You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Roberto Parra-Saldívar.
Corn is the fourth largest crop in the world, and its residues represent a potentially renewable feedstock for industrial lactic acid production through simultaneous saccharification and fermentation (SSF). Different frameworks of bioconversion surged as alternatives for biomass disposal, which depend on the nature of the residue.
- second-generation lactic acid
- corn stover
- corncob
- simultaneous saccharification and fermentation
- lignocellulose revalorization
1. Introduction
Biorefinery is based on the fermentation of the carbohydrates present in biomass, such as glucose and xylose, to produce a bioproduct of higher value. Lactic acid (LA) is a product of the central carbon metabolism of different bacteria and fungi species [7,8][1][2]. LA is considered a commodity chemical, with a wide range of industrial applications that are determined by: its acidic character in aqueous medium, its bifunctional reactivity linked to the presence of a hydroxyl and a carboxyl group, and its optical activity [9][3].
L-LA is generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) and used in the food and beverage industry [10][4]. Additional applications in the cosmetics and hygiene industry have been documented [9,10,11,12][3][4][5][6]. The leading demand for LA is in the production of polymers as precursor of polylactic acid (PLA), a biodegradable alternative to petroleum-based plastics [13][7]. The degradation activity of L-lactate dehydrogenase inside the human body allows for biomedical [7][1], pharmaceutical applications [14,15][8][9] and drug delivery matrices [16][10].
Mass production of LA uses fermentation of corn, beet and other first-generation (1G) feedstocks for the simplicity of the upstream operations. On the other hand, second- (2G) and third-generation (3G) feedstocks, such as LB and algae biomass, respectively, are cheaper substrate options that do not compete with food, and are instead often considered waste [17][11].
Different approaches for 2G-LA production have emerged (Figure 21). The first one is called separate hydrolysis and fermentation (SHF) and involves enzymatic hydrolysis of the polysaccharides and subsequent fermentation of the released sugars. In contrast, simultaneous saccharification and fermentation (SSF) and consolidated bioprocess (CBP) are one-pot methods, where enzymatic hydrolysis and microbial fermentation occur in parallel [18,19][12][13]. The main advantage of combining both operations is that hydrolytic activity is increased due to reduction in feedback inhibition (main limitation of SHF) by immediate product consumption [20][14].
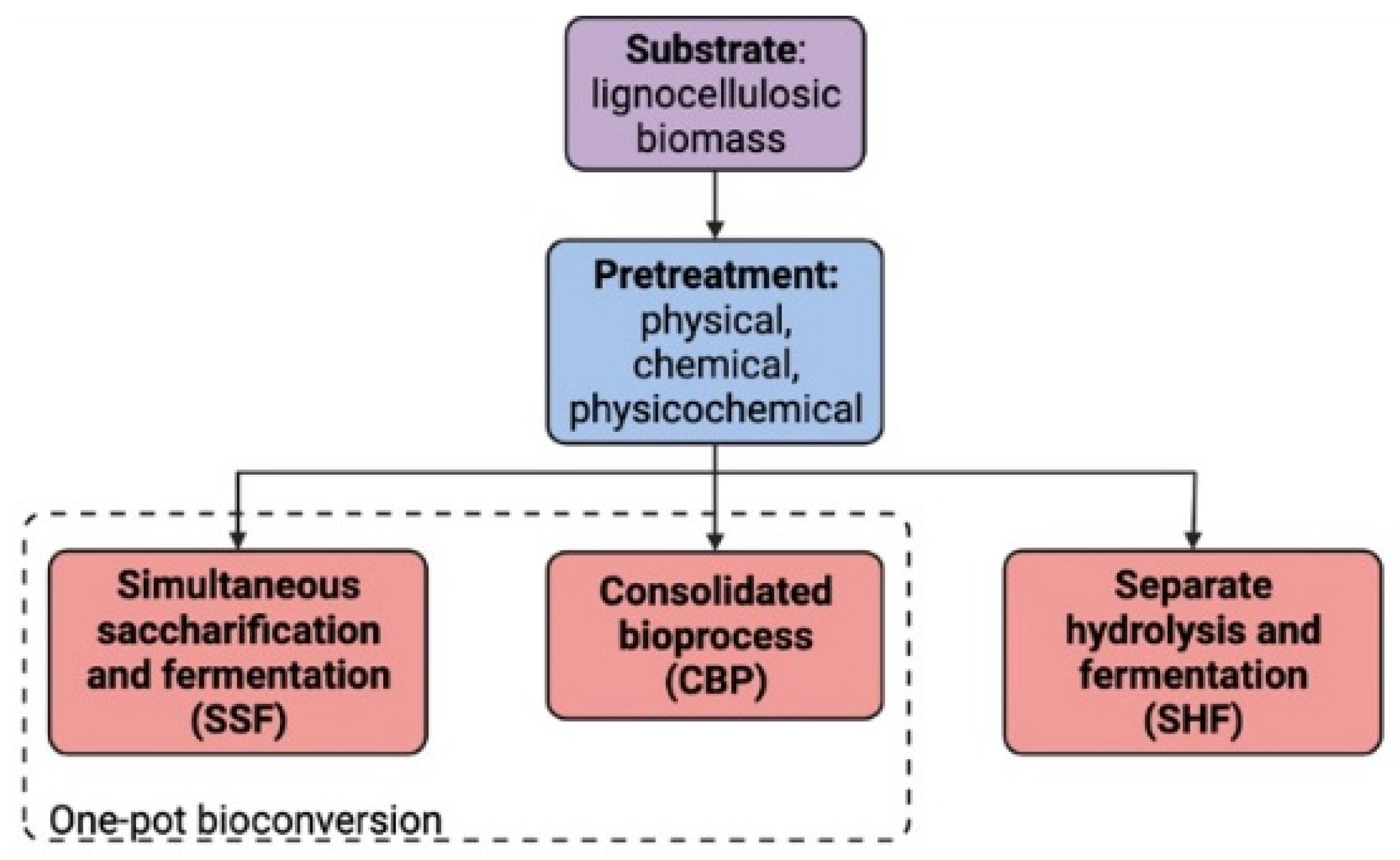
Figure 21. Lignocellulose revalorization methods. The lignocellulosic feedstock enters as a substrate, goes through a pretreatment process and either the SHF, CBP or SSF may be applied for the obtention of LA.
2. Bioconversion Process
Bioconversion is the use of the biochemical energy stored in waste biomass to produce chemicals of higher value through fermentation. Different frameworks of bioconversion surged as alternatives for biomass disposal, which depend on the nature of the residue. Bioconversion of LB includes three key operations: pretreatment, saccharification and fermentation. When each operation is performed independently, the process is called SHF. On the other hand, the saccharification and fermentation operations can be coupled into a single vessel operation, called SSF (Figure 21).
