Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Chaiyavat Chaiyasut.
Aromatic essential oils play a significant role in pharmaceuticals, food additives, cosmetics, and perfumery. Essential oils mostly comprise aliphatic hydrocarbons, monoterpenoids, sesquiterpenoids and diterpenes. Plant extracts comprise a complex mixture of terpenes, terpenoids, aliphatic and phenol-derived aromatic components. Terpenes are a significant class of hydrocarbons with numerous health benefits. Monoterpenes are the most important constituents of essential oils. α-phellandrene (α-PHE) is a cyclic monoterpene with two double bonds in a heterocyclic ring (endocyclic).
- α-phellandrene
- essential oils
- terpenes
1. Introduction
Plant-based medicines are playing a vivid role in health care. Plant-derived essential oils are one of the prominent sources of bioactive molecules, including volatile lipophilic compounds with strong aromas. Essential oils are composed of hydrocarbons and their derivatives, such as alcohols, acids, esters, aldehydes, ketones, amines, nitrogen and sulfur compounds, oxygenated terpenes, terpenoid hydrocarbons and sesquiterpenes [1,2][1][2]. Essential oils are isolated from plants through various distillation processes, such as hydro and steam distillation, effleurage, maceration, supercritical, microwave-assisted, and solvent extractions [1]. Other chromatographic techniques, such as column, thin layer, high-pressure liquid chromatography, mass spectrometry (MS), nuclear magnetic resonance (NMR) spectroscopy, gas chromatography/mass spectrometry (GC/MS), liquid chromatography/mass spectrometry, and Fourier-transform infrared spectrophotometry were also used in the characterization and identification of compounds in the essential oils [1].
Monoterpenes are the most important constituents of essential oils. α-phellandrene (α-PHE) is a cyclic monoterpene with two double bonds in a heterocyclic ring (endocyclic) [3]. It was named after Eucalyptus phellandra (E. phellandra), the plant from where α-PHE was first isolated. α-PHE is a natural plant-derived compound with various medicinal properties, found useful in the pharmaceutical, food, cosmetic and perfume industries [4,5][4][5]. Phellandrene (PHE) compounds are prevalently used in fragrances [6]. PHE is found in plants, such as Angelica and Eucalyptus, and due to its pleasant scent, it has been used in fragrances [7]. E. microtheca, E. viminalis, and E. dives primarily emit α-PHE into the atmosphere, and the α-PHE produces particle nucleation due to monoterpene oxidation, observed over Eucalypt forests [8,9][8][9]. In the indoor environment, α-PHE is used in cleansing products, detergents, and room fresheners [10]. α-PHE interacts with the local environment when it is emitted. α-PHE is an important component for ozonolysis and produces a significant, blue-colored haze due to nocturnal nucleation over the Eucalypt forests [11].
2. Properties of Phellandrene
Phellandrenes comprise alpha-phellandrene (α-PHE) and beta-phellandrene (β-PHE), prominently found in eucalyptus plant species. PHEs are also present in other plants, such as mint, black pepper, parsley, cinnamon, lavender, pine, ginger grass, water fennel, and dill [13][12]. PHEs are herbaceous with different aromas, which have been known for their anti-fungal [14][13], anti-inflammatory, anti-hyperalgesic, anti-depressant [15][14], analgesic [16][15] and anti-cancer activity [17,18][16][17]. Plant-derived secondary metabolites comprise different active components, most prevalently, terpenes, alkaloids, and phenolic compounds [19][18]. The compounds are extracted from the medicinal plants via various methods, which yield a complex liquid mixture of chemicals, including terpenes, terpenoids, aliphatic and aromatic compounds and characteristic volatile properties, and are mostly lipophilic [4,16][4][15]. The monoterpene α-PHE is one of the predominant constituents of many plant species. The list of the representative plant species containing α-PHE is summarized in Table 1, and pictures of the few representative plant species are displayed in Figure 1.
Figure 1. The pictures of the few representative plant species reported to have α-phellandrene [38].
Table 1.
