Horticultural products display fast senescence after harvest at ambient temperatures, resulting in decreased quality and shorter shelf life. As a gaseous signal molecule, nitric oxide (NO) has an important physiological effect on plants. Specifically, in the area of NO and its regulation of postharvest senescence, tremendous progress has been made. This re followingview summarizes NO synthesis; the effect of NO in alleviating postharvest senescence; the mechanism of NO-alleviated senescence; and its interactions with other signaling molecules, such as ethylene (ETH), abscisic acid (ABA), melatonin (MT), hydrogen sulfide (H2S), hydrogen gas (H2), hydrogen peroxide (H2O2), and calcium ions (Ca2+). The aim of this treviextw is to provide theoretical references for the application of NO in postharvest senescence in horticultural products.
- nitric oxide
- postharvest
- senescence
- molecular interaction
1. Introduction
2. NO Delays Postharvest Senescence
Horticultural plants are prone to rapid senescence after postharvest storage at ambient temperature. Postharvest senescence is affected by several factors, such as temperature [20][26], light [21][22][27,28], and some plant growth regulators [23][24][25][29,30,31]. Multiple studies have shown that NO is an effective way to delay postharvest senescence.2.1. Exogenous NO Delays Postharvest Senescence
The effect of NO on alleviation of postharvest senescence can be demonstrated by the exogenous application of NO on postharvest plants. In exogenous NO treatment, three methods are available: fumigation, immersion, and spraying. Fumigation with direct NO gas delayed the senescence in postharvest mangoes and peaches [26][27][32,33]. The immersion of NO gas solution and NO donor sodium nitroprusside (SNP) or S-nitrosoglutathione (GSNO) solution also delays the postharvest senescence in some fruits by inhibiting ethylene production and reducing respiration rates [28][29][34,35]. Additionally, spraying NO donor GSNO solution is commonly used to extend the postharvest life of blueberries by improving their concentrations of ascorbic acid and glutathione [30][36]. The effects of exogenous NO on delaying postharvest senescence in horticultural products are listed in Table 1.| Species | Treatment | NO-Mediated Effect | References |
|---|---|---|---|
| Pear | 100 μM L−1 SNP |
Decreased the transcript levels of cell wall- and ethylene synthetase-related genes; reduced respiration rate and ethylene production | [31][37] |
| Apple | 100 μM L−1 GSNO |
Activated nucleocytoplasmic MdERF5 and suppressed ethylene biosynthesis | [18] |
3.5. The Promotion of Energy Metabolism
The lack of energy caused by the impaired respiratory chain and reduced ATP synthesis leads to cellular breakdown and dysfunction during the postharvest senescence stage [46][52]. The maintenance of cellular ATP and energy levels can thus maintain the normal physiological activities of the tissues, thereby postponing postharvest senescence and prolonging the shelf life of horticultural products. Several studies have established that NO-delayed postharvest senescence is ascribed to the optimization of energy metabolism. NO treatment, for example, delayed the softness and weight loss of water bamboo by maintaining the integrity of the mitochondrial ultrastructure and enhancing ATP levels [17]. Furthermore, NO donor SNP treatment enhanced ATP synthase activity, ATP synthase CF1 alpha subunit (AtpA) content, and AtpA expression levels in the postharvest freshness of cut lilies [47][53].3.6. The Induction of SAGs
Various external and internal signals are likely to activate a set of SAGs that drive postharvest senescence. During postharvest senescence, SAGs can be induced by NO to delay senescence. These NO-induced SAGs include various transcription factors (ERFs) and some structural genes encoding enzymes related to cell wall metabolism, ethylene biosynthesis, and antioxidants. The SAGs regulated by NO during the postharvest senescence process are listed in Table 2.| Horticultural Products | Species | SAGs | References |