2.1. Pretreatment
Valorization of crop residues requires a pretreatment step to increase the permeability of the matrix for the subsequent enzymatic hydrolysis. Chemical factors of LB, namely the content of cellulose, hemicellulose, lignin, and the interaction among these biopolymers, create a heterogeneous matrix that is naturally recalcitrant to enzymatic conversion. This is further influenced by physical factors of LB, such as crystallinity, particle size, specific surface area and pore size [27][15]. Both polymers, cellulose and hemicellulose, are potential biofactory feedstocks, which leaves lignin and its interlinkages with both polysaccharides as the main targets of the pretreatment.
In addition to enabling maximum access of hydrolytic enzymes to their substrate, the following set of considerations (adapted from the one originally proposed in [28][16]) should serve as a guideline for the selection of an appropriate pretreatment method: (a) it should minimize energy demands, time and unit operations in the overall process, (b) hemicellulose fraction must be preserved if subsequent stages allow its bioconversion, (c) degradation byproducts must be minimized, (d) the pretreatment catalyst should be low-cost itself and/or its recycling should be inexpensive, and (e) its environmental impact and water usage should be kept to a minimum. The approaches for LB pretreatment fit within one of the following categories: physical, chemical, or physicochemical operations; being the combination of two or more methods as a recurring strategy.
2.1.1. Physical Pretreatment
Methods for physical pretreatment of LB usually generate fewer waste residues and fermentation inhibitors compared to chemical and physicochemical pretreatments. Physical methods can be further categorized into size reduction operations, thermal (pyrolysis) and field-assisted treatments [28][16]. Detailed information on the latter two can be found in [29][17]. The reviewed SSF experiments were limited to size reduction operations such as ball milling and extrusion. Except for the experiments in [30][18], size reduction was always followed by a chemical or physicochemical pretreatment.
Size reduction increases the surface area accessible to hydrolytic enzymes, and it can also decrease crystallinity of lignocellulose. Although it hardly produces any chemical changes on its complex polymeric matrix [28][16], size reduction is still necessary to enhance digestibility and fluidity of LB [31][19]. For SSF, corn-derived feedstocks are milled between 0.075 [32][20] and 10 mm [23,33,34,35][21][22][23][24]. Finer milling is often associated to improved cellulose conversion efficiency. However, this translates to increasing operational costs. Moreover, hydrolysis yield remains approximately constant in saccharification of corn stover milled to fractions between 0.21 and 1.42 mm [36][25]. This indicates that particle size is a minor conversion factor in SSF, and its optimization should be limited to the selection of low- energy-demanding milling methods.
2.1.2. Chemical Pretreatment
Chemical pretreatment of LB can include dilute acid, mild alkali, ozonolysis, and deep eutectic solvents, among others. Discussion in this section is limited to acidic and alkali methods, because only these have been used as part of the bioconversion framework under investigation.
Acid pretreatment at low temperatures (<100 °C) requires concentrated solutions (30–70%). On the other hand, dilute acids (0.1–10%) can also be used under higher temperatures (100–250 °C). Both sets of conditions are harsh to the substrate and might cause the generation of inhibitory compounds, such as phenolic compounds, furfurals and aldehydes [37][26]. These substances are responsible for large amounts of wastewater generation. Dilute acid pretreatment is preferred because of its diminished toxicity and corrosion risks, as well as its lower maintenance costs. Acid pretreatment is also particularly effective against hemicellulose, which has a positive impact on the recovery of the cellulosic fraction.
Contrary to dilute acid, alkali pretreatment under mild conditions has been demonstrated to maintain most of the hemicellulose fraction of LB, while at the same time having a significant impact on the solubilization of lignin. Xylose (hemicellulose) utilization must be pondered to attain maximum substrate utilization. For example, 5% (w/w) NaOH-pretreated corn stover showed an increase of 61.66% in cellulose content, 66.97% decrease in lignin content, and 7.67% decrease in hemicellulose composition, with respect to the raw feedstock. After a fed-batch SSF, up to 0.77 g of LA/g of stover were obtained, demonstrating the potential results when a xylose fermenting microorganism is used [38][27].
Alkali reagents act against the uronic acid linkages between hemicellulose and lignin [39][28]. Moreover, glycosides and other intermolecular ester bonds are also affected. These solutions also promote cellulose swelling and decrystallization, which increases internal surface area [28][16]. Dilute NaOH is the most employed alkali catalyst in pretreatment of corn crop residues, because it has been proven efficient for pretreatment of LB with less than 26% lignin [28][16].
By far, dilute acid and mild alkali methods are the most popular pretreatments for corn crop residues bioconversion into LA. Their implementation requires low investment, easy operation, and they effectively reduce the recalcitrance of corn crop residues. Unfortunately, scaling-up remains environmentally and industrially challenging, considering the large amounts of water needed to remove fermentation inhibitors from the pretreated feedstock. The main approaches to address scalability limitations of chemical pretreatment are discussed in Section 3.1.1later.
2.1.3. Physicochemical Pretreatment
Physicochemical methods include: ammonia-based pretreatments, autohydrolysis (also referred to as steam explosion), liquid hot water, oxidative operations, etc. These methods are less frequently used for the pretreatment of corn crop residues. Soaking aqueous ammonia and autohydrolysis pretreatments have been followed by SSF of corn stover [40][29] and corncob [41][30], respectively.
Ammonia-based pretreatments are differentiated according to the temperature and pressure at which they take place. Soaking aqueous ammonia (SAA) occurs at temperatures between 30 and 70 °C and at atmospheric pressure. Ammonia induces selective delignification, resulting in minor hemicellulose and cellulose degradation [42][31]. Ammonia also acts by swelling the lignocellulosic matrix. An environmental advantage of this method is that aqueous ammonia can be reused for pretreatment of subsequent batches [43][32]. On the other hand, the nature of this catalyst requires a washing step of the biomass before its saccharification.
A protocol of corn stover soaking in aqueous ammonia, where long treatment time and a high temperature were selected (90 °C for 24 h), showed a significant decrease in lignin content (from 17.2 to 7.7%). In absolute terms, most of the glucan and xylan fractions were maintained, whereas in relative terms these fractions went from 36.8 to 54.4% and from 21.7 to 24.9%, respectively [40][29].
Autohydrolysis pretreatment is carried out with saturated steam, at pressures between 0.69 and 4.83 MPa, and temperatures of 160 and up to 260 °C. These extreme conditions allow water to permeate into the lignocellulosic structure. This way, a sudden drop of pressure induces the absorbed water to swiftly escape, causing an explosion that affects the lignocellulosic fibers at a structural level. The action mechanism allows short treatment times, in the order of seconds to minutes. In addition to said mechanical effect, acetyl groups from side chains of hemicellulose generate acetic acid. Acetic acid then acts on the glycosidic bonds of hemicellulose, to release glucose and xylose [29][17].
This pretreatment method requires fewer chemicals (acidic catalysts might be added), and its short treatment times are reflected in low energy usage. Corncobs were pretreated by autohydrolysis in a Parr reactor at 202 °C, to obtain a hemicellulose-free substrate for SSF. The high temperature resulted in a decrease in hemicellulose content, from 39.0 to 10.4%. The relative decrease of hemicellulose caused an increase in cellulose and lignin, from 39 to 59.1%, and from 14.4 to 22.6%, respectively. This means that the lignin was not completely digested. In the same experiment, 8 g of water/g of dried corncob were used for the autohydrolysis operation [41][30]. Therefore, another main disadvantage of this technique is high water usage. This is further impacted because, as with the rest of the chemical and physicochemical methods, fermentation inhibitors might be generated at high temperatures, which calls for a washing step.