The list of representative plant species that are reported to have α-Phellandrene.
| S. No. | Plant Species | α-Phellandrene Content (%) # | References |
|---|---|---|---|
| 1 | Curcuma zedoaria (Christm) | 14.93 | [20] |
| 2 | Xylopia aromatica L. | 2.2–6.4 | [21] |
| 3 | Rosmarinus officinalis L. | 0.1–0.4 | [22] |
| 4 | Eucalyptus dives | 17.4 | [23] |
| 5 | Eucalyptus staigeriana | 8.8 | [23] |
| 7 | Schinus terebinthifolius Raddi | 15.7 | [24] |
| 34.38 | [25] | ||
| 6 | Schinus molle L. | 46.52 | [25] |
| 8 | Solanum erianthum D. Don. | 17.5 | [26] |
| 9 | Thymus kotschyanus Boiss. and Hohen. | 10.8 | [27] |
| 10 | Cupressus atlantica Gaussen | 5.5 | [28] |
| 11 | Anethum graveolens L. | 32 | [29] |
| 12 | Myrica gale L. | 8 | [30] |
| 13 | Ligusticum mutellina L. Crantz | 23.4 | [31] |
| 14 | Ligusticum marginatum | 50.2 | [32] |
| 15 | Artemisia feddei | 5.78 | [33] |
| 16 | Bursera morelensis | 1 | [34] |
| 17 | Monodora myristica (Gaertn.) | 34.4 | [35] |
| Monodora myristica (Gaertn.) Dunal | 53 | [36] | |
| 18 | Piper nigrum | 8.56 | [37] |
# α-PHE content (%) in the essential oil of the respective plants.
 The chemical transformation of α-PHE by photo-oxidation and electrochemical processes has been reported [53][52]. (−)-(R)-α-PHE was also bio-transformed by fungal species such as Fusarium heterosporium (F. heterosporium), Aspergillus alliaceus (A. alliaceus), Yarrowia lipolitica (Y. lipolitica), Alternaria alternata (A. alternata), Saccharomyces cerevisiae (S. cerevisiae), Kluyveromyces lactis (K. lactis), Neurospora crassa (N. crassa), Fusarium solani (G. solani), Fusarium culmorum (F. culmorum), Botrytis cinerea (B. cineria), Aspergillus flavus (A. flavus), Phanerochaete chrysosporium (P. chrysosporium), and Devosia riboflavina (D. riboflavina) strains [5].
The chemical transformation of α-PHE by photo-oxidation and electrochemical processes has been reported [53][52]. (−)-(R)-α-PHE was also bio-transformed by fungal species such as Fusarium heterosporium (F. heterosporium), Aspergillus alliaceus (A. alliaceus), Yarrowia lipolitica (Y. lipolitica), Alternaria alternata (A. alternata), Saccharomyces cerevisiae (S. cerevisiae), Kluyveromyces lactis (K. lactis), Neurospora crassa (N. crassa), Fusarium solani (G. solani), Fusarium culmorum (F. culmorum), Botrytis cinerea (B. cineria), Aspergillus flavus (A. flavus), Phanerochaete chrysosporium (P. chrysosporium), and Devosia riboflavina (D. riboflavina) strains [5].
3. Chemistry and Synthesis of α-PHE
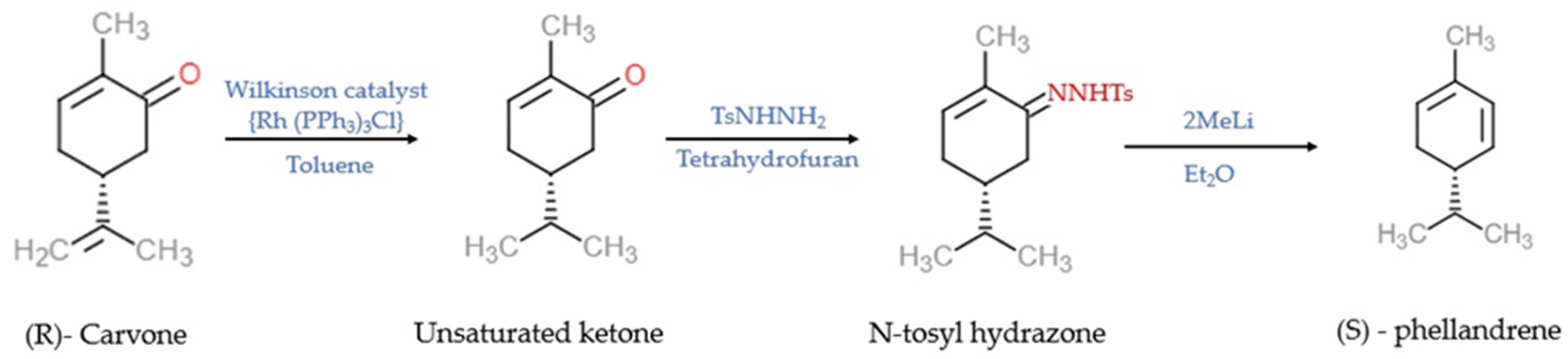
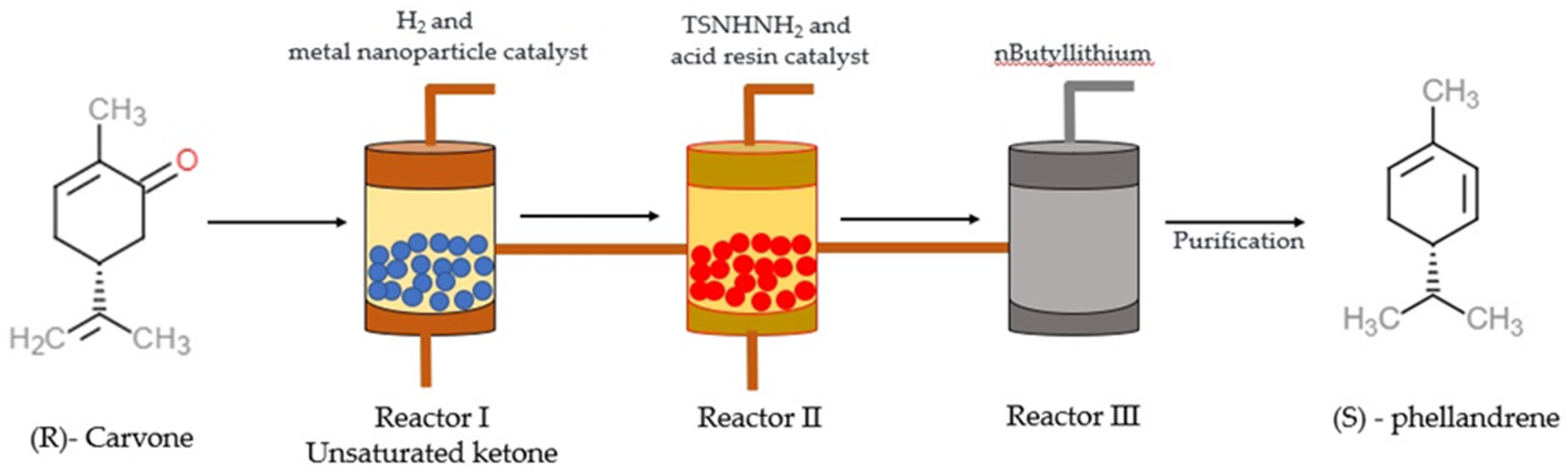