|---|---|---|---|
| Fruits | Pear | PcPG, PcCel, PcACO1, PcACO2, PcACS1, PcNOS, PcNR1, and PcNR2 | [31][37] |
| Apple | MdACS1, MdACO1, MdERF5, and MdPP2C57 | [18] | |
| Strawberry | 5 μM L−1 SNP |
Inhibited ethylene production, respiration rate, and activity of ACC synthase; reduced the content of ACC | |
| Mango | MiACO, MiACS, MiETR1, MiERS1, MiEIN2, and MiERF | [48][54] | [32][38] |
| Peach | 10 μL L−1 NO |
Maintained higher sucrose content but decreased glucose and fructose to lower levels during late storage | [27][33] |
| Carnation | |||
| Table grape | VvSOD, VvCAT, VvPOD2, and VvGR | [45][51] | 0.1 mM L−1 SNP |
| Kiwifruit | PG, PL | Maintained water metabolism and antioxidative enzyme activity and mass-eliminated ROS as well as cell membrane stability | , β-Gal, PE, ACO, ERS1, ETR2, ERF016, ERF7, ERF010, ERF062, ERF110, ERF037, ERF008, ERF113, ERF12, ERF095, [33][39] |
| Rose | 200 μM L−1 SNP |
Decreased ethylene output by inhibiting ACO activity in cut rose flowers | [16] |
| Lily | 100 μM L−1 SNAP |
Increased Ca2+/CaM contents, enhanced Ca2+-ATPase activity, and up-regulated gene expression of CaM, CBL1, and CBL3 | [15] |
| Consolida ajacis L. | 40 μM L−1 SNP |
Alleviated deteriorative postharvest changes by modulating physiological and biochemical mechanisms underlying senescence | [34][40] |
| Calendula officinalis L. |
100 μM L−1 SNP |
Improved flower longevity by delaying neck bending, inhibited bacterial growth, and increased activities of antioxidant enzymes | [35][41] |
| Tomato | 1 mM L−1 SNP |
Retarded pericarp reddening of tomato fruit, suppressed ethylene production, and influenced quality parameters during storage | [28][34] |
| Water bamboo shoots | 30 μL L−1 NO |
Delayed softness and weight loss and enhanced ATP levels by activating the expression and activity of SDH, MDH, and CCO | [17] |
| Lettuce | 100 and 200 ppm NO | Inhibited the accumulation of H2O2, delayed senescence, and prolonged shelf life | [36][42] |
2.2. Endogenous NO Production during Postharvest Senescence Process
The effect of NO on postharvest senescence can also be revealed by endogenous NO production during postharvest senescence processes delayed by environmental factors and some chemical substances. For example, UV-B treatment can maintain decreased fruit firmness and delay postharvest senescence in mangoes by enhancing endogenous NO levels [37][43]. Endogenous NO production and NOS activity were induced by 1-methylcyclopropene (1-MCP) in the senescence process in cut roses [16]. Likewise, melatonin (MT) led to an increase in NO content through an increase in NOS activity and upregulation of PcNOS transcript levels, which subsequently delayed senescence in peaches [31][37]. However, in cold-stored peaches, abscisic acid (ABA) can induce endogenous NO synthesis via the NR pathway [38][44]. Similarly, NO production was also triggered by hydrogen gas (H2) by enhancing NR activity, which mitigated postharvest senescence in cut rose flowers [39][45].3. The Mechanism of NO-Regulated Postharvest Senescence
NO delays postharvest senescence by regulating various metabolism pathways, including ethylene biosynthesis, respiratory metabolism, cell wall metabolism, reactive oxygen species (ROS) metabolism, and energy metabolism (Figure 1). Moreover, a set of senescence-associated genes (SAGs) that drive postharvest senescence are regulated by NO during postharvest senescence processes.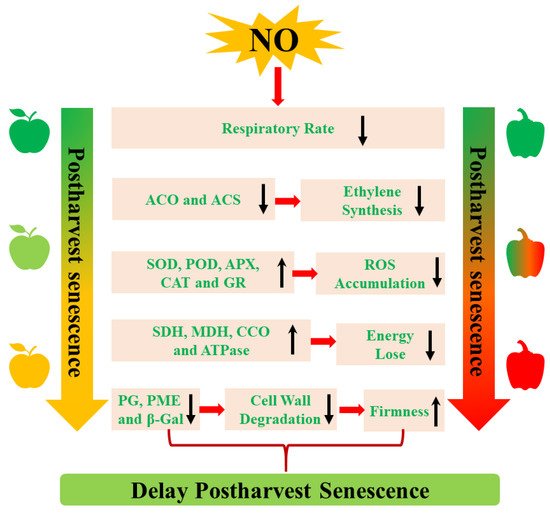
3.1. The Inhibition of Ethylene (ETH) Biosynthesis