The pretreatment step represents an area of opportunity in the bioconversion of LB. As explored in this section, this operation is associated with the generation of harmful byproducts and high demand of resources. Additionally, the pretreatment of corn stover requires an investment twice the value of what would be required for the bioconversion of third-generation feedstock (seaweed). First-generation (glucose) substrates do not require a pretreatment step [44][33]. Therefore, the development of a framework capable of reducing pretreatment-associated costs would have a direct impact in increasing the economic competitiveness of 2G-LA.
2.2. Simultaneous Saccharification and Fermentation
-
It avoids the need to physically separate hydrolysate from biomass, an operation that would inevitably lead to sugar loss;
-
In comparison to SHF, larger amounts of pretreated LB can be fed into the bioreactor at once;
-
Performing two steps simultaneously in the same reactor has a direct impact on reducing overall process duration;
-
Investment costs are reduced because fewer reaction vessels are needed;
- It avoids the need to physically separate hydrolysate from biomass, an operation that would inevitably lead to sugar loss;
- In comparison to SHF, larger amounts of pretreated LB can be fed into the bioreactor at once;
- Performing two steps simultaneously in the same reactor has a direct impact on reducing overall process duration;
- Investment costs are reduced because fewer reaction vessels are needed;
On the other hand, one of the main design challenges is finding an optimal combination of enzymes and microorganisms. Ideally, the microorganisms and the hydrolytic enzymes need to fit within each other’s temperature and pH range. Otherwise, a trade-off between enzymatic yield and fermentation yield would be observed [24][35]. Most saccharification enzymes show their highest activity at temperatures between 45 and 50 °C [46][36]. Additionally, materials and equipment are the same in SHF and SSF. Therefore, favoring SSF over SHF for LA production should be the case as long as a thermotolerant LAB strain is available.
The selected microorganism must produce high-titer and optically pure LA, with low formation of byproducts. The fermentation operation requires a minimum LA titer of 100 g/L and over 99% optical purity for an industrial-scale process to be economically feasible. This value enables for cost-efficient downstream operations [23][21].
2.2.1. Enzymatic Hydrolysis
Once the pretreatment step is over, polysaccharides from LB are more readily accessible for hydrolysis. This operation consists of the monomerization of cellulose and hemicellulose into fermentable sugars by hydrolytic enzymes. Saccharification of LB is performed using different free enzymes, which focus on hydrolyzing different linkages that comprise the polymeric structure of cellulose and hemicellulose. Enzymatic mixtures are thus needed to enable maximum and efficient co-hydrolysis of the polysaccharide matrix.
The main group of enzymes used for complete saccharification of cellulose are cellulases. This term includes three different types of enzymes, which are endoglucanase (E.C. 3.2.1.4), exoglucanase (also referred to as cellobiohydrolase) (E.C. 3.2.1.91) and β-glucosidase (also referred to as cellobiase) (E.C. 3.2.1.21) [47][37]. Endoglucanase acts on the β-1,4 D-glycosidic bonds in cellulose. Exoglucanase also hydrolyzes β-1,4 D-glycosidic bonds but its activity is limited to cleave off cellobiose units from the ends of the polymeric chain. Lastly, β-glucosidase transforms each cellobiose molecule into two glucose molecules [48][38]. Additionally, β-glucosidase enhances the activity of the other two enzymes, as they are inhibited by cellobiose [49][39].
Hemicellulases are often regarded as accessory enzymes for saccharification, but have been proven essential to assist on the retrieval of fermentable sugars. They are needed to facilitate cellulase access to cellulose fibers, by breaking the hemicellulose barrier. Moreover, their hydrolysate from corn crop residues has the potential to be converted into LA, given that a pentose-fermenting microorganism is introduced. When this is the case, the process is often called simultaneous saccharification and co-fermentation (SSCF). Xylose is the main monosaccharide in the hemicellulose fraction of corn crop residues [50][40], and it is present in its polymeric form as xylan. Therefore, the appropriate hemicellulases for this kind of feedstock are endoxylanase and β-xylosidases. The first one hydrolyzes the xylan backbone of hemicellulose into short oligosaccharides, while the latter one catalyzes the monomerization of these oligosaccharides into xylose [51][41]. Thus, effective lignocellulose deconstruction is a product of the synergistic work of a broad spectrum of hydrolytic enzymes.
Follow-up studies should assess different enzymatic formulations, based on specific feedstocks and fermentation configurations. Since the saccharification rate has been demonstrated to be a limiting step in SSF, taking the time to find an optimal composition could lead to an efficient and cost-effective bioconversion [52][42]. One example of such experiments was conducted in [53][43], where hemicellulose-free corncob residue (from acid pretreatment) was hydrolyzed with different formulations of cellulases (which included endo- and exogluganase) and β-glucosidase. Under identical SSF conditions, the highest LA titer was achieved at loadings of 2:1, in terms of U/g cellulose. Out of the reviewed works, this was the only one where the proportion of the enzymes was a factor under investigation.
Most of the studies on SSF of herbaceous residues utilize commercial mixtures of enzymes. Manufacturers rarely make information about the enzymatic composition of their products available to the customer. This limits the factors related to saccharification that can be tested and therefore, hydrolysis optimization strategies are also narrowed. Furthermore, Cellic® CTec2 (Novozymes, Bagsværd, Denmark) was used as an enzymatic cocktail in 50% of the experiments selected herein. According to the supplier (Novozymes, Application sheet Luna No. 2010-01668-01), the optimal temperature and pH of this product are 45–50 °C and 5.0–5.5, respectively.
Enzymatic cocktails available in the market are composed of cellulases from highly productive filamentous fungi such as Aspergillus niger, Trichoderma longibrachiatum and Trichoderma reesei [54][44]. The proteome analysis of these kind of organisms has allowed for the identification and improvement of saccharification cocktails. For instance, over 30 LB degrading enzymes were found in the fermentation broth of T. reesei TUT C-30, when pretreated corn stover was used as fermentation feedstock [55][45]. Out of these, six enzymes are considered essential for deconstruction of pretreated corn stover: cellobiohydrolase I and II (CBHI and CBHII), endoglucanase I (EGI), β-glucosidases (βG), endo-β-1,4-xylanases (EX) and β-xylosidases (βX) [55,56][45][46]. In addition to these, other lignocellulosic and accessory enzymes might be present in the benchmark formulations. Among them are endoglucanase III, endoglucanase IV, endoglucanase VII, exoglucanase II and chinitase [57][47]. The formulation and sources of the commercial cocktails are not available to consult. However, Cellic® CTec2 (Novozymes, Bagsværd, Denmark) has demonstrated strong exoglucanase, β-glucosidase and xylanase activities at 50 °C and pH 4.8 [58][48].