Tea and lemon tree oils have high p-cymene and other monoterpenoids [39,40][38][39]. γ-Terpinene (1-isopropyl-4-methyl-1,4-cyclohexadiene) and α-PHE (5-isopropyl-2-methyl-1,3-cyclohexadiene) with the molecular formula C10H16 are grouped under the class of monoterpenes. In α-PHE, the cis-1, 3-diene chain is inserted into the 6-membered ring. Photooxidation of α-PHE revealed that the ring structure of α-PHE opens to produce a cyclohexatriene-type product [41][40]. Like γ-terpinene, α-PHE can also exhibit three structural conformations due to the internal rotation of the exocyclic isopropyl group. The three conformers can be identified using experimental IR spectra. The UV-induced photoreaction results in the reorganization of the π-system. At λ > 200 nm, new bands are produced in the spectrum of α-PHE; this shows that the α-PHE seems to be photolabile under UV-C light [41][40]. In solution, α-PHE exists in folded conformation with the left-handed diene helix [42][41]. The diene helix is left handed in the axial conformer, and in the equatorial conformer, it is right handed. In α-PHE, (R)-(−)-5-isopropyl-2-methyl-1,3-cyclohexadiene possesses the quasi-axial-isopropyl group to sustain in solution [43][42]. α-PHE exists as an axial-isopropyl conformer in conformational equilibrium, and its stability is due to the presence of the CH/π hydrogen bond [44][43]. The electron transfer catalysis reaction of (R)-α-PHE with 4-methoxystyrene produces mixed cycloaddition products [45][44]. Upon photoexcitation, the ground state and excited state dynamics of α-PHE were studied using broadband femtosecond UV absorption spectroscopy [46][45]. The ring-opening dynamics of α-PHE were examined using picosecond time-resolved UV resonance Raman spectroscopy [47][46]. In the matrix isolation experiment, α-PHE photochemistry and ground state conformational size were intermediate between 1,3-cyclohexadiene (CHD) and 7-dehydrocholesterol, provitamin D3 (DHC) [41][40]. Synthesis of α-PHE from (R)-carvone through the batch process was reported by Sen and Grosch [48][47] and, through the continuous-flow process, by [49][48]. In Sen and Grosch’s model of α-PHE synthesis, R-carvone converted to the unsaturated ketone with the help of Wilkinson’s catalyst (Rh (PPh3)3Cl) and toluene. Unsaturated ketone converted to N-tosyl hydrazone in the presence of tosyl hydrazide resin (TsNHNH2) and tetrahydrofuran (THF). With the help of methyllithium (2MeLi) and diethylether (Et2O), N-tosyl hydrazone converted into (S)-α-PHE, and the yield was 48% [48][47] (Figure 2). In the continuous-flow process model, R-carvone was subjected to reduction with the help of a metal nanoparticle as a catalyst in the first reactor. Then the condensation was carried out in the second reactor with TSNHNH2 and acid resin as the catalyst. The steam from the second reactor was linked with the third reactor, which contains a strong base, n-butyllithium (nBuLi). The organic phase was collected and dried in the presence of disodium sulphate (Na2SO4) and filtered with alumina, which yielded crude α-PHE. The product was then purified using flash chromatography and distilled to produce 96% pure α-PHE [49][48] (Figure 3).
