Despite progress in the development of such interesting materials, there are still some issues that need to be resolved, such as biocompatibility, high efficiency, selectivity of the action, stability, long-term and multiple use, and the temperature of the transition close to physiological temperatures (appropriate transition temperature). Therefore, there is a constant need for new approaches to design surfaces that could meet all the desired requirements. The mechanisms of their temperature-induced reactions are one of the most crucial elements that affect the characteristics of temperature-sensitive grafted brush coatings.
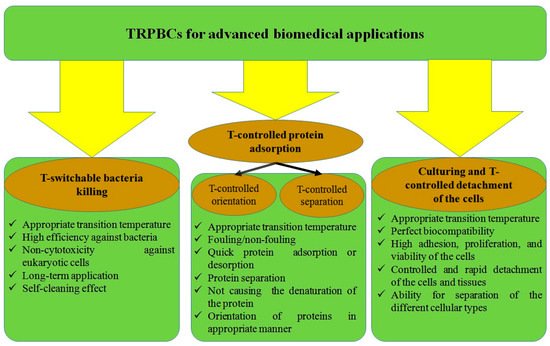
Each desired application places some specific requirements for polymer coatings, and TRPBCs for advanced biomedical applications must meet the required criteria, as presented in
Figure 1.
Figure 1.
Advanced biomedical applications of the TRPBCs and the specific criteria required for these applications.
Traditional antibacterial coatings are generally constructed based on either an ‘active strategy’ to kill bacteria by using antibacterial agents or a ‘passive strategy’ to prevent bacterial adhesion with the objective of reducing the viability or number of attached bacteria
[20][21][20,21]. Antibacterial agent-loaded coatings often suffer from the problems associated with the accumulation of dead bacteria, which might trigger undesired inflammation and reduce the efficiency of killing, leading to the formation of biofilms, as even a few bacteria attached to the coating can colonize quickly
[20][21][20,21]. On the other hand, it remains challenging to achieve a ‘perfect’ antifouling coating with 100% bacterial prevention efficiency. According to this, the TRPBCs for temperature-switchable bacteria-killing should change their physicochemical properties from a strongly antibacterial state to a cell repellant state at the appropriate temperature, namely, to be highly effective against bacterial cells, and to have a self-cleaning effect using the switching on/off of the temperature, with an appropriate transition temperature, and long-term application including multiple cycles of temperature-induced transitions. Moreover, such coatings should be nontoxic toward eukaryotic cells, which is a very challenging issue, as usually the mechanisms that are responsible for the death of bacteria cells are effective also toward other cell types.
Advanced applications for temperature-controlled protein adsorption can be divided into two subcategories: temperature-controlled protein fouling/non-fouling or protein separation
[22][23][24][22,23,24] and temperature-controlled protein orientation
[25]. Controlling the interaction between surfaces and proteins in an aqueous environment is important for the prediction of the protein adsorption during the first step of surface fouling. Moreover, in recent years, temperature-controlled protein separation turned out to be a powerful method for separating a given protein from a protein mixture. The TRPBCs for advanced application as the temperature-controlled protein fouling/non-fouling materials should undergo the transition from a highly adsorbing state to a strongly non-fouling state in response to small changes in the temperature in the physiological environment. In addition, TRPBCs should be able to separate a single protein from the mixture of the proteins and trigger very rapid adsorption/desorption of the protein in response to small changes in temperature. Another approach is the advanced application of TRPBCs for temperature-controlled protein orientation. The orientation of proteins on the surface plays a crucial role in the binding of cell receptors and ultimately defines their targeting efficiency
[26]. TRPBCs for temperature-controlled protein orientation, first, should not cause denaturation of the adsorbed proteins, and second, the temperature of the transition should be close to physiological (appropriate temperature).
The damaged tissue of the patient can hypothetically be recovered by introducing the external cells or by implantation of the external tissue. However, the application of external cells to restore damaged tissue requires a long incubation time, whereas many of the introduced cells may lose their functions in a much shorter time. Therefore, the introduction of external tissues is a promising strategy for the restoration of damaged tissues. The most suitable approach for tissue engineering is cell sheet engineering employing TRPBCs. The main requirements for TRPBCs for advanced cell culturing and detachment are the following: perfect biocompatibility, high adhesion, proliferation and viability of the cells on the coatings, controlled and rapid cell or tissue detachment, appropriate transition temperature, and the ability for separation of the different cellular types. Application of the TRPBCs for temperature-modulated separation of the different cellular types is based on the adhesion and detachment of different types of cells on the same coatings, which is enabled by the change of physicochemical properties at different temperatures. This separation method is very important for various applications in biotechnology and biomedicine, because it is possible to not use reagents that damage and deactivate biological compounds, proteins, and cells.