It is important to note that out of the reviewed experiments that used Cellic® CTec2, only [59][49] was performed at 50 °C, and the experiment in [60][50] was conducted within the optimal pH range. Thus, most experiments prioritize fermentation over hydrolysis conditions. In some cases, such experimental decisions might be empirical, where residual carbohydrates might be relatively present when fermentation is over. No cases have been reported where pH and temperature are experimental factors with varying levels in a SSF experiment. Instead, using corn-derived feedstock, some researchers have opted to include a 6 h saccharification step at 50 °C prior to SSF at 43 °C [23,33,34,35,61][21][22][23][24][51]. This strategy seems to be associated with high LA titers and yields.
With the available reports, it is difficult to define an optimal enzymatic cocktail for SSF of corn crop residues. The reviewed experiments focused the attention on the end product variables rather than intermediate operations. However, Cellic® CTec2 (Novozymes, Bagsværd, Denmark) has been showed to outperform other formulations in the hydrolysis of multiple substrates [54][44], including corn crop residues [62][52].
2.2.2. Fermentative Microorganisms
To maximize fermentation yield and LA titer, a variety of wild-type and genetically engineered microorganisms have been studied for bioconversion of corn crop residues. The main interest lies in LA bacteria (LAB). LAB ferment glucose to LA through glycolysis (or the Embden–Meyerhof–Parnas pathway; EMPP), and some pentoses through the pentose phosphate pathway (PPP); additionally, the presence of phosphoketolase enzymes might redirect the latter to produce acetate and ethanol (Figure 32). Depending on their metabolic profile, they are classified as homofermentative (hexoses uptake and LA production), facultatively heterofermentative (pentoses/hexoses uptake and production of LA and other products) and heterofermentative (pentoses/hexoses uptake and production of LA, side products and CO2) [63][53]. Mixed bacterial and mixed fungal fermentations have also been explored as a means to maximize saccharification and fermentation yields.
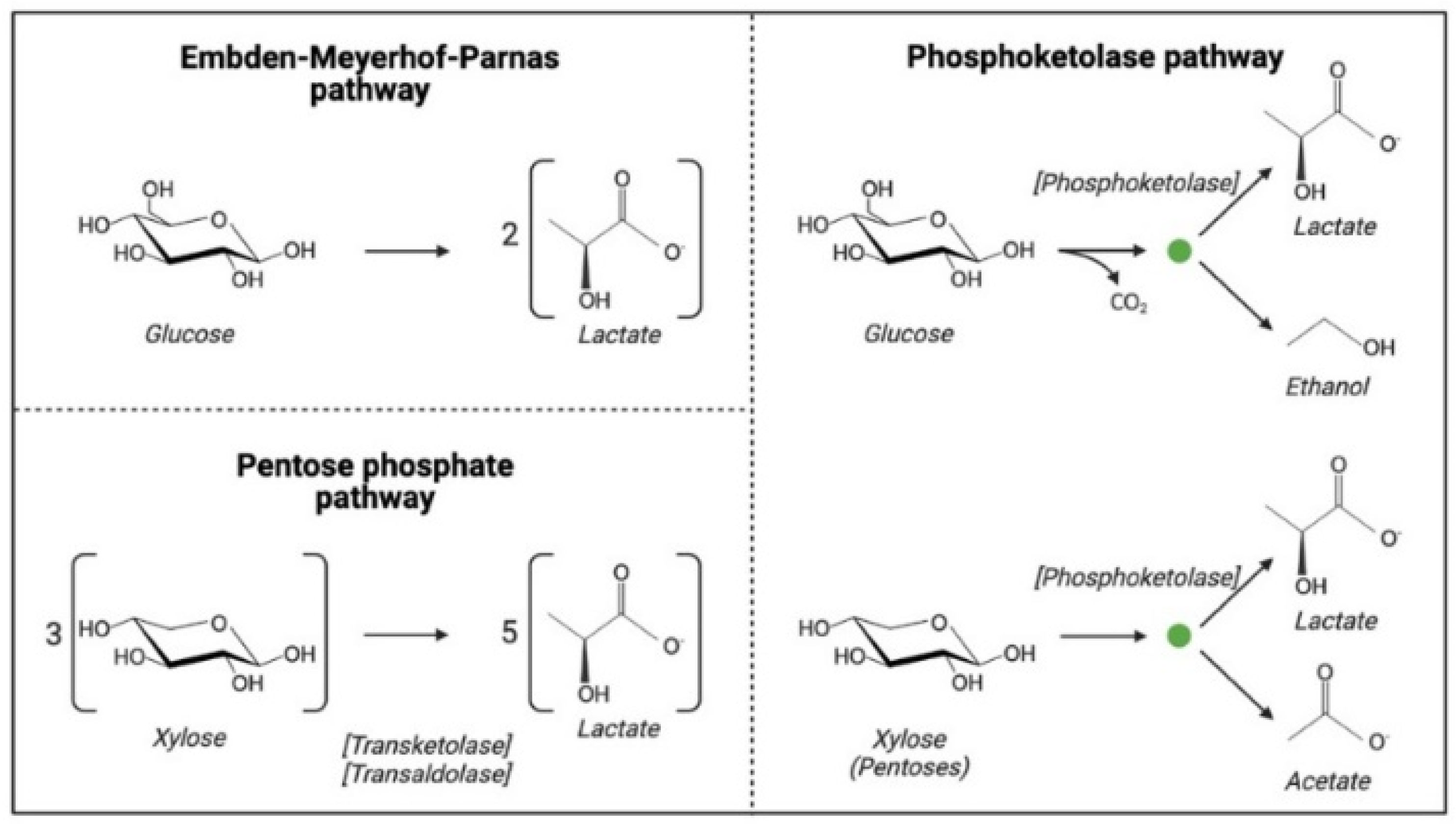
Figure 32. LAB shows production of LA through three main pathways, which might share some molecular intermediates. The green dot (∙) represents the divergence from the PPP. Only the overall substrates and products of each pathway are shown. The potential metabolic input and output is commonly used as a classification criterion in LAB strains.
Wild-Type Microorganisms
Lactobacillus pentosus is a mesophilic species, with a facultative heterofermentative metabolism [64][54]. In the experiments in [40][29], it was reported that the glucose and arabinose uptake rate remained high during batch SSF experiments using L. pentosus ATCC 8041. However, significant xylose consumption occurred only after glucose depletion, which was attributed to diauxic growth. At the same time, LA production slowed down, while acetic acid productivity increased. In the fed-batch culture, LA accumulated rapidly before 72 h, but a sudden drop in productivity was observed before a concentration of 60 g/L was reached. The researchers concluded that this was due to the inhibitory effect of the lactate ions on L. pentosus.
In contrast, L. pentosus FL0421 was tested in a fed-batch SSF experiment, and simultaneous consumption of glucose and xylose was observed [65][55]. In the same study, the authors presumed that the concentration of xylose dissolved in the medium had an effect on the preferred metabolism pathway for this pentose. It was noted that at a high concentration of xylose, the productivity of acetic acid was significantly higher compared to that of a low xylose concentration. A final concentration of 34.27 g/L of acetic acid was produced. They proposed a low-rate initial saccharification stage to overcome this phenomenon.
