agrosilvică Bucureşti: Bucureşti, Romania, 1969; pp. 63–78.
- Bârlănescu, E.; Costea, A.; Armăşescu, S.; Tănăsescu, S.; Vârbănescu, M.; Ruţă, S.; Baciu, V. Experimentări Privind Aplicarea Răriturilor în Salcâmetele din Oltenia. Cercetări Privind Cultura Salcâmului; Editura Agrosilvică Bucureşti: Bucureşti, Romania, 1969; pp. 111–154.
- Costea, A.; Lăzărescu, C.; Bârlănescu, E.; Ivanschi, T.; Armăşescu, S.; Trantescu Gr Latiş, L.; Pârvu, E. Recomandări Privind Cultura Salcâmului—Robinia pseudoacacia L.; Institutul de Cercetări Studii şi Proiectări Silvice: Bucureşti, Romania, 1969; 39p.
- Hernea, C.; Poşta, D.S.; Dragomir, P.I.; Corneanu, M.; Sărac, I. Aspects Concerning Germination Tests of Robinia pseudoacacia var. Oltenica. J. Hortic. For. Biotechnol. 2010, 14, 138–142.
- Technical Norms for Forestry; Ministry of Environment, Water and Forests: Bucharest, Romania, 2000.
- Abrudan, I.V. Impăduriri; Editura Universitaţii Transilvania din Braşov: Brașov, Romania, 2000; 200p, ISBN 973-635-688-4.
- Popa, B. Analiza lucrărilor de substituire şi refacere în arboretele din Podişul Covurlui. Rev. Pădurilor 2003, 2, 13–17.
- Constandache, C.; Sanda, N.; Virgil, I. Împădurirea terenurilor degradate ineficiente pentru agricultura din sud-estul ţării. Ann. For. Res. 2006, 49, 187–204.
- Bîrlănescu, E.; Aurelian, C.; Cristian, S. O nouă varietate de salcîm identificată în România—Robinia pseudacacia L. var oltenica Birl. Bost. et Stoic. Rev. Pădurilor 1966, 89, 483–486.
- Hernea, C.; Netoiu, C.; Corneanu, M. Auxological Research Concerning Robinia Pseudoacacia var. Oltenica; Editura Editura Universitaria Craiova: Craiova, Romania, 2008.
- Corneanu, M.; Corneanu, G.C.; Iliev, I.; Danci, O.; Stefanescu, I.; Popa, M. Micropropagation of Robinia pseudoacacia var. oltenica Selected Stress Resistant Clones on Media with Deuterium Depleted Water. J. Hortic. For. Biotechnol. 2010, 14, 141–145.
- Lăzărescu, C. Rezultatele privind înmulţirea salcâmului prin butăşire repetată. Rev. Pădurilor 1968, 83, 57–59.
- Costea, A.; Lăzărescu, C.; Bârlănescu, E. Contribuţii privind selecţia salcâmului şi tipurile de cultură cu salcâm în terenuri forestiere. Ann. For. Res. 1970, 27, 163–168.
- Bolea, V.; Catrina, I.; Popa, A.; Afrenie, F.; Cioloca, N.; Nicolescu, L. Particularităţi ecologice ale salcîmului—Robinia pseudacacia L.—relevate prin mediul variaţiei sezoniere a fotosintezei în raport cu factorii de mediu. Rev. Pădurilor 1995, 110, 33–41.
- Enescu, V.; Bîrlănescu, E.; Costea, A. Selection de Certaines Populations D’elite du Robinier Faux Acacia et Possibilityes de Leur Multiplication par Voie Vegetative; FAO—Consultation Mondiale sur la Genetique Forestiere et l’Amelioration des arbres, Stokholm; FAO FORGEN: Rome, Italy, 1963; pp. 63–64.
- Mihai, G.; Dincă, L. In situ conservation of forest genetic resources from the Southern Carpathians. Int. J. Conserv. Sci. 2010, 11, 1051–1058.
- Bârlanescu, E.; Diaconu, M.; Costea, A.; Cojocaru, I. Cercetări privind ameliorarea salcâmului. Ann. For. Res. 1977, 34, 41–54.
- Milescu, I.; Armăşescu, S. Unele particularităţi dendrometrice ale arboretelor de salcâm în raport cu provenienţa. Rev. Pădurilor 1960, 75, 414–417.
- Holban, C. Culturi specializate de salcâm pe soluri aluviale. Rev. Pădurilor 1969, 84, 359–360.
- Bularca, M. Cercetări cu privire la utilizarea lemnului de salcîm şi salcie şi a unor materiale lignocelulozice (coarde de viţă de vie şi puzderii de in) ca materie primă la fabricarea plăcilor din fibre de lemn (PFL)—procedeu umed. Rev. Pădurilor 1985, 36, 193–200.
- Porojan, M. Contribuţii la Studiul Proprietatilor Fizico-Mecanice si Tehnologice ale Lemnului de Salcâm. Ph.D. Thesis, Universitatea Transilvania din Braşov, Facultatea de Industria Lemnului, Brașov, Romania, 2007.
- DeGomez, T.; Wagner, M.R. Culture and Use of Black Locust. HortTechnology 2001, 11, 279–288.
