The research field of liquid crystals and their applications is recently changing from being largely focused on display applications and optical shutter elements in various fields, to quite novel and diverse applications in the area of nanotechnology and nanoscience. Functional nanoparticles, such as ferroelectric and magnetic particles, have recently been used to a significant extent to modify the physical properties of liquid crystals. Also, intriguing photonic functionalities can be realized by adding nanoparticles such as quantum dots, metal particles, semiconductors, etc. into liquid crystals. The self-organization of liquid crystal molecules is exploited to used as order templates to orient nanoparticles. Similarly, anisodiametric nanoparticles such as rods, nanotubes and flakes are shown to form lyotropic liquid crystal phases in the presence of isotropic host solvents at a certain concentration.
- Liquid crystals
- ferroelectric nanoparticles
- quantum dots
- graphene oxides
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
The research field of liquid crystals and their applications is recently changing from being largely focused on display applications and optical shutter elements in various fields, to quite novel and diverse applications in the area of nanotechnology and nanoscience. Functional nanoparticles, such as ferroelectric and magnetic particles, have recently been used to a significant extent to modify the physical properties of liquid crystals. Also, intriguing photonic functionalities can be realized by adding nanoparticles such as quantum dots, metal particles, semiconductors, etc. into liquid crystals. . The self-organization of liquid crystal molecules is exploited to used as order templates to orient nanoparticles. Similarly, anisodiametric nanoparticles such as rods, nanotubes and flakes are shown to form lyotropic liquid crystal phases in the presence of isotropic host solvents at a certain concentration.
1. Introduction
For many materials, the transition between the liquid and the solid phase is not a single-step process, but a range of various mesophases, which are called liquid crystals (LCs). LCs are self-organized anisotropic fluids that are thermodynamically located between the isotropic liquid and the crystalline phase, exhibiting the fluidity of liquids as well as the long-range lattice order that can only be found in crystalline solids [1,2,3][1][2][3]. Generally, LCs are composed of anisotropic building blocks (usually of rod or disc shape), which are spontaneously oriented along a specific direction, called the director n [4]. Without an external alignment force, the director of a nematic LC, the simplest phase of LC, whose molecules are only orientationally ordered, is usually spatially changed continuously but randomly over large spatial extensions (except for defects, where the director may vary suddenly and drastically) [5,6][5][6].
Normally, one can split LCs into two typical categories, i.e., thermotropic LCs and lyotropic LCs [7,8,9,10][7][8][9][10]. Thermotropic LCs are usually further distinguished according to the shape of their constituent molecules, being called calamitic for rod-like, discotic for disk-like and sanidic for brick- or lath-like molecules (Figure 1a) [11]. A typical calamitic mesogen is generally composed of a rigid core, often incorporating phenyl and biphenyl groups, and two flexible endgroups, often alkyl or alkoxy chains. A common structure of discotic mesogens is a rigid, disk-like core to which six flexible endgroups are attached. Apart from these conventional mesogens, research attention has been recently focused on the so-called non-conventional LCs [12], which are neither rod- nor disk-shaped. Among them, bent-core LCs [13], in particular, have received attention due to their unique effects of the observation of chirality from achiral molecules, resulting from sterically induced packing of the bent-core mesogens [10], such as ferroelectricity or the formation of helical superstructures in the B7 phase [14]. Thermotropic LCs are commonly constituted by single organic entities or mixtures thereof, which exhibit various mesophases at different temperatures or pressures [15], illustrated in Figure 1b. As the temperature rises, a typical thermotropic LC passes through higher ordered phases, also called soft crystals, the hexatic smectic phases with positional order as well as bond orientational order, through the fluid smectic phases (SmC and SmA), which exhibit both positional and orientational order, and finally to the nematic phase (N) with purely orientational order, into the isotropic phase. The number of different phases observed depends on the chemical composition, symmetry and order of the LC molecules. About 25 different thermotropic phases are known to date, and they are still increasing in number.
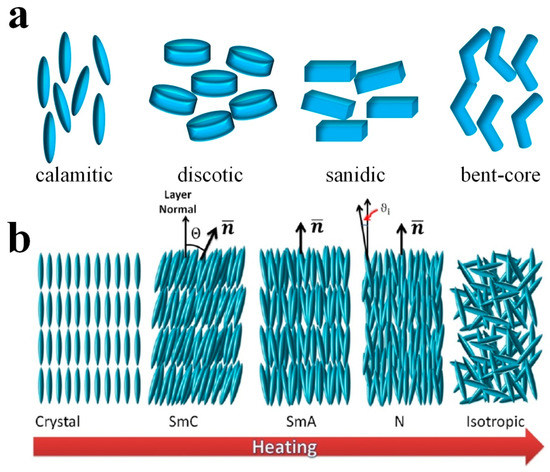
(
) The various shapes of LC molecules. (
) A typical illustration of different thermotropic LC phases observed on heating from crystalline state. Reproduced with permission from [7]. Copyright (2017) MDPI.