Other LAB have also been tested for their attractive fermentation features. Pediococcus acidilactici had already been used for production of bacteriocin [66][56], but it was not until recently that the species attracted attention for its high-titer LA production. The first reported SSF of pretreated corn residue, using a P. acidilactici strain, was performed in [24][35]. The fermentation of pretreated corn stover by P. acidilactici DQ2, resulted in a downstream-cost-efficient LA concentration of 101.9 g/L. This isolated strain also showed optimal LA productivity at 48 °C and low metabolic inhibition by compounds generated in acidic pretreatment of LB. However, optical purity of the product was poor (L-LA at 63.4%) and xylose utilization was observed only at non-optimal growth conditions. These issues were addressed through genetic engineering in later experiments that will be discussed in the following section. Similar LA titer results were obtained using P. acidilactici PA204 (104.11 g/L) [38][27]. In this case, LA productivity seemed to be affected by alkali pretreatment inhibitors of corn stover. This strain was able to convert xylose into LA, but at a much slower rate compared to glucose conversion. Even so, more than half of the fed xylose fraction remained at the end of the experiment. It is worth noting that acetic acid formation by P. acidilactici PA204 was minimal.
The most studied wild-type bacteria for LA production is Bacillus coagulans. Different strains have shown optimal growth and LA production at 50 °C [53,59,67,68][43][49][57][58]). This physiological characteristic is compatible with the temperature needed for high rate saccharification by cellulase activity and it also enables the maintenance of axenic cultures under non-sterile conditions. B. coagulans is also associated with: xylose uptake [32][20], low carbon catabolite repression [59][49] and low byproduct formation [53][43]. It has also been proven to produce enantiomeric pure L-LA (>99%), and to be resistant to growth inhibitors that are generated by the acidic pretreatment of corn fiber [67][57]; whereas phenolic compounds formed during alkali pretreatment seem to hinder the fermentation [32][20].
Less studied microorganisms include Lactobacillus delbrueckii, which was selected based on its ability to produce optically pure D-LA (99.9%) under anaerobic conditions. However, it was not able to utilize xylose, and its growth temperature and pH did not match those of the selected saccharification enzymes [69][59]. This is also the case with Lactobacillus rhamnosus, which instead exhibited a metabolism of cellobiose, avoiding the need to include β-glucosidase in the enzymatic cocktail while at the same time reaching a cellulose conversion of up to 97.5% [41][30].
Co-culture methods have been suggested as a means to widen the range of fermentable sugars from cellulose and hemicellulose hydrolysate. The simultaneous inoculation of Lactobacillus rhamnosus and Lactobacillus brevis showed an increase of 18.6 and 29.6% in LA yield (with respect to their monocultures) from a batch fermentation of pretreated corn stover. The selection was made on the argument that L. brevis is able to utilize glucose and xylose simultaneously, while L. rhamnosus competed for glucose intake and avoided high byproduct formation [70][60]. Under the same logic, a mixed culture of L. brevis ATCC 367 and Lactobacillus plantarum 21028 was tested [71][61]. In this case, L. plantarum was inoculated 24 h prior to L. brevis. This approach allowed maximum glucose conversion to LA, and utilization of xylose. In both experiments, the SSCF temperature was kept at 37 °C, which reduced hydrolytic enzymes activity by approximately 40% when compared to hydrolysis under optimal temperature (45–50 °C).
Acremonium thermophilus is a thermophilic fungus that can grow at extreme temperatures and has not been as studied as much as other thermophilic species but is recognized as a potential source of enzymes with scientific and commercial benefits [72][62]. In particular, the A. thermophilus ATCC 24622 strain produces cellulose by hydrolysis and has proved its efficiency for LA production [73][63]. In [30][18] culture samples of the fungus were used, thus obtaining the highest yields of L-LA from the untreated substrate. LA was produced using Acremonium cellulase and its enhanced production was achieved by SSF with a mixed culture of A. thermophilus and Rhizopus sp., without the addition of cellulase preparation.
Rhizopus oryzae NLX-M-1 is a filamentous fungus that has been used to ferment pretreated corncobs with low hemicellulose content [74][64]. Although the fungus produces optically-pure L-LA from glucose as well as from xylose (showing carbon catabolite repression), it also exhibits important drawbacks with respect to its bacterial counterparts. First, R. oryzae is an aerobic organism, which inevitably increases manufacturing costs and second, its growth temperature is around 30 °C, a value at which cellulase activity is hindered. Finally, a considerable amount of other fermentation products, such as ethanol could be generated (up to 0.24 g of ethanol for each gram of LA in a high-solids loading experiment).
Moreover, the saccharification of corn straw can be achieved with Trichoderma koningii, a saprotroph fungus often used as a biopesticide [76][65] (Tripathi et al., 2010). It can produce cellulases in anaerobic conditions. These enzymes can degrade the corn straw to glucose, cellobiose and xylose. For the SSF process, the manipulated parameters were the solid to liquid ratio, fermentation time, size of the inoculum and pH [75][66].
Genetically Modified Strains
Genetic engineering has also been explored in strains used in SSF of corn crop residues (Figure 43 and Table 21). The already modified strain of L. plantarum NCIMB 8826 ΔIdhL1 was designed with the purpose of producing optically pure D-LA, by the deletion of its L-lactic acid dehydrogenase gene [77][67]. This strain was further modified by Y. Zhang et al. (2016) with a recombinant xylose assimilation plasmid. The new L. plantarum NCIMB 8826 ΔIdhL1 -pLEM-xylAB strain was able to simultaneously ferment both glucose and xylose into D-LA, with a high yield of 0.77 g/g of pretreated corn stover.
