Antibiotics are regularly used in animal husbandry to treat diseases. This practice is beneficial to animals’ health and helps ensure food security. However, the misuse of antibiotics, especially in food-producing animals, has resulted in the advent of antimicrobial resistance (AMR) and its dissemination among foodborne pathogens. The occurrence of AMR in bacteria pathogens that cause infections in animals and those associated with food spoilage is now considered a global health concern affecting humans, animals and the environment. The search for alternative antimicrobial agents has kindled the interest of many researchers. Among the alternatives, using plant-derived nanoparticles (PDNPs) for treating microbial dysfunctions in food-producing animals has gained significant attention. In traditional medicine, plant extracts are considered as safe, efficient and natural antibacterial agents for various animal diseases. Given the complexity of the AMR and concerns about issues at the interface of human health, animal health and the environment, it is important to emphasize the role of a One Health approach in addressing this problem.
- alternative therapy
- antibiotics
- antimicrobial resistance
- foodborne pathogens
- green synthesis
- multidrug-resistant bacteria
- One Health Concept
- phyto-nanomedicine
1. Introduction
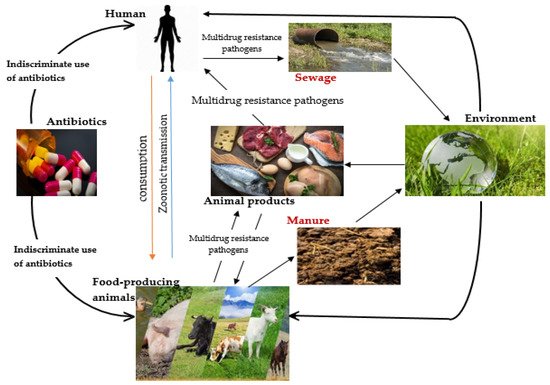
2. Emergence of Multidrug Resistance (MDR) Pathogens in the Food Chain
A leading public health issue in recent decades has been the growth of multi-drug resistant (MDR) bacteria. The prevalence of these pathogens in animal-derived products, including milk and meat, has risen considerably and their potential to evolve new features, notably MDR, is significant . The upsurge in MDR bacteria has hitherto remained undisclosed to the animal food-service sector because there has previously been virtually no communication of their occurrence in animal-based products. However, more recently, new exceptions, such as mobile colistin-resistant (mcr) strains and New Delhi metallo-β-lactamase-1 (NDM-1)-producing variants in food-producing animals, have been surfacing as discrete pools of colistin and β-lactam resistance, along with the alternative carbapenem antibiotic-resistant strain . The use of antibiotics has long been linked to the emergence of drug resistance . When an antibiotic is ingested, it kills vulnerable bacterial cells, while the resistant ones continue to proliferate and become the dominant strains . This provides opportunities for the transfer of resistant genes to their offspring . Given that the food supply chain is an ecological niche made up of diverse biological points in which significant amounts of drugs are utilized and scores of bacteria coexist, food-producing animals, seafood, meat and milk are regarded as significant pools for the proliferation of antimicrobial-resistant bacteria . Antibiotic resistance may occur in one of two ways. Firstly, it can occur as intrinsic resistance, in that an existing natural composition in the bacterial species provides that specific species with the potential to resist the action of an antibiotic [1]. During their developmental stages, bacterial cells amass genetic flaws in their chromosomes and/or plasmids and pass down the same to their daughter cells through vertical gene transfer (VGT), thus accounting for natural or inherent resistance [1]. The other mechanism, termed “acquired resistance”, involves the transfer of genetic materials between and within bacterial species. This mechanism involves the lateral transfer of the genetic materials in a process called horizontal gene transfer (HGT). These codes are carried on or within selfish genetic elements, including transposons .Use of Antibiotics in Animal Agriculture, Their Mode of Action and Resistance Mechanisms
Antibiotics are routinely utilized in animal production to support the health and development of the animals. Producers and consumers as a whole gain certain financial advantages from this strategy. For a very long time, antibiotics have been thought of as the first line of defence against bacterial infections in animal husbandry. They are still essential medical drugs that must be handled with caution when treating sick animals, thus ethical livestock production does not have to completely forego their use. Antibiotics can be categorized according to their modes of action, which include the inhibition of cell wall synthesis, the suppression of nucleic acid synthesis, the repression of ribosome function, the inhibition of cell membrane function, and the inhibition of folate metabolism (Table 1). However, there are certain issues connected to the use of antibiotics in animal agriculture. Given that the antibiotics used are identical to or substitutes for the antibiotics used in human treatment procedures, there has been great worry that repeatedly exposing these animals to low dosages of antibiotics adds considerably to antimicrobial resistance. Livestock alone consumes 50–80% of all antibiotics produced in the majority of the developed countries . Animals are frequently given less antibiotics than are used for therapeutic purposes when using them as a growth promoter. Due to the frequent exposure of bacteria to sub-lethal doses of antibiotics and the favourable conditions for the selection and maintenance of resistance features, this approach is more likely to exert significant pressure on the emergence of antimicrobial resistance mechanisms (Table 1).| Antibiotic Family | Mode of Action | Mechanism of Resistance | Reference |