Cell adhesion and release, bacteria attachment, and protein adsorption are usually governed by nonspecific physical interactions with TRPBCs. Nonspecific physical interactions include van der Waals, electrostatic, steric, hydration, and hydrophobic forces. In some cases, specific biological active motifs can be inserted into TRPBCs, which essentially improves the efficiency of these systems. In the work
[27], copolymer TRPBC, including
N-isopropylacrylamide (
NIPAM) as temperature responsive units and 2-lactobionamidoethyl methacrylate as specific biological active motifs, was synthesized. In turn, in the works
[28][29][28,29], copolymer TRPBCs of poly(
NIPAM)-
block-poly(acrylic acid) and poly(2-(2-methoxyethoxy)ethyl methacrylate) with RGD motifs responsible for the acceleration of cell attachment were synthesized. Motivated by the strong progress in TRPBCs, in this
rpape
search researchersr we made an attempt to systematize the recent advances in the engineering of advanced biomedical TRPBCs. Additionally, the mechanisms of the temperature-induced transition of TRPBCs and methods of their fabrication and characterization were discussed in detail. Finally, three groups of advanced biomedical applications of the TRPBCs: temperature-stimulated bacteria killing, temperature-controlled proteins adsorption, as well as culturing and temperature-controlled detachment of the cells, were complexly described. The crucial requirements for TRPBCs suitable for advanced biomedical applications have been analyzed and listed.
2. Mechanisms of the Temperature-Induced Transition of TRPBCs
The TRPBCs exhibit the response to temperature governed by different mechanisms attributed to intermolecular interactions of the macromolecular chains between themselves and with the environment
[1][25][30][31][1,25,30,31]. The mechanism responsible for the temperature-dependent properties of polymer brushes is strongly dependent on the chemical nature of the macromolecular chains.
The first group of transitions is related to critical solution temperatures (Upper and Lower Critical Solution Temperatures (UCST and LCST)) and cannot be realized without surrounding solvents
[32][33][34][32,33,34]. For advanced biomedical applications, TRPBSs with LCST based on
NIPAM, oligo(ethylene glycol)ethyl ether methacrylate with Mw = 246 (OEGMA246), di(ethylene glycol)methyl ether methacrylate (other names 2-(2-methoxyethoxy)ethyl methacrylate or OEGMA188) (DEGMA) and their copolymers with various monomers are manufactured and exploited
[1].
The second group of transitions is related to temperature-induced changes in the physical state of polymers, where the presence of solvent is not required (glass–glass, glass-rubber (Tg), nematic-isotropic temperature transitions in polymers)
[35]. In this
rpape
search, researchersr, we focus on transitions based on LCST and Tg, which are most suitable for fabrication of the biomedical devices. These transitions are schematically sketched in
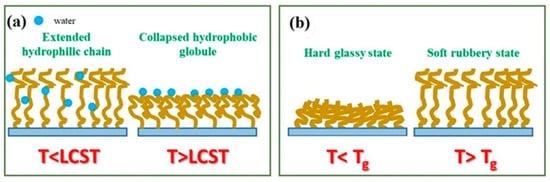
Figure 2.
Figure 2. Schematic view of the transition of the TRPBCs from the extended hydrophilic chain to the collapsed hydrophobic globule caused by LCST (a) and the transition from the hard glassy state to the soft rubbery state (Tg) (b).