- Pleșca, I.M.; Blaga, T.; Dincă, L.; Breabăn, I.G. Prioritizing the potential of non-wood forest products from Arad County by using the analytical huerarchy process. Present Environ. Sustain. Dev. 2019, 13, 225–233.
- Mădaş, M.N. Cercetări privind indicii de calitate şi autenticitate ai mierii de salcâm (Robinia pseudoacacia L.). Doctoral Thesis, Facultatea de Zootehnie şi Biotehnologii, Universitatea de Ştiinţe Agricole şi Medicină Veterinară, Cluj-Napoca, Romania, 2013; 34p.
- Chirilă, M.; Chirilă, P. Tratament Homeopatic. Îndreptar de Simptome şi Semne; Editura Ştiinţifică şi Enciclopedică Bucureşti: Bucuresti, Romania, 1986; 253p.
- Pârvu, C. Universul Plantelor—Mică Enciclopedie, Ediţia a III a; Editura Enciclopedica: Bucuresti, Romania, 2000.
- Vasile, D.; Enescu, M.; Dincă, L. Which are the main medicinal plants that could be harvested from Eastern Romania? Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2018, 18, 523–528.
- Constandache, C.; Dincă, L.; Tudor, C. The bioproductive potential of fast-growing forest species on degraded lands. Sci. Papers. Ser. E. Land Reclam. Earth Obs. Surv. Environ. Eng. 2020, IX, 87–93.
- Neţoiu, C.; Hernea, C.; Tomescu, R. Vegetaţia forestieră din bazinul mijlociu al Jiului. In Bazinul Mijlociu al Jiului. Implicaţii de mediu şi Sociale ale Industriei Extractive şi Energetice. Studiu Monographic; Editura Universitaria Craiova: Craiova, Romania, 2011; pp. 191–219.
- Mantovani, D.; Veste, M.; Freese, D. Black locust (Robinia pseudoacacia L.) ecophysiological and morphological adaptations to drought and their consequence on biomass production and water-use efficiency. New Zealand J. For. Sci. 2014, 44, 29.
- Cântar, I.C.; Chisăliță, I.; Dincă, L. Structure of some stands installed on tailing dumps: Case study from Moldova Noua, Romania. Int. Multidiscip. Sci. GeoConference SGEM 2018, 18, 757–764.
- Ianculescu, M. Studiu Privind Reconstrucţia Ecologică a Pădurilor Afectate de Poluare din Zona Copşa Mică; Tema ICAS nr. 6.6.—A.38; Scientific report (Unpublished); Arhiva ICAS Bucureşti: Bucuresti, Romania, 1994.
- Abrudan, I.V.; Blujdea, V.; Pahonţu, C. Împădurirea terenurilor degradate în contextul eforturilor de diminuare a schimbărilor climatice. Rev. Pădurilor 2002, 117, 1–5.
- Ciuvăţ, A.L. Monitorizarea Proiectului de Împădurire a Terenurilor Degradate, Estimarea Acumulării de Carbon, Raportarea şi Resimularea Acumulării—Referat Ştiinţific; Scientific Report (Unpublished); Institute for Research and Management in Forestry Bucharest: Bucuresti, Romania, 2011.
- Ducci, F.; De Rogatis, A.; Proietti, R.; Curtu, L.A.; Marchi, M.; Belletti, P. Establishing a baseline to monitor future climate change-effects on peripheral populations of Abies alba in central Apennines. Ann. For. Res. 2021, 64, 33–66.
- Kutnar, L.; Kermavnar, K.; Pintar, A.M. Climate change and disturbances will shape future temperate forests in the transitionzone between Central and SE Europe. Ann. For. Res. 2021, 64, 67–86.
- Ianculescu, M. Perdelele forestiere de protecţie în contextual majorării suprafeţei pădurilor şi al atenuării modificărilor climatic. In Silvologie Vol. IVA; Giurgiu, V., Ed.; Editura Academiei Române Bucureşti: Bucharest, Romania, 2005; pp. 201–223.
- Costăchescu, C.; Dănescu, F.; Mihăilă, E. Perdele Forestiere de Protecţie; Editura Silvică: Bucharest, Romania, 2010; 262p, ISBN 978-606-8020.
- Mihăilă, E.; Costăchescu, C.; Dănescu, F. Sisteme Agrosilvice; Editura Silvică: Bucharest, Romania, 2010; 190p, ISBN 978-606-8020-06-8.
- Ciuvăţ, A.L.; Abrudan, I.V.; Blujdea, V. Biomass Production in Young Black Locust Plantations in Southern Romania. In Proceedings of the Proceedings International Conference Ecology-Interdisciplinary Science and Practice, Sofia, Bulgaria, 25–26 October 2012; pp. 70–74, ISBN 978-954-749-096-3.
- Purcelean, Ş. Specii şi varietăţii decorative de Robinia indicate pentru spaţii verzi. Rev. Pădurilor 1954, 69, 369–372.
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; Von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640.
- Constandache, C.; Peticilă, A.; Dincă, L.; Vasile, D. The usage of Sea Buckthorn (Hippophae rhamnoides L.) for improving Romania’s degraded lands. AgroLife Sci. J. 2016, 5, 50–58.
More