Figure 2. The batch process model of the synthesis of (S)—phellandrene [48][47]. Chemical structures were drawn using free online ChemDoodle Web software (https://web.chemdoodle.com/demos/2d-sketcher, accessed on 22 August 2022).

Figure 3. The illustration describes the process of (S)—phellandrene synthesis using a continuous-flow process [49][48]. Chemical structures were drawn using free online ChemDoodle Web software (https://web.chemdoodle.com/demos/2d-sketcher, accessed on 22 August 2022).
4. Biotransformation of α-PHE
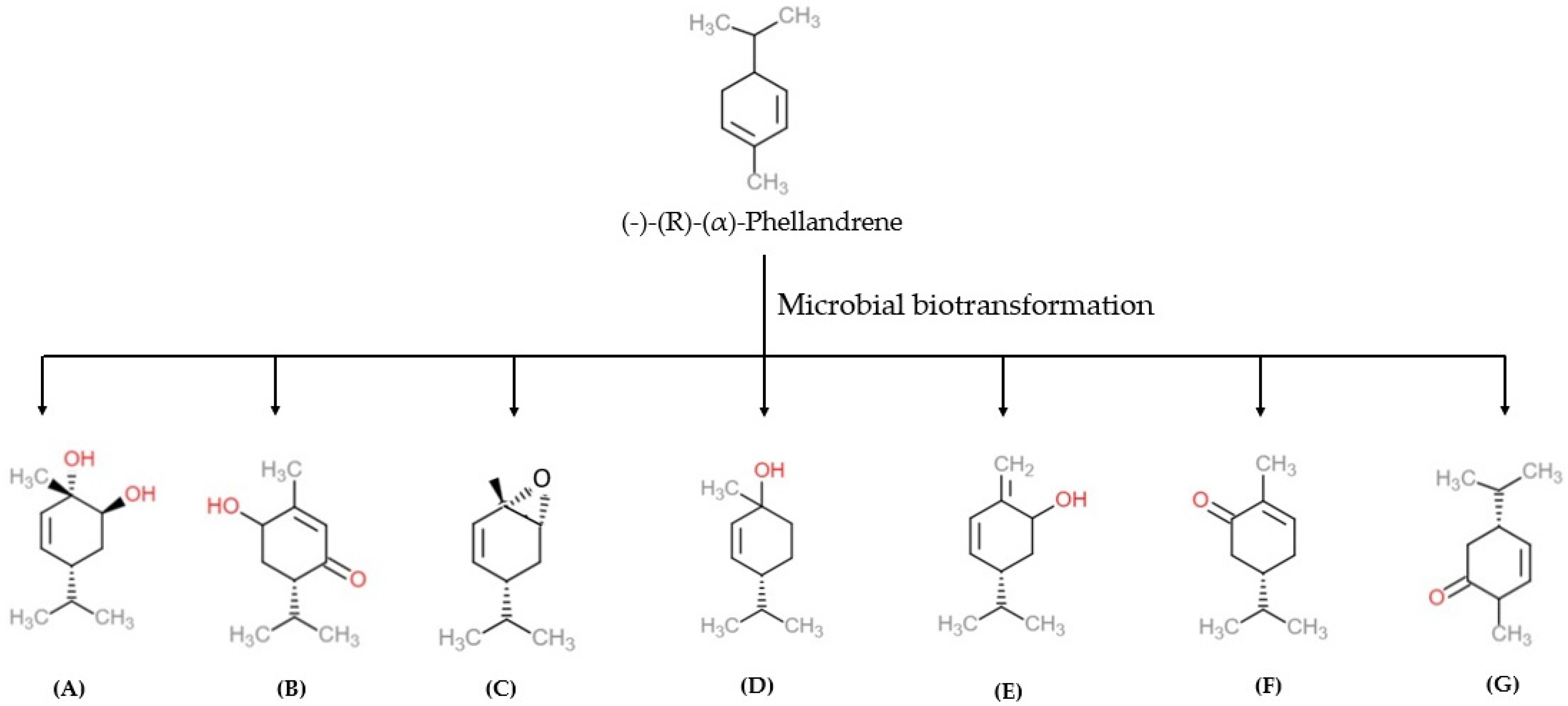
Generally, fragrance compounds exist as single enantiomers. The diversity in sensory properties of fragrance is associated with different types of enantiomers. The chemical synthesis or derivatizations of stereo-, enantio- and regioselective forms can be made by the biotransformation method. The stereo- and enantioselective chiral compound biotransformations were found useful in pharmaceutical and chemical industries in commercial-scale applications [50][49]. α-PHE exists in two enantiomer forms, such as (−)-(R) and (+)-(S), with varied physicochemical and olfactory properties [5]. İşcan et al. bio-transformed enantiomerically pure (−)-(R)-α-PHE using 16 microorganisms, including bacteria, yeasts, and fungi. Organisms, such as Escherichia coli (E. coli), Escherichia coli O157-H7, Staphylococcus aureus (S. aureus), Staphylococcus aureus MRSA, Staphylococcus epidermidis (S. epidermis), Pseudomonas aeruginosa (P. aeruginosa), Enterobacter aerogenes (E. aerogenes), Proteus vulgaris (P. vulgaris), Salmonella typhimurium (S. typhimrium), Bacillus cereus (B. cereus), Bacillus subtilis (B. subtilis), Agrobacterium tumefaciens (A. tumefaciens), Erwinia carotovora subsp. carotovora (E. carotovora subsp. carotovora), Peudomonas syringae pv. Phaseolicola (P. syringae pv. phaseolicola), and Pseudomonas syringae pv. tomato (P. syringae pv. tomato) and Serratia marcescens, were used in the biotransformation of (−)-(R)-α-PHE [5]. (−)-(R)-α-PHE biotransformation yielded six metabolites namely, 5-p-menthene-1,2-diol, 6-hydroxypiperitone, α-PHE epoxide, cis-p-menth-2-en-1-ol, p-mentha-1(7), 5-dien-2-ol and carvotanacetone. The resulting metabolites were screened by chromatographic techniques, and their biological activities were examined against pathogenic microorganisms. The anti-bacterial activity of α-PHE and the compound 5-p-menthene-1,2-diol were analyzed. Among these two metabolites, 5-p-menthene-1,2-diol showed significant anti-microbial and anti-candidal (anti-fungal) activity compared to α-PHE [5]. In other studies, α-PHE was bio-transformed into other metabolites using different organisms. Three different metabolites, 5-p-menthene-1,2-diol, p-mentha-1(7), 5-dien-2-ol and 5-p-menthen-2-one, were synthesized by Corynespora cassiicola (C. cassiicola) (DSM 62474) (Figure 4) [51][50]. Biotransformation of α-PHE by Alternaria alternata produces 5-p-menthen-1, 2-diol with anti-microbial activity [52][51].
Figure 4. The microbial biotransformation of (−)-(R)-(α)-phellandrene produces different products namely (A) 5-p-menthene-1,2-diol, (B) 6-hydroxypiperitone, (C) α-PHE epoxide, (D) cis-p-menth-2-en-1-ol, (E) p-mentha-1(7), 5-dien-2-ol, (F) carvotanacetone, (G) 5-p-menthen-2-one [5]. Chemical structures were drawn using free online ChemDoodle Web software (https://web.chemdoodle.com/demos/2d-sketcher, accessed on 22 August 2022).