2.1. TRPBCs with Critical Solution Temperatures
The LCST (UCST) is the critical temperature below (above) which the components of a mixture are miscible for all concentrations. Transitions governed by LCST (and UCST) of polymers result in a change in the thickness and wettability of polymer brush coatings
[1][36][1,36]. Below LCST, macromolecular chains are in an extended hydrophilic chain conformation, miscible with the environment
[37]. When the temperature increases above the LCST, the macromolecules collapse and transform into collapsed hydrophobic globules weakly miscible with the environment
[37]. This scenario is opposite for polymers with UCST, where the macromolecular chains are in the collapsed hydrophobic state below UCST and in the extended hydrophilic chain conformation above UCST
[33][38][39][40][41][42][33,38,39,40,41,42]. In polymer chemistry, the phenomenon of the LCST is related to the systems based on polymer-solvent mixtures that are miscible below a given critical temperature and turn to two-phase unmixed systems above this critical temperature. The Gibbs free energy change (ΔG) related to the mixing of these two phases is negative below the LCST and positive above it, and the entropy change ΔS = −(dΔG/dT) is negative for the mixing process. This is in contrast to the more common and intuitive case, in which the entropy change promotes mixing because of the increased volume accessible to each component upon mixing. In general, the unfavorable entropy of mixing responsible for the LCST may have two physical origins. The first is related to interactions between the two components, such as strong polar interactions or hydrogen bonds, which prevent random mixing. The second physical factor that can lead to LCST is compressibility effects, especially in polymer-solvent systems
[43][44][43,44]. In contrast to the LCST, the UCST is the critical temperature above which the components of a mixture are miscible in all proportions. Phase separation at the UCST is driven by unfavorable energetics; in particular, interactions between components favor a partially demixed state
[43][44][43,44].
The TRPBCs most commonly used in biomedicine include OEGMA- or
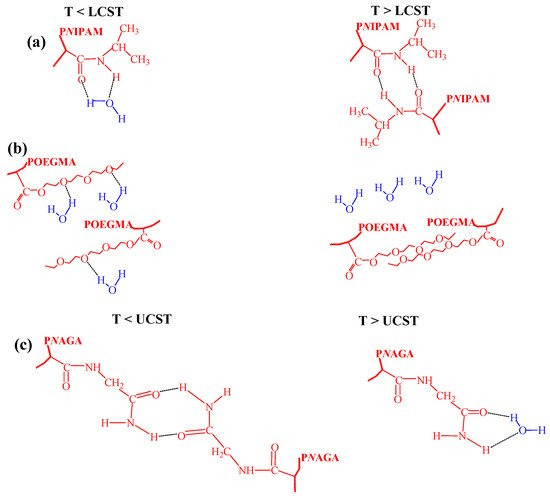
NIPAM-based polymer systems. The conformations of the macromolecular chains for these polymers are governed by hydrogen bonding, as sketched in
Figure 3. For P
NIPAM and copolymers with
NIPAM fragments, hydrogen bonds between hydrophilic amide groups of
NIPAM and water are established at T < LCST. Once the temperature increases above LCST, these bonds break, and other hydrogen bonds are established between the amide groups in the
NIPAM chains
[1][45][46][47][1,45,46,47] (
Figure 3a). In case the of POEGMA, hydrogen bonds occur between the ether oxygen of poly(ethylene glycol) and water hydrogens at T < LCST, while at T > LCST the hydrogen bonds between the ethers in polymer chains are dominant (
Figure 3b)
[47][48][49][50][47,48,49,50]. Transitions are accompanied by changes in the volume and surface hydrophilicity. In the work
[51], a series of dense water-swollen polymer brushes was studied using contact angle measurements, ellipsometry and quartz crystal microbalance. Diagrams of surface versus volume hydrophilicity of the brushes allow one to identify two types of behavior: strongly water-swollen brushes exhibit a progressive decrease in volume hydrophilicity with temperature, while surface hydrophilicity changes moderately; weakly water-swollen brushes have a close-to-constant volume hydrophilicity, while surface hydrophilicity decreases with temperature. Thermoresponsive brushes abruptly switch from one behavior to the other and do not exhibit an abrupt change of surface hydrophilicity throughout their collapse transition. In general, there is no direct correlation between surface hydrophilicity and volume hydrophilicity, because surface properties depend on the details of conformation and composition at the surface, while volume properties are averaged over a finite region within the brush
[51]. In contrast to results reported in the work
[51],
our
esearchers' works
[1][47][48][49][1,47,48,49] always showed strong changes in surface hydrophilicity at temperature-induced transitions. These differences may be related to the different structures of TRPBCs (thickness, grafting density, or other factors).
Figure 3.
The simplified mechanisms of the LCST or UCST transitions for
N
IPAM (
a
), OEGMA (
b
) and
N
AGA (
c
) based TRPBCs.
Similar mechanisms of the temperature-induced response for LCST-based systems were shown for TRPBCs based on
N,
N-dimethylaminoethyl methacrylate, 4-vinyl pyridine, derivatives of amino acids and polypeptides, and pentaerythritolmonomethacrylate
[52][53][54][55][56][52,53,54,55,56].