|---|
| Pathogen | Class of antibiotic Resistance |
Transmission Route |
Food Product Susceptible to Contamination | Reference |
|---|---|---|---|---|
| β-lactams β-lactamase inhibitors Fluoroquinolones Macrolides, Lincosamides and Streptogamin (MLS) Aminoglycosides Tetracyclines Sulfonamides (Folate pathway inhibitors) |
||||
| Nontyphoidal Salmonella Campylobacter jejuni Escherichia coli Staphylococcus aureus, Methicillin- resistant Staphylococcus aureus (MRSA) and other staphylococci Listeria monocytogenes and other Listeria species | Cell wall synthesis inhibitors. Binds transpeptidase also known as penicillin binding proteins (PBPs) that help form peptidoglycan Inactivates the enzyme; beta-lactamase Hydrolysis of the beta-lactam ring Binds DNA-gyrase or topoisomerase II and topoisomerase IV; enzymes needed for supercoiling, replication and separation of circular bacterial DNA. Binds the bacterial 50S ribosomal subunits; inhibit protein synthesis Bind to the bacterial 30S ribosomal subunit thus inhibit bacterial protein synthesis Bind reversibly to the 30S ribosomal subunit as such blocks the binding of the aminoacyl-tRNA to the acceptor site on the mRNA-ribosome complex Inhibit the bacterial enzyme dihydropteroate synthetase (DPS) in the folic acid pathway, thereby blocking bacterial nucleic acid synthesis |
Cephalosporin a,b Fluoroquinolone b Tetracycline b,c Penicillin a,b Sulfonamide b,c Fluoroquinolone b Macrolide a,b Cephalosporin a,b Fluoroquinolone b Carbapenem a Cephalosporin c Methicillin a,b Vancomycin a Cephalosporin a,b Penicillin a,b Fluoroquinolone b Tetracycline b,c Aminoglycoside a,b Carbapenem a | Beta-lactamase production primarily - bla genes, Expression of alternative PBPs Production of extended spectrum beta-lactamases (ESBLs) Target modification, Decreased membrane permeability, Efflux pumps Target site modification, Active drug efflux Target site modification (via the action of 16S rRNA methyltransferases (RMTs)), Enzymatic Drug Modification (adenylation, acetylation and phosphorylation), Efflux systems Efflux systems, Target modification, Inactivating enzymes, Ribosomal protection Excessive bacterial production of dihydrofolate reductase (DHFR), Reduction in the ability of the drug to penetrate the bacterial cell wall, Production of altered forms of the dihydropteroate synthetase (DPS) enzyme with a lower affinity for sulfonamides, Hyperproduction of para-amino benzoic acid (PABA), which overcomes the competitive substitution of the sulfonamides |
3. Annals of One Health Antimicrobial Resistance
Antibiotic resistance is a growing issue of severe public health concern worldwide and is now regarded as a critical One Health issue. Based on a concise historical record, two accounts of some of the antimicrobial resistance issues that have resulted from the use of the same antibiotic classes in humans and animals, as well as the associated complications with competing interests, are described. The first scenario, which focuses on third-generation cephalosporins, demonstrates One Health concerns with an antibiotic that is primarily used for therapeutic purposes in animals and is also used prophylactically in some key conditions. The second scenario is colistin, an older type of antibacterial agent that has long been utilized in animals for medicinal, preventive and growth-promotion objectives, but has only lately gained prominence in the human health arena.3.1. Third-Generation Cephalosporins
Broad-spectrum beta-lactam antibiotics, known as third-generation cephalosporins, are routinely utilized in humans and animals. Cefotaxime, ceftriaxone and other members of this group are employed to treat a wide range of infections in humans, including urinary tract, abdominal, lung, and bloodstream infections caused by E. coli, Klebsiella pneumoniae, and other bacteria, as well as infections caused by Neisseria gonorrhoeae . This class of antibiotics has been designated as “critically essential” for human health because of its critical role in the treatment of numerous bacterial infections, in which resistance has become a serious issue . Extended-spectrum beta-lactamases (ESBLs) and AmpC beta-lactamases are responsible for resistance to third-generation cephalosporins. ESBL genes are easily spread by plasmids, transposons and other genetic elements . Originally thought to be chromosomally