5. α-PHE in Atmospheric Emission
Naturally, the monoterpenes play an important function in the atmosphere’s chemistry and influence the atmosphere’s oxidative capacity, which results in the formation and development of the tropospheric ozone and the secondary organic aerosol (SOA), which cause changes in climate and health [54,55][53][54]. The biogenic emission sources exceed the emission level of volatile compounds globally into the atmosphere; in this scenario, the monoterpenes produce a significant proportion of non-methane hydrocarbons [56][55]. α-PHE is the most reactive monoterpene, known for its tropospheric degradation and aerosol formation [57][56]. The ozonolysis changes of α-PHE and other associated mechanisms were studied. The results showed that when α-PHE reacts with ozone, one of the double bonds in α-PHE opens and releases SOA with 10 carbons. Then, the second addition of ozone causes fragmentation and produces SOA with three or seven carbons [57][56].References
- Baser, K.H.C. Analysis, and Assessment of Essential Oils. In A Manual on the Essential Oil Industry; De Silva, K.T., Ed.; United Nations Industrial Development Organization: Vienna, Austria, 1995; pp. 155–178.
- Guan, X.; Ge, D.; Li, S.; Huang, K.; Liu, J.; Li, F. Chemical composition and antimicrobial activities of Artemisia argyi Levl. et Vant essential oils extracted by simultaneous distillation-extraction, subcritical extraction and hydrodistillation. Molecules 2019, 24, 483.
- Kim, H.; Park, J. A density functional theory study on the reaction mechanism of α-Phellandrene with NO3. Bull. Korean Chem. Soc. 2015, 36, 2139–2142.
- Bizzo, R.H.; Hovell, A.M.C.; Rezende, C.M. Brazilian essential oil: General view, developments, and perspectives. Quim. Nova 2009, 3, 588–594.
- İşcan, G.; Kirimer, N.; Demirci, F.; Demirci, B.; Noma, Y.; Başer, K.H. Biotransformation of (−)-(R)-α-phellandrene: Antimicrobial activity of its major metabolite. Chem. Biodivers. 2012, 9, 1525–1532.
- Gaich, T.; Mulzer, J. Chiral Pool Synthesis: Starting from Terpenes. In Comprehensive Chirality, 1st ed.; Carreira, E.M., Yamamoto, H., Eds.; Elsevier: Amsterdam, The Netherland, 2012; Volume 2, pp. 163–206.
- New Directions Aromatics. Available online: https://www.newdirectionsaromatics.com/tags/phellandrene/essential-oils/ (accessed on 13 June 2022).
- Lee, S.H.; Young, L.H.; Benson, D.R.; Suni, T.; Kulmala, M.; Junninen, H.; Campos, T.L.; Rogers, D.C.; Jensen, J. Observations of nighttime new particle formation in the troposphere. J. Geophys. Res. 2008, 113, D10210.
- Suni, T.; Kulmala, M.; Hirsikko, A.; Bergman, T.; Laakso, L.; Aalto, P.P.; Leuning, R.; Cleugh, H.; Zegelin, S.; Hughes, D.; et al. Formation and characteristics of ions and charged aerosol particles in a native Australian Eucalypt Forest. Atmos. Chem. Phys. 2008, 8, 129–139.
- Clarke, S. Essential Chemistry for Aromatherapy, 2nd ed.; Churchill Livingstone: London, UK, 2009; pp. 123–229.
- Mackenzie-Rae, F.A.; Liu, T.; Deng, W.; Saunders, S.M.; Fang, Z.; Zhang, Y.; Wang, X. Ozonolysis of α-phellandrene—Part 1: Gas- and particle-phase characterisation. Atmos. Chem. Phys 2017, 17, 6583–6609.
- Trulieve. Available online: https://www.trulieve.com/discover/blog/everything-you-need-to-know-about-a-phellandrene-and-its-benefits (accessed on 20 July 2022).
- Zhang, J.H.; Sun, H.L.; Chen, S.Y.; Wang, T.T. Anti-fungal activity, mechanism studies on α-Phellandrene and nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13.
- Piccinelli, A.C.; Santos, J.A.; Konkiewitz, E.C.; Oesterreich, S.A.; Formagio, A.S.; Croda, J.H.; Ziff, E.B.; Kassuya, C.A. Antihyperalgesic and antidepressive actions of (R)-(+)-limonene, α-phellandrene, and essential oil from Schinus terebinthifolius fruits in a neuropathic pain model. Nutr. Neurosci. 2015, 18, 217–224.
- Lima, D.F.; Brandão, M.S.; Moura, J.B.; Leitão, J.M.; Carvalho, F.A.; Miúra, L.M.; Leite, J.R.; Sousa, D.P.; Almeida, F.R. Antinociceptive activity of the monoterpene α-phellandrene in rodents: Possible mechanisms of action. J. Pharm. Pharmacol. 2012, 64, 283–292.