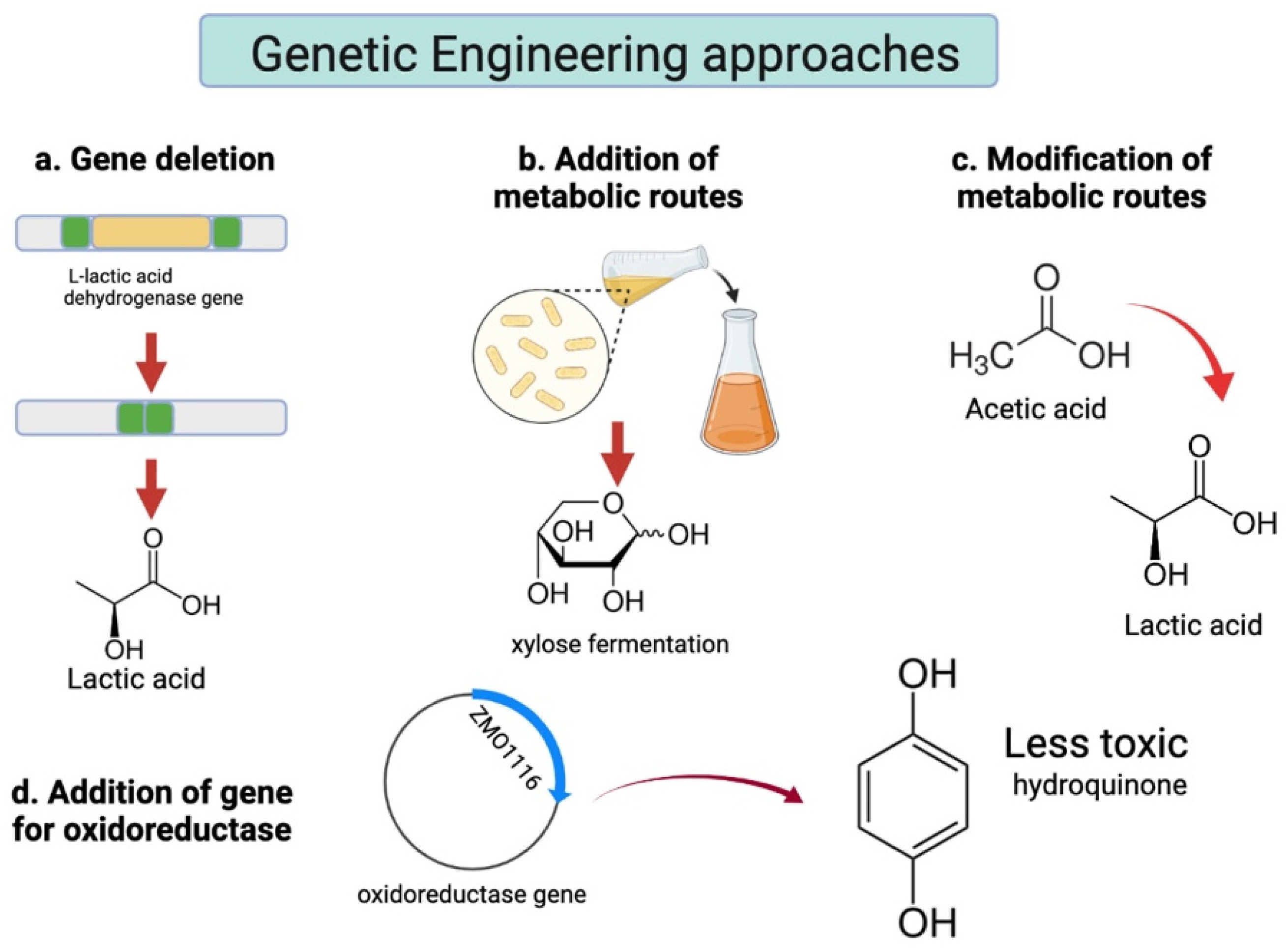
Figure 43. Summary of the genetic engineering approaches that have been explored. (a) Gene deletion is focused on the production of optically pure LA. P. acidilactici ZP26 and P. acidilactici TY112 are a product of this modification. (b) The insertion of xylose uptake genes resulted in the strains P. acidilactici ZY15, P. acidilactici ZY271 and L. plantarum NCIMB 8826 ΔIdhL1 -pLEM-xylAB. (c) P. acidilactici ZY15 and P. acidilactici ZY271 were additionally modified to decrease acetic acid production. (d) P. acidilactici ZY15 was transformed to express an oxidoreductase enzyme, which catalyzes the reduction of the harmful p-benzoquinone into a safer hydroquinone.
The performance shown by the P. acidilactici DQ2 strain in [24][35] attracted interest for further experimentation using genetic engineering techniques. Two genetically modified strains were created from P. acidilactici DQ2 by knocking out the L-lactic acid and D-lactic acid dehydrogenase genes, respectively. P. acidilactici ZP26 was able to produce D-LA with 99.32% optical purity, while P. acidilactici TY112 produced L-LA with optical purity of 99.89% [34][23].
Table 21. Methodology and results from SSF experiments using genetically-engineered microorganisms.
| Microorganism | Feedstock | Pretreatment | Enzymes | Fermentation Conditions | Lactic Acid | Xylose Utilization |
Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | Titer (g/L) | Productivity (g/L⋅h) |
Optical Purity (%) |
||||||||||||||||||
| Lactobacillus plantarum | NCIMB 8826 𝛥 | IdhL1 | -pLEM- | xylAB | Corn stover | NaOH pretreatment at 121 °C for 30 min and water washed | Cellic | ® | CTec2 at 5.6 FPU/g DM | Fed-batch culture at 37 °C and pH 5 | 0.77 | a | 61.4 | 0.32 | >99 | e, g | Yes | g | [60] | [50] | |
| Pediococcus acidilactici | TY112 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellulase Youtell #6 at 15 FPU/g DM Saccharification 6 h prior to inoculation |
Batch culture at 45 °C, pH 5.5 and 30% ( | w | / | w | ) solids loading | 71.5 | b | 104.5 | 1.45 | N. R. | No | [23] | [21] | ||||
| Pediococcus acidilactici | ZY271 | Ensiled corn stover | Dry dilute sulphuric acid pretreatment, biodetoxification and partial saccharification. | Cellic | ® | CTec2 at 5 mg protein/g DM. Saccharification 6 h prior to inoculation |
Batch culture at 42 °C, pH 5.5 and 30% ( | w | / | w | ) solids loading | N. R. | 139.0 | 1.93 | f | 99.7 | d | Yes | g | [33] | [22] |
| Pediococcus acidilactici | TY112 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellulase Youtell #6 at 15 FPU/g DM. Saccharification 6 h prior to inoculation |
Batch culture at 45 °C, pH 5.5 and 25% ( | w | / | w | ) solids loading | 65 | b | 77.66 | 1.06 | 99.89 | d, g | No | [34] | [23] | |||
| Pediococcus acidilactici | ZP26 | 58 | b | 76.76 | 1.02 | 99.3 | e, g | No | |||||||||||||
| Pediococcus acidilactici | ZY15 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellic | ® | CTec2 at 10 mg protein/g cellulose. Saccharification 6 h prior to inoculation |
Batch culture at 42 °C, pH 5.5 and 25% ( | w | / | w | ) solids loading | 64.7 | c | 97.3 | 1.01 | 99.2 | e, g | Yes s | g | [35] | [24] |
| Pediococcus acidilactici | ZY15-𝛥 | ackA2::ZMO1116 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellic | ® | CTec2 at 10 mg protein/g cellulose. Saccharification 6 h prior to inoculation |
Batch culture at 42 °C, pH 5.5 and 30% ( | w | / | w | ) solids loading | N.R. | 123.8 | N.R. | N.R. | Yes | g | [61] | [51] |
a g LA/g loaded biomass. b % with respect to maximum theoretical yield from cellulose. c % with respect to maximum theoretical yield from cellulose and xylose. d %L-LA. e %D-LA. f Calculated given the data from the article. g Derived from genetic modification. DM: dry matter. FPU: filter paper units. N. R.: Not reported.