It is well known that an increase in endogenous ETH is a sign of senescence. Thus, inhibiting endogenous ethylene production is considered a useful method to delay postharvest senescence. Exogenous NO can inhibit ETH production, which delays postharvest senescence of horticultural products, including mangoes [26][27][32,33], peaches [27][33], and cut rose flowers [16]. In addition, the inhibition of ETH biosynthesis-related enzymes 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase (ACO) and ACC synthase (ACS) activity and their expression levels is associated with NO-induced decreases in the endogen ETH during the postharvest senescence process [26][28][32,34]. Therefore, the positive effect of NO on postharvest senescence is largely dependent on the inhibition of the ETH biosynthesis pathway.3.2. The Decrease of Respiratory Metabolism
Climacteric transition is generally regarded as an important signal of the initiation of senescence in climacteric plants, which affects the storage life of postharvest plants. Reducing the respiratory rate can effectively delay postharvest senescence and prolong the shelf life of horticultural crops. A previous study showed that NO treatment restrained the increase in the respiration rate and extended the postharvest life of water bamboo shoots [17]. The application of 10 μL of NO gas fumigation significantly inhibited the respiratory rate of climacteric plums and peaches, thereby extending their shelf life [27][40][33,46]. Rather than suppressing the respiratory rate of climacteric fruits, NO was also shown to depress the respiratory rate of non-climacteric fruit. For example, the respiratory rate was significantly inhibited by NO treatment throughout the entire storage period of winter jujube fruit [41][47].3.3. The Activation of Cell Wall Metabolism
Generally, senescent fruits exhibit the symptom of softening as a result of cell wall degradation. Several degrading enzymes, including polygalacturonase (PG) and pectin methylesterase (PME), are involved in the degradation of the cell wall [42][48]. Changes in cell wall metabolism-related enzyme activity are responsible for the decrease in firmness affected by a set of abiotic factors. The application of exogenous NO maintains the decrease in firmness and extends the postharvest life of blueberries [30][36]. Similarly, cornelian cherries treated with 500 μM of NO donor SNP exhibited higher firmness, possibly resulting from the lower activity of PE and PME, which degrade cell walls [43][49]. A decrease in the NO-induced activities of PG, PME, and β-galactosidase (β-Gal) delayed postharvest winter jujube fruit softening as well [44][50]. At the transcript level, NO treatment suppressed the softening of postharvest tomatoes by downregulating the gene expression levels of LePG, LePhy1, and LePME [28][34]. In summary, NO can inhibit cell wall metabolism-related enzyme activities, which maintains the decrease in firmness of horticultural products during the NO-delayed postharvest senescence process.3.4. The Regulation of ROS Metabolism
Postharvest senescence is often accompanied by increased ROS, followed by the induction of some SAGs. The increase in ROS level occurs in parallel with increases in lipid peroxidation in senescent cells. In addition to endogenous ROS, ROS-related antioxidant enzymes are also related to postharvest senescence. Extensive research has shown that NO can delay postharvest senescence by decreasing ROS levels and enhancing antioxidant activities. Exogenous NO fumigation reduces ROS, O2•−, and hydrogen peroxide (H2O| CNGC1 | |||
| , | |||
| CPK1 | |||
| , | |||
| CIPK2 | |||
| , | CML31, CML48, and ZIFL1 | [49][55] | |
| Wax apple | PAL, POD, GLU, C3H, CA, F5H, 4CL, CCoAOMT, and C4H | [50][56] | |
| Peach | PpaSOD, PpaCAT, PpaPOD, PpaPOD-1, PpaAPX, and PpaPAL | [51][57] | |
| Cut flowers | Gladiolus | GgCyP1 and GgDAD1 | [52][58] |
| Lily | CaM, CBL1, CBL3, and LlatpA | [15][47][15,53] | |
| Vegetables | Tomato | LeACS2, LeACS4, LeACO1, LePME, LePG, LePhy1, and LeGAPDH | [28][34] |
| Water bamboo shoots | ZlH+-ATPase, ZlNa+-K+-ATPase, ZlCa2+-ATPase, ZlMDH, ZlSDH, and ZlCCO | [17] |
