Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Stamatis Gregoriou and Version 4 by Rita Xu.
Onychomycosis is a common nail disease caused by fungi. The primary pathogens are dermatophytes, yeasts, non-dermatophyte moulds, and mixed fungal populations may also contribute to the development of a recalcitrant condition, usually accompanied by difficulties in everyday life and severe emotional stress. Treatment failure and relapse of the infection are the most frequent problems. Resistance to antifungals, an increasing number of comorbidities, and polydrug use among the ageing population are imperatives that impose a shift to safer drugs. Topical antifungals are considered less toxic and minimally interact with other drugs.
- onychomycosis
- topical
- antifungals
- nail
- treatment
- Efinaconazole
1. Introduction
Onychomycosis is a term that encompasses all the nail pathologies caused by fungi and accounts for approximately 50% of all nail diseases [1]. The reported mean prevalence in Europe and North America is 4.3–8.9% [2]. The epidemiologic data for onychomycosis exhibit remarkable geographical variance, depicting differences regarding the ecology of fungi and the characteristics of the affected population (i.e., genetics, culture, climate, and lifestyle). In Western countries, the primary culprit pathogens are dermatophytes (up to 80–90% of cases), whilst yeasts and non-dermatophyte moulds (NDMs) account for 5–17% and 2–3% of cases. The prevalence of dermatophyte infection is lower in South Europe and Asia/the Middle East, accounting for 40–68% and 40–48%, respectively. Yeasts are isolated in 21–55% of cases in South Europe and 43–46% in the Middle East. NDMs attribute to 8–11% of infections in Asian countries [3]. According to a recent Iranian report [4], samples collected by two laboratories affiliated with Tehran University exhibited 33.7%, 21.8%, and 44.4% rates for yeast, dermatophyte, and NDM onychomycoses, respectively. This is a remarkably different epidemiologic pattern compared to that demonstrated in the Western world.
For dermatophyte onychomycosis, tinea pedis is usually a precondition, and a familial pattern was described by Zaias et al. in 1996, postulating a genetic basis in the susceptibility of developing the infection [5]. NDM onychomycoses are opportunistic infections and are typically characterised by the absence of concomitant interdigital space infection. Exposure to NDMs may lead to onychomycosis in the presence of numerous predisposing factors [6]: high temperatures and humidity due to climate conditions, occlusive footwear and hyperhidrosis; advanced age; nail deformity due to acute or chronic trauma; chronic cutaneous diseases, such as psoriasis; comorbidities with impaired vascular and immune function; environmental exposure due to occupational or leisure activities; toenail damage presenting as asymmetric gait nail unit signs (AGNUS) [7][8][9][7,8,9]. The latter provides a plausible explanation for the formation of a nail unit space, which is colonised by opportunistic fungi and serves as a niche for the constant presence of the NDM in the toenail environment. Therefore, it can be extrapolated that the infection is not the primary one in a sequence of events which usually starts with the formation of a cleft between the nail plate and bed, generated by chronic or acute trauma and habitual or occupational injury. Mixed infections by dermatophytes and non-dermatophytes are increasing in prevalence, either on the basis of impaired anatomy or not. They establish a refractory microenvironment, which necessitates a thorough assessment in terms of clinical and laboratory evaluations [10].
Onychomycosis has a severe impact on patients’ quality of life and poses a challenge to doctors’ practice. Onychomycotic nail deformity and dystrophy often present as both aesthetic and functional disorders that may result in difficulty in proper shoe fitting and subsequent inconvenience or even pain during daily activities. Treatment failure has been reported in 20–50% and recurrence rates in 10–53% of the cases [11][12][13][11,12,13]. Socks and shoes comprise the main reservoirs of fungi and sources of reinfection. The nail growth rate is low, and treatment, either systemic or topical, is required over long periods of time. Compliance with long-term treatments is often poor. Adherence to systemic therapy for months is a practice that increases safety and drug-to-drug interaction issues, especially in the case of elderly patients or patients with multiple comorbid clinical entities. Topical treatments of onychomycosis, used either as a monotherapy or adjuvant therapy, are generally characterised by low efficacy. The drug concentration in the tissue is highly dependent on the permeability/penetration qualities of the topically applied agents. Improving transungual distribution is considered as one of the key targets for the development of effective new agents.