Although at the time P. acidilactici TY112 could not ferment hemicellulose, the strain achieved an industrially attractive L-LA titer (104.5 g/L) [23][21]. More recently, heterologous genes encoding xylose assimilation enzymes were introduced into P. acidilactici ZP26 [35][24] and TY112 [78][68]. In these experiments, the metabolic pathway responsible for acetic acid production was re-directed to the LA producing PPP. The new strains were termed P. acidilactici ZY15 and P. acidilactici ZY271, respectively. P. acidilactici ZY15 showed a xylose conversion yield of pretreated corn stover of 92.6% and a final acetic acid titer of 0.50 g/L. On the other hand, P. acidilactici ZY271 was first tested in SSF of wheat straw, and reached a xylose conversion of 94.9%, with no carbon catabolite repression. The final acetic acid titer was less than 1.0 g/L. When using corn stover, in a solid-state fermentation followed by SSF, P. acidilactici ZY271 reached the highest LA titer out of the reviewed literature (139.2 g/L) [33][22]. Later, P. acidilactici ZY15 was transformed with a plasmid expressing the oxidoreductase gene ZMO1116, from Zymomonas mobilis. The enzyme converts p-benzoquinone, a toxic inhibitor originated in acidic pretreatment of lignocellulose, into less toxic hydroquinone. After SSF at identical conditions, a remarkable 21.4% increase in D-LA titer was observed when compared to its parental strain [61][51].
References
- Kumar, A.; Thakur, A.; Panesar, P.S. Lactic acid and its separation and purification techniques: A review. Rev. Environ. Sci. Biotechnol. 2019, 18, 823–853.
- Tan, J.; Abdel-Rahman, M.A.; Sonomoto, K. Biorefinery-Based Lactic Acid Fermentation: Microbial Production of Pure Monomer Product. Adv. Polym. Sci. 2018, 279, 27–66.
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de S. Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83.
- Ameen, S.M.; Caruso, G. The Importance of Lactic Acid in the Current Food Industry. An Introduction. In Lactic Acid in the Food Industry; Parisi, S., Ed.; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–5. ISBN 978-3-319-58144-6.
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of optically pure lactic acid by microbial fermentation: A review. Environ. Chem. Lett. 2020, 18, 539–556.
- Abedi, E.; Mohammad, S.; Hashemi, B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974.
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138.
- Ajala, E.O.; Olonade, Y.O.; Ajala, M.A.; Akinpelu, G.S. Lactic Acid Production from Lignocellulose—A Review of Major Challenges and Selected Solutions. ChemBioEng Rev. 2020, 7, 38–49.
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46.
- Lassalle, V.; Ferreira, M.L. PLA nano- and microparticles for drug delivery: An overview of the methods of preparation. Macromol. Biosci. 2007, 7, 767–783.
- Zhang, Z.; Tsapekos, P.; Alvarado-Morales, M.; Zhu, X.; Zervas, A.; Jacobsen, C.S.; Angelidaki, I. Enhanced fermentative lactic acid production from source-sorted organic household waste: Focusing on low-pH microbial adaptation and bio-augmentation strategy. Sci. Total Environ. 2022, 808, 152129.
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39.
- Carrillo-Nieves, D.; Rostro Alanís, M.J.; de la Cruz Quiroz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74.
- Gauss, W.F.; Suzuki, S.; Takagi, M. Manufacture of Alcohol from Cellulosic Materials Using Plural Ferments. U.S. Patent No. 3990944, 1976.
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874.
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7.
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206.
- Miura, S.; Arimura, T.; Itoda, N.; Dwiarti, L.; Feng, J.B.; Bin, C.H.; Okabe, M. Production of L-lactic acid from corncob. J. Biosci. Bioeng. 2004, 97, 153–157.
- Oyedeji, O.; Gitman, P.; Qu, J.; Webb, E. Understanding the Impact of Lignocellulosic Biomass Variability on the Size Reduction Process: A Review. ACS Sustain. Chem. Eng. 2020, 8, 2327–2343.
- Zhang, Z.; Xie, Y.; He, X.; Li, X.; Hu, J.; Ruan, Z.; Zhao, S.; Peng, N.; Liang, Y. Comparison of high-titer lactic acid fermentation from NaOH-and NH3-H2O2-pretreated corncob by Bacillus coagulans using simultaneous saccharification and fermentation. Sci. Rep. 2016, 6, 37245.
- Liu, G.; Sun, J.; Zhang, J.; Tu, Y.; Bao, J. High titer l-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour. Technol. 2015, 198, 803–810.
- Han, X.; Hong, F.; Liu, G.; Bao, J. An Approach of Utilizing Water-Soluble Carbohydrates in Lignocellulose Feedstock for Promotion of Cellulosic L-Lactic Acid Production. J. Agric. Food Chem. 2018, 66, 10225–10232.
- Yi, X.; Zhang, P.; Sun, J.; Tu, Y.; Gao, Q.; Zhang, J.; Bao, J. Engineering wild-type robust Pediococcus acidilactici strain for high titer l- and d-lactic acid production from corn stover feedstock. J. Biotechnol. 2016, 217, 112–121.
- Qiu, Z.; Gao, Q.; Bao, J. Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer D-lactic acid fermentation from corn stover feedstock. Bioresour. Technol. 2017, 245, 1369–1376.
- Li, H.; Ye, C.; Liu, K.; Gu, H.; Du, W.; Bao, J. Analysis of particle size reduction on overall surface area and enzymatic hydrolysis yield of corn stover. Bioprocess Biosyst. Eng. 2015, 38, 149–154.
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141.
- Zhang, Z.; Li, Y.; Zhang, J.; Peng, N.; Liang, Y.; Zhao, S. High-titer lactic acid production by Pediococcus acidilactici PA204 from corn stover through fed-batch simultaneous saccharification and fermentation. Microorganisms 2020, 8, 1491.
- Qing, Q.; Zhou, L.; Guo, Q.; Gao, X.; Zhang, Y.; He, Y.; Zhang, Y. Mild alkaline presoaking and organosolv pretreatment of corn stover and their impacts on corn stover composition, structure, and digestibility. Bioresour. Technol. 2017, 233, 284–290.
- Zhu, Y.; Lee, Y.Y.; Elander, R.T. Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and cofermentation. Appl. Biochem. Biotechnol. 2007, 137–140, 721–738.
- Rivas, B.; Moldes, A.B.; Domínguez, J.M.; Parajó, J.C. Lactic acid production from corn cobs by simultaneous saccharification and fermentation: A mathematical interpretation. Enzyme Microb. Technol. 2004, 34, 627–634.
- Gao, J.; Yang, X.; Wan, J.; He, Y.; Chang, C.; Ma, X.; Bai, J. Delignification Kinetics of Corn Stover with Aqueous Ammonia Soaking Pretreatment. BioResources 2016, 11, 2403–2416.
- Zhao, C.; Shao, Q.; Chundawat, S.P.S. Recent advances on ammonia-based pretreatments of lignocellulosic biomass. Bioresour. Technol. 2020, 298, 122446.
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.V.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable bio-succinic acid production: Superstructure optimization, techno-economic, and lifecycle assessment. Energy Environ. Sci. 2021, 14, 3542–3558.