associated, AmpC beta-lactamases have also been found on plasmids and demonstrated to have been propagated through horizontal transfers throughout Enterobacteriaceae . Unfortunately, resistance to third-generation cephalosporins is frequent in E. coli and K. pneumoniae, both emanating from serious human infections in many countries and forcing clinicians to rely more heavily on the few remaining antimicrobial classes, such as carbapenems. According to a study by the World Health Organization (WHO) , as opposed to susceptibility to infections, patients with third-generation cephalosporin-resistant E. coli infections showed a two-fold increase in all-cause deaths, bacterium-attributable mortality and 30-day mortality. Salmonella species have also been found to harbour resistance, which is mediated mostly by the CMY-2 AmpC beta-lactamase genes that are usually remotely-hosted with genes encoding resistance to other antimicrobial classes, such as tetracyclines, aminoglycosides and sulfonamides . Although much of the proliferation of E. coli with ESBL and other β-lactamases is assumed to be clonal, the relevant genes have been found in a range of bacteria from humans, animals and the environment . From a One Health perspective, third-generation cephalosporins are favourably considered to be critically essential for both human and animal health (Table 2). As a result, third-generation cephalosporins are widely used either as therapeutic or prophylactic agents, which facilitates the spreading of resistance from animals to humans (Table 1). Another family of antibiotics, the fluoroquinolones, has been used in similar approaches and has thus led to resistance to these antimicrobial agents. Following the mass treatment of chicken flocks, resistance to key antimicrobials has evolved among Campylobacter jejuni isolates .3.2. Colistin
Colistin is an antibiotic that belongs to the family polymyxin, which has been utilized in human and animal care for more than five decades . Polymyxins, which are toxic to the neurons and nephrons of humans, were hitherto primarily used as colistimethate sodium by inhalation in humans for topical applications and in the nursing of cystic fibrosis patients . Colistin is becoming more popular as a last resort for treating multi-drug-resistant Gram-negative infections, such as carbapenem-resistant Pseudomonas aeruginosa, Acinetobacter baumannii, K. pneumoniae, and E. coli, primarily in intensive care units in several countries . Most often, colistin is administered orally to herds of pigs, poultry, and in certain circumstances, calves, for its therapeutic or prophylactic benefits in food-producing animals . Colistin is also used as a growth promoter in animals in several countries . Owing to technical problems in phenotypic susceptibility testing, compulsory checks for colistin resistance in Salmonella and E. coli from animals and some food products began in Europe as recently as 2014 . A study reported that among the 162 colistin-resistant E. coli isolates from chicken, MDR was found in 91.4% of the cases . In the recent past, acquired colistin resistance was assumed to be limited to chromosomal mutations and was basically non-transferable . However, in 2015, findings from a study in China revealed the presence of a colistin resistance gene, mcr-1, in E. coli isolates from animals, food, and human bloodstream infections . Colistin differs from third-generation cephalosporins in some critical One Health aspects of antibiotic resistance. These are associated with the accounts and style of colistin usage in humans and animals, as well as with the successive establishment of resistance to the polymyxin group of antibiotics, which were most likely triggered by the massive amounts of colistin used in animals rather than in humans . In addition, the use of Avoparcin in animals has been linked to the choice and proliferation of vancomycin-resistant Enterococcus (VRE) species and glycopeptide-resistant genes in enterococci from animals, food, humans and the environment .| Monobactam | ||||
| a | Macrolide a,b Lincosamide c,d |
Faecal shedding into the environment Waste water, faeces and urine Water Contact with carrier animals; indiscriminate use of antibiotics in animals; negligence resulting in cross-infections within the confines of and amid farms; foreign trade in animal, food or supplementary outputs Sewage, effluent, faeces of man and animal, soil water |
Meat and poultry products, fruits and vegetables Meat and poultry products Milk, meat and eggs Bacon, meat, milk and eggs Unpasteurized milk and its derivatives, meat, fish, chicken, poultry products, vegetables and salads |
References
- Ajose, D.J.; Oluwarinde, B.O.; Abolarinwa, T.O.; Fri, J.; Montso, K.P.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Combating bovine mastitis in the dairy sector in an era of antimicrobial resistance: Ethnoveterinary medicinal option as a viable alternative approach. Front. Vet. Sci. 2022, 9, 287.