2. New Topical Agents for Onychomycosis
The topical agents that have been most recently approved include Efinaconazole, Tavaborole, and Luliconazole. Data published on their safety and efficacy come from a limited number of countries where the drugs have become available.2.1. Efinaconazole
Efinaconazole (molecular formula C18H22F2N4O, chemical structure as seen in Figure 1), a next-generation triazole, has been available in the USA and Japan since 2014. It is FDA-approved for the treatment of onychomycosis due to T. rubrum and T. mentagrophytes for patients aged 18 years and older. In 2020, this indication was extended to children 6 years of age and older. It is administered as a 10% solution and blocks ergosterol synthesis by inhibiting the enzyme lanosterol 14-dimethylase. Nail penetration of Efinaconazole is enhanced by an efficient combination of physicochemical properties, such as low surface tension, low affinity to keratin, and poor aqueous solubility [14][25]. Efinaconazole displays a low interaction with the nail keratin, as measured in bovine hoof membranes, a widely used model for the human nail [15][26]. Accordingly, it has been demonstrated that the site of action for this drug is the nailbed and fails to achieve a high concentration within the nail plate. In two phase III randomised, double-blinded studies, Efinaconazole 10% solution exhibited mycological cure rates (negative potassium hydroxide, KOH, direct examination and negative culture) of 55.2% and 53.4% in patients with distal–lateral subungual onychomycosis after treatment for 48 weeks (superior to vehicle, p < 0.001). Complete cure rates (mycologic cure and absence of clinical involvement) occurred in 17.8% and 15.2% vs. 3.3% and 5.5% in the vehicle-receiving group of subjects [16][15]. A retrospective survey of the treatment results documented a 25% complete cure rate in patients with severe involvement (Scoring Clinical Index for Onychomycosis, SCIO = 21–30) [17][27]. Efinaconazole is fungistatic, not fungicidal; therefore, it may induce resistance in dermatophytes such as other azole derivatives. Actually, it has been observed that T. rubrum strains with resistance to Efinaconazole may show cross-resistance to Itraconazole but not Amorolfine or Ciclopirox [15][26].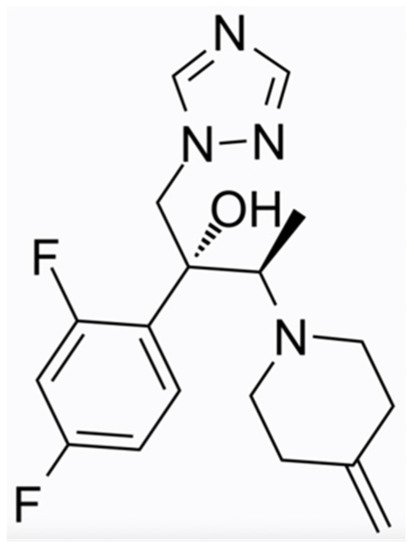
Figure 1. Efinaconazole chemical structure.
2.2. Tavaborole
Tavaborole (molecular formula C7H6BFO2, chemical structure as seen in Figure 2) has been US FDA-approved since 2014. This oxaborole drug inhibits the enzyme leucyl-tRNA synthetase and interferes with the process of protein synthesis, thus exerting its action to a broad spectrum of microorganisms, i.e., fungi (dermatophytes, yeasts, and non-dermatophyte moulds) and some bacteria. The efficacy and safety of tavaborole were evaluated in two identical phase III multicentre, randomised, double-blinded, vehicle-controlled trials [18]. Adults with distal subungual onychomycosis that affected 20–60% of the great toenail were enrolled for a 48-week intervention with once-daily applications. An assessment of the results was performed at week 52. Tavaborole solution 5% was significantly superior to the vehicle in all endpoint efficacy parameters. The complete cure rates for the target great toenail were 6.5% and 9.1% for Tavaborole vs. 0.5% and 1.5% for the vehicle (p = 0.001 and p < 0.001), respectively. The mycological cure rates (negative culture and KOH direct microscopy) were 31.1% and 35.9% for tavaborole-treated patients vs. 7.2% and 