- Hsieh, S.L.; Li, Y.C.; Chang, W.C.; Chung, J.G.; Hsieh, L.C.; Wu, C.C. Induction of necrosis in human liver tumor cells by α-phellandrene. Nutr. Cancer 2014, 66, 970–979.
- Lin, J.J.; Hsu, S.C.; Lu, K.W.; Ma, Y.S.; Wu, C.C.; Lu, H.F.; Chen, J.C.; Lin, J.G.; Wu, P.P.; Chung, J.G. Alpha-phellandrene-induced apoptosis in mice leukemia WEHI-3 cells in vitro. Environ. Toxicol. 2016, 31, 1640–1651.
- Kennedy, D.O.; Wightman, E.L. Herbal Extracts and Phytochemicals: Plant Secondary Metabolites and the Enhancement of Human Brain function. Adv. Nutr. 2011, 2, 32–50.
- POWO Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2022. Available online: http://www.plantsoftheworldonline.org/ (accessed on 17 September 2022).
- Singh, G.; Singh, O.P.; Maurya, S. Chemical and biocidal investigations on essential oils of some Indian Curcuma species. Prog. Cryst. Growth Charact. Mater. 2002, 45, 75–81.
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Analysis of volatile secondary metabolites from Colombian Xylopia aromatica (Lamarck) by different extraction and headspace methods and gas chromatography. J. Chromatogr. A 2004, 1025, 105–113.
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening of the essential oil composition of wild Sicilian rosemary. Biochem. Syst. Ecol. 2010, 38, 659–670.
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of essential oil of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737.
- Nascimento, A.F.; Camara, C.A.; Moraes, M.M.; Ramos, C.S. Essential oil composition and acaricidal activity of Schinus terebinthifolius from Atlantic Forest of Pernambuco, Brazil against Tetranychusurticae. Nat. Prod. Commun. 2012, 7, 129–132.
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472.
- Essien, E.E.; Ogunwande, I.A.; Setzer, W.N.; Ekundayo, O. Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm. Biol. 2012, 50, 474–480.
- Asbaghian, S.; Shafaghat, A.; Zarea, K.; Kasimov, F.; Salimi, F. Comparison of volatile constituents, and antioxidant and antibacterial activities of the essential oils of Thymus caucasicus, T. kotschyanus and T. vulgaris. Nat. Prod. Commun. 2011, 6, 137–140.
- Arjouni, M.Y.; Bahri, F.; Romane, A.; El Fels, M.A. Chemical composition and antimicrobial activity of essential oil of Cupressus atlantica. Nat. Prod. Commun. 2011, 6, 1519–1522.
- Hajhashemi, V.; Abbasi, N. Hypolipidemic activity of Anethum graveolens in rats. Phytother. Res. 2008, 22, 372–375.
- Popovici, J.; Bertrand, C.; Bagnarol, E.; Fernandez, M.P.; Comte, G. Chemical composition of essential oil and headspace-solid micro extracts from fruits of Myrica gale L. and antifungal activity. Nat. Prod. Res. 2008, 22, 1024–1032.
- Brandt, J.J.; Wulf, S. Composition of the Essential Oils of Ligusticum mutellina (L.) Crantz (Apiaceae). J. Essen. Oil. Res. 1995, 7, 231–235.
- Thakuri, B.C.; Padalia, R.C.; Tiwari, A.; Chanotiya, C.S.; Mathela, C.S. α-Phellandrene rich essential oil from Ligusticum marginatum C.B. Clarke. J. Essent. Oil Bear. Plants 2007, 10, 1–4.
- Cha, J.D.; Jung, E.K.; Kil, B.S.; Lee, K.Y. Chemical composition, and antibacterial activity of essential oil from Artemisia feddei. J. Microbiol. Biotechnol. 2007, 17, 2061–2065.
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound healing activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488.
- Koudou, J.; Etou, A.W.; Ossibi, K.; Abena, A.A.; Gbeassor, M.; Bessiere, J.M. Chemical composition and hypotensive effects of essential oil Monodora myristica Gaertn. J. Biol. Sci. 2007, 7, 937–942.
- Onyenekwe, P.C.; Stahl, M.; Adejo, G. Post-irradiation changes of the volatile oil constituents of Monodora myristica (Gaertn) Dunal. Nat. Prod. Res. 2012, 26, 2030–2034.
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J. Chromatogr. A 2002, 976, 265–275.
- Hausen, B.M.; Reichling, J.; Harkenthal, M. Degradation products of monoterpenes are the sensitizing agents in tea tree oil. Am. J. Contact Dermat. 1999, 10, 68–77.