- Hans, M.; Kumar, S.; Chandel, A.K.; Polikarpov, I. A review on bioprocessing of paddy straw to ethanol using simultaneous saccharification and fermentation. Process Biochem. 2019, 85, 125–134.
- Zhao, K.; Qiao, Q.; Chu, D.; Gu, H.; Dao, T.H.; Zhang, J.; Bao, J. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour. Technol. 2013, 135, 481–489.
- Zhang, C.Q.; Qi, W.; Wang, F.; Li, Q.; Su, R.X.; He, Z.M. Ethanol From Corn Stover Using SSF: An Economic Assessment. Energy Sources Part B Econ. Plan. Policy 2011, 6, 136–144.
- Singh, J.; Kundu, D.; Das, M.; Banerjee, R. Enzymatic Processing of Juice from Fruits/Vegetables: An Emerging Trend and Cutting Edge Research in Food Biotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132807.
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82.
- Sun, F.F.; Hong, J.; Hu, J.; Saddler, J.N.; Fang, X.; Zhang, Z.; Shen, S. Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzyme Microb. Technol. 2015, 79–80, 42–48.
- Okeke, B.C.; Obi, S.K.C. Lignocellulose and sugar compositions of some agro-waste materials. Bioresour. Technol. 1994, 47, 283–284.
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480.
- Guo, H.; Chang, Y.; Lee, D.J. Enzymatic saccharification of lignocellulosic biorefinery: Research focuses. Bioresour. Technol. 2018, 252, 198–215.
- Jiang, S.; Xu, P.; Tao, F. l-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour. Technol. Rep. 2019, 6, 131–137.
- Contreras, F.; Pramanik, S.; Rozhkova, A.M.; Zorov, I.N.; Korotkova, O.; Sinitsyn, A.P.; Schwaneberg, U.; Davari, M.D. Engineering robust cellulases for tailored lignocellulosic degradation cocktails. Int. J. Mol. Sci. 2020, 21, 1589.
- Culbertson, A.; Jin, M.; Da Costa Sousa, L.; Dale, B.E.; Balan, V. In-house cellulase production from AFEXTM pretreated corn stover using Trichoderma reesei RUT C-30. RSC Adv. 2013, 3, 25960–25969.
- Balan, V.; Jin, M.; Culbertson, A.; Uppugundla, N. The Saccharification Step: Trichoderma Reesei Cellulase Hyper Producer Strains. In Lignocellulose Conversion; Springer: Berlin, Heidelberg, 2013; pp. 65–91. ISBN 9783642378614.
- Liu, J.; Cai, Y.; Liu, L.; Zhan, R.; Zhao, S.; Liang, Y.; Peng, N.; Zhu, J.; Li, H.; Xiao, N.; et al. Enhanced lactic acid production by Bacillus coagulans through simultaneous saccharification, biodetoxification, and fermentation. Biofuels Bioprod. Biorefining 2020, 14, 533–543.
- Yang, J.; Kim, J.E.; Kim, J.K.; Lee, S.H.; Yu, J.H.; Kim, K.H. Evaluation of commercial cellulase preparations for the efficient hydrolysis of hydrothermally pretreated empty fruit bunches. BioResources 2017, 12, 7834–7840.
- Hu, J.; Zhang, Z.; Lin, Y.; Zhao, S.; Mei, Y.; Liang, Y.; Peng, N. High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresour. Technol. 2015, 182, 251–257.
- Zhang, Y.; Vadlani, P.V.; Kumar, A.; Hardwidge, P.R.; Govind, R.; Tanaka, T.; Kondo, A. Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 2016, 100, 279–288.
- Qiu, Z.; Fang, C.; He, N.; Bao, J. An oxidoreductase gene ZMO1116 enhances the p-benzoquinone biodegradation and chiral lactic acid fermentability of Pediococcus acidilactici. J. Biotechnol. 2020, 323, 231–237.
- Chekushina, A.V.; Dotsenko, G.S.; Sinitsyn, A.P. Comparing the efficiency of plant material bioconversion processes using biocatalysts based on Trichoderma and Penicillium verruculosum enzyme preparations. Catal. Ind. 2013, 5, 98–104.
- Salvetti, E.; Harris, H.M.B.; Felis, G.E.; O’Toole, P.W. Comparative genomics of the genus Lactobacillus reveals robust phylogroups that provide the basis for reclassification. Appl. Environ. Microbiol. 2018, 84, AEM-00993.
- Salvetti, E.; Torriani, S.; Felis, G.E. The Genus Lactobacillus: A Taxonomic Update. Probiotics Antimicrob. Proteins 2012, 4, 217–226.
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80.
- Altuntas, E.G.; Cosansu, S.; Ayhan, K. Some growth parameters and antimicrobial activity of a bacteriocin-producing strain Pediococcus acidilactici 13. Int. J. Food Microbiol. 2010, 141, 28–31.
- Bischoff, K.M.; Liu, S.; Hughes, S.R.; Rich, J.O. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 2010, 32, 823–828.
- Chen, H.; Su, Z.; Wang, Y.; Wang, B.; Si, Z.; Lu, J.; Su, C.; Ren, W.; Chen, H.; Cai, D.; et al. Lactic acid production from pretreated corn stover with recycled streams. Process Biochem. 2020, 91, 132–140.
- Zhang, Y.; Vadlani, P.V. D-lactic acid biosynthesis from biomass-derived sugars via lactobacillus delbrueckii fermentation. Bioprocess Biosyst. Eng. 2013, 36, 1897–1904.
- Cui, F.; Li, Y.; Wan, C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 2011, 102, 1831–1836.
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699.
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic Fungi: Their Physiology and Enzymes. Microbiol. Mol. Biol. Rev. 2000, 64, 461–488.
- Bhatt, S.M. Economical Lactic Acid Production and Optimization Strategies. In Fungal Biorefineries; Springer: Cham, Switzerland, 2018; pp. 85–105.
- Zhang, L.; Li, X.; Yong, Q.; Yang, S.T.; Ouyang, J.; Yu, S. Simultaneous saccharification and fermentation of xylo-oligosaccharides manufacturing waste residue for l-lactic acid production by Rhizopus oryzae. Biochem. Eng. J. 2015, 94, 92–99.
- Tripathi, R.M.; Gupta, R.K.; Shrivastav, A.; Singh, M.P.; Shrivastav, B.R.; Singh, P. Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 35005.
- Wang, Q.; Zou, D.; Ma, H.; Ji, Y.; Wang, X. Simultaneous Saccharification and Fermentation of Corn Straw to Lactic Acid. Chem. Biochem. Eng. Q. 2010, 24, 371–376.
- Okano, K.; Zhang, Q.; Shinkawa, S.; Yoshida, S.; Tanaka, T.; Fukuda, H.; Kondo, A. Efficient production of optically pure D-lactic acid from raw corn starch by using a genetically modified L-lactate dehydrogenase gene-deficient and α-amylase-secreting Lactobacillus plantarum strain. Appl. Environ. Microbiol. 2009, 75, 462–467.
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic L-lactic acid fermentation. Bioresour. Technol. 2018, 249, 9–15.
More