12.2% for the vehicle group (p < 0.001 for both), respectively. For both studies, treatment-related adverse events were higher in the Tavaborole arms, but the discontinuations attributed to them were almost equal. The low molecular weight (152 Da) of Tavaborole offers a clear penetration advantage and results in enhanced distribution through the nail plate compared to Ciclopirox 8%, as was demonstrated by Hui et al. [19][28]. It has been assumed that Tavaborole is not likely to induce resistance owing to its mechanism of action [20][29]. However, the in vitro resistance of T. rubrum strains and isolates to Tavaborole has been developed by propagating them on Sabouraud dextrose agar media containing low, non-inhibitory concentrations of the drug (0.5 × MIC) [21][30]. As mentioned above, the favourable pharmacokinetic profile of Tavaborole contributes to high concentrations in the ungual apparatus in vitro, but it is unknown whether host or disease-related factors could reverse this merit, leading to the development of resistant strains in vivo. Moreover, a low efficacy of Tavaborole against yeasts and moulds compared to Terbinafine and azoles (except Fluconazole) has been reported [22][31].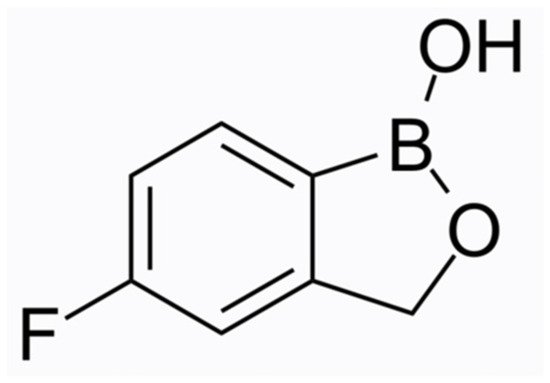
Figure 2. Tavaborole chemical structure.
2.3. Luliconazole
The most recently approved antifungal topical agent for onychomycosis is Luliconazole 10% solution (molecular formula C14H9Cl2N3S2, chemical structure as seen in Figure 3), a member of the imidazole subfamily, which became available in Japan in 2016. Even though the FDA has approved Luliconazole cream 1% for the treatment of tinea pedis, tinea cruris, and tinea corporis, approval of the solution for onychomycosis is pending in the USA. The molecular target of Luliconazole is lanosterol 14a-demethylase, an enzyme involved in the biosynthesis of fungal cell membranes, which is inhibited by the drug [23][24][20,32]. Luliconazole is administered on the nail as a 5% w/w solution. The drug concentration increases in a dose-dependent manner, as it is positively associated with the duration of application. Luliconazole penetrates the nail plate and rapidly achieves fungicidal levels in the nail unit independent of the nail plate thickness [25][33]. Luliconazole was reported to achieve a 14.9% complete cure rate (0% clinical involvement and negative KOH test under direct microscopy), which was significantly higher compared to the vehicle (5.1%). The efficacy was evaluated in a multi-centre, double-blinded, randomised phase III study after 48 weeks of daily application in adults with distal–lateral subungual onychomycosis with 20–50% clinical involvement of the great toenails [23][20]. Shimoyama et al. documented a complete cure rate of 15.8% in patients with SCIO 21-30 affected by various types of onychomycoses (superficial white onychomycosis, distal–lateral subungual onychomycosis, proximal subungual onychomycosis, and dermatophytoma) after long-term treatment (mean duration 12.4 months) [17][27]. The safety and efficacy of luliconazole 10%, not 5%, solution is being evaluated in a currently active, open-label, phase I study in patients with moderate to severe distal subungual onychomycosis (NCT05110638). The different approach is that the application is performed by the study personnel once daily for 29 consecutive days to all toenails and periungual areas, regardless of whether they are affected or not.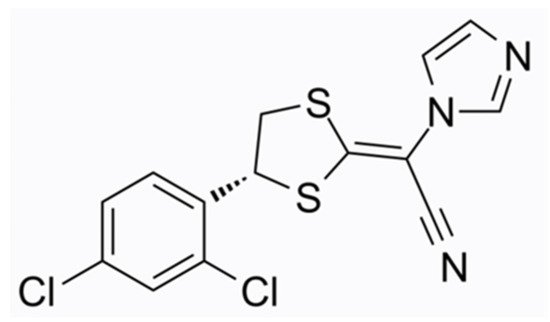
Figure 3. Luliconazole chemical structure.