- Iwanami, Y.; Tateba, H.; Kodama, N.; Kishino, K. Changes of lemon flavor components in an aqueous solution during UV irradiation. J. Agric. Food Chem. 1997, 45, 463–466.
- Marzec, K.M.; Reva, I.; Fausto, R.; Proniewicz, L.M. Comparative matrix isolation infrared spectroscopy study of 1,3- and 1,4-diene monoterpenes (α-phellandrene and γ-terpinene). J. Phys. Chem. A 2011, 115, 4342–4353.
- Burgstahler, A.W.; Ziffer, H.; Weiss, U. The configurations of levopimaric acid and α-phellandrene; Interpretation of their rotatory dispersions. J. Am. Chem. Soc. 1961, 83, 4660–4661.
- Horsman, G.A.; Emeis, C.A. Low-temperature optical rotatory dispersion. Tetrahedron 1965, 22, 167–173.
- Takahashi, O.; Yamasaki, K.; Kohno, Y.; Ueda, K.; Suezawa, H.; Nishio, M. Origin of the axial-alkyl preference of (R)-α-phellandrene and related compounds investigated by high-level ab initio MO calculations. Importance of the CH/π hydrogen bond. Tetrahedron 2008, 64, 5773–5778.
- Sevov, C.S.; Wiest, O. Selectivity in the electron transfer catalyzed Diels-Alder reaction of (R)-alpha-phellandrene and 4-methoxystyrene. J. Org. Chem. 2008, 73, 7909–7915.
- Arruda, B.C.; Smith, B.; Spears, K.G.; Sension, R.J. Ultrafast ring-opening reactions: A comparison of alpha-terpinene, alpha-phellandrene, and 7-dehydrocholesterol with 1,3-cyclohexadiene. Faraday Discuss. 2013, 163, 159–275.
- Reid, P.J.; Doig, S.J.; Wickham, S.D.; Mathies, R.A. Photochemical ring-opening reactions are complete in picoseconds: A time resolved UV resonance Raman study of 1,3 cyclohexadiene. J. Am. Chem. Soc. 1993, 115, 4754–4763.
- Sen, A.; Grosch, W. The synthesis of enantiomerically pure (S)(+)-a-phellandrene from (R)(−)-carvone. Flavour Fragr. J. 1990, 5, 233–234.
- Miller, S.J.; Ishitani, H.; Furiya, Y.; Shu, K. High-Throughput Synthesis of (S)-α-Phellandrene through Three-Step Sequential Continuous-Flow Reactions. Org. Process Res. Dev. 2021, 25, 192–198.
- Straathof, A.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2002, 13, 548–556.
- Abraham, W.R.; Stumpf, B.; Kieslich, K. Microbial Transformations of Terpenoids with 1-p-Menthene Skeleton. Appl. Microbiol. Biotechnol. 1986, 24, 24–30.
- Lai-King, S.Y.; Saunders, R.M.K.; Brown, G.D. Phytochemistry of Illicium dunnianum and the systematic position of the illiciaceae. Phytochemistry 1997, 44, 1099–1108.
- Montiel, V.; Lopez-Segura, M.; Aldaz, A.; Grande, M.; Barba, F. Electrooxidation of terpenes-I. Synthesis of dihydrocarvone and 1-hydroxyneodihydrocarveol by anodic oxidation of limonene. Electrochim. Acta 1984, 29, 1123–1126.
- Hoffmann, T.; Odum, J.R.; Bowman, F.; Collins, D.; Klockow, D.; Flagan, R.C.; Seinfeld, J.H. Formation of organic aerosols from the oxidation of biogenic hydrocarbons. J. Atmos. Chem. 1997, 26, 189–222.
- Griffin, R.J.; Cocker, D.R.; Flagan, R.C.; Seinfeld, J.H. Organic aerosol formation from the oxidation of biogenic hydrocarbons. J. Geophys. Res. 1999, 104, 3555–3567.
- Schurgers, G.; Arneth, A.; Holzinger, R.; Goldstein, A.H. Process-based modelling of biogenic monoterpene emissions combining production and release from storage. Atmos. Chem. Phys. 2009, 9, 3409–3423.
- Mackenzie-Rae, F.A.; Wallis, H.J.; Rickard, A.R.; Pereira, K.L.; Saunders, S.M.; Wang, X.; Hamilton, J.F. Ozonolysis of α-phellandrene—Part 2: Compositional analysis of secondary organic aerosol highlights the role of stabilised Criegee intermediates. Atmos. Chem. Phys. 2017, 18, 4673–4693.
More