Poly(
N-acryloylglycinamide) (P
NAGA) is the most known polymer with UCST
[33][41][57][33,41,57]. In the previous works, it was shown that at T < UCST the hydrogen bonds between the carbonyl and amine groups of the P
NAGA were observed. They were broken at T > UCST where the interaction with water prevailed for both groups (see
Figure 3c).
The crucial parameters determining the properties of the thermoresponsive grafted polymer brushes include polymer density, molecular weight, topology, and chemical component dissolved in the solvent or included in the structure of the macromolecules. The behavior of poly(
N-isopropylacrylamide) (P
NIPAM) grafted brushes at low grafting densities and molecular weights, as well as at high grafting densities and molecular weights, was described by Leckband et al.
[58]. At low densities of grafting and molecular weights, the formation of lateral aggregates or “octopus micelles” was demonstrated. In contrast, at high grafting densities and molecular weights, the P
NIPAM-grafted brush coatings collapsed uniformly. In turn, Benetti
[59][60][59,60] showed the impact of macromolecular topology (linear versus cyclic polymer brushes) on colloidal stability and bio-inertness as well as temperature responsivity. It was noted that the properties and behavior of the examined polymer brushes were significantly different, even at the same temperature.
Another interesting work
[61] reported the synthesis and detailed characterization of thermoresponsive poly(
N,
N-dimethylaminoethyl methacrylate) TRPBC with well-controlled molecular weight and grafting density (0.08–0.20 chains/nm
2). For this material, a well-pronounced LCST transition is observed with a reduction in brush layer thickness of more than 40% by spectroscopic ellipsometry at intermediate grafting densities (0.12–0.20 chains/nm
2) in 5 mM NaCl solution. In turn, the UCST transition, induced by multivalent [Fe(CN)
6]
3− ions, reaches a remarkable change in layer thickness of ~80% already at the lowest investigated grafting density of 0.08 chains/nm
2.
A chemical component dissolved in the solvent or included in the structure of the macromolecules has an important impact on the temperature-responsive properties of the TRPBCs. In the works
[1][47][48][1,47,48], carboxylic groups in the structure of the multifunctional initiator, which initiates the grafting from the surface, blocked the temperature-responsive properties of the P
NIPAM or POEGMA-based grafted brushes at acidic pH. Recently, it was shown
[49][62][63][49,62,63] that the LCST value of POEGMA or P
NIPAM TRPBCs in buffer differs from that determined in water because the ions of the buffer contribute to the interactions between the macromolecules themselves as well as between macromolecules and the buffer. Moreover, additional components introduced into TRPBCs may influence their LCST. Incorporation of silver nanoparticles in POEGMA brushes leads to a decrease in LCST from 29.7 to 21.6 °C, while at a high concentration of silver nanoparticles the temperature-responsive properties of POEGMA-based TRPBCs were blocked
[64].
2.2. TRPBCs with Tg
The glass-rubber transitions of the polymers or α-relaxation influence the elasticity of the polymeric surface
[25][31][35][65][66][67][25,31,35,65,66,67]. Below the glass-rubber transition temperature, the polymer is in the hard glassy state; above the glass-rubber transition temperature, the polymer is in the soft rubbery state. In the glassy state, the neighboring macromolecules interact quite strongly, tending to link together. Above the glass-rubber transition, the neighboring macromolecules interact weaker. This also affects the morphology of the polymer grafted brush coatings, which are strongly transformed from highly rough and structured at T < Tg to relatively smooth at T > Tg.
It should be noted that temperature-induced transitions, such as glass–glass transitions or β-relaxation might have a weak influence on surface elasticity while showing a considerable influence on wettability, heat capacity, and refractive index
[35][66][67][35,66,67]. Glass–glass transitions can be attributed to a particular molecular rearrangement in the glassy polymer state, significantly less expressed than in the glass-rubber transition. Liquid crystalline polymers form orientationally ordered anisotropic liquid crystalline phases in a well-defined temperature range
[35][66][67][68][69][35,66,67,68,69]. Polymers that undergo these transitions are used for temperature-controlled orientation of proteins
[25], aligning of liquid crystals
[66], and are promising materials for temperature-stimulated cell detachment
[31][67][31,67].
The glass transition was reported for poly(butyl methacrylate) (PBMA) TRPBCs
[31], where it is shown that Tg is depends on the thickness of the brush coating. The dependence of transition temperature on the thickness of the coatings was also demonstrated for poly(methyl methacrylate) and polystyrene TRPBCs
[70][71][72][70,71,72].