In contrast, lyotropic LCs consist of at least two different kinds of components: a collection of anisodiametric molecules and particles dispersed in a suitable solvent (Figure 2). These systems will thus always be mixtures, with the main control variable being the concentration. Unlike thermotropics, the phase transitions of lyotropic LCs are not merely dependent on the temperature of the system, but also, mainly, on the relative concentration of each component [15]. Being mixtures, there will always be two-phase regions at the phase boundaries between two different lyotropic phases. One can distinguish lyotropic LCs into three different kinds: (i) amphiphilic lyotropics, (ii) colloidal lyotropics, and (iii) chromonics, where the constituent molecules are dye molecules in a suitable solvent. As the name indicates, amphiphilic lyotropic LCs are usually composed of amphiphilic molecules upon addition of a solvent [16], often water. As Figure 2 shows, with increasing amphiphile concentration, due to the segregation of hydrophobic and hydrophilic regions, these molecules self-assemble into spherical or rod-like micelles, leading to the formation of the hexagonal phase, cubic phase or lamellar phase. Nevertheless, the solute component of a lyotropic LC need not always be molecular in nature, but may also consist of much larger (solid) particles with anisotropic shapes. It can also be of colloidal size [7], as we will discuss further in Section 3[7]. Such materials would then be known as inorganic liquid crystals, LC clays, but also nanotubes, graphene oxide or biological structures such as viruses (Figure 3).
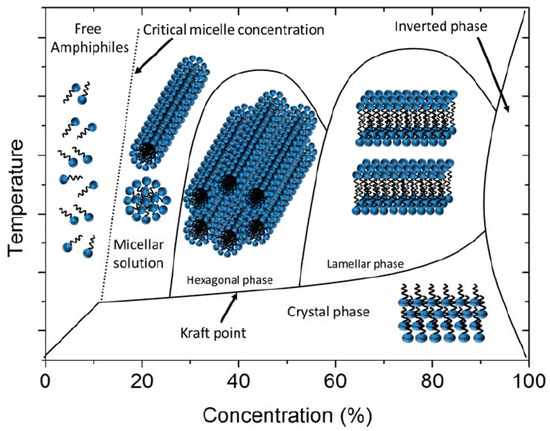
A typical lyotropic LC phase diagram of amphiphilic molecules dissolved in a solvent. Cubic phases may be observed at different positions in the phase diagram. At very high concentrations, the inverse phases are located. Reproduced with permission from [7]. Copyright (2017) MDPI.
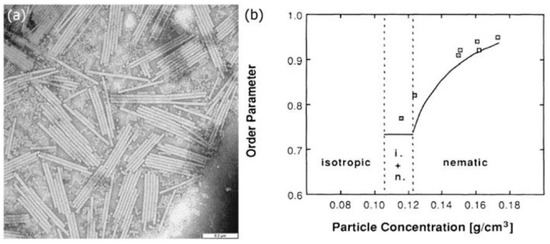
(
) SEM image of a collection of tobacco mosaic virus particles. Scale bar: 0.2 μm. Reproduced from Wikimedia Commons, with no author name supplied. (
) The orientational order parameter of the viruses increases as their concentration in the solvent is increased. Reproduced with permission from [17]. Copyright (1988) American Physical Society.
The fact that the director of LCs, which is often equivalent to the optic axis of the material, is easily influenced by a variety of external stimuli such as mechanical, magnetic, electric, or optic fields, as well as temperature, makes liquid crystals attractive to both the industry and academia [6[6][18][19][20][21][22][23][24][25][26][27],17,18,19,20,21,22,23,24,25,26], as exemplified by the $100 billion industry built around displays and large-screen, flat-panel consumer electronics. Due to the great achievement in displays since the 1970s, LCs are one of the most popular materials around the world [27][28]. However, with the rapid development and impressive advantages of organic light-emitting displays (OLEDs), there is some competition emerging. Over the last decade, more and more scientists have moved their attention away from display materials to a diversity of new fields—for instance, new optical devices, telecommunication, information storage, energy conservation, elastomer robots, sensors, biotechnologies, nano-/micromanipulation, just to name a few [15,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45][15][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46]. These new fields can be quite different from displays and more stimulating due to their novelty, leading to a new era for LCs, materials design and technology.
At the same time, revolutionary developments have been made in the fields of nanotechnology and nanoscience, leading to the birth of a series of novel nanomaterials [10,47,48,49,50,51,52,53,54,55][10][47][48][49][50][51][52][53][54][55]. These nanostructured materials, whose size in at least one dimension is in the range from 1 nm to 100 nm, have gained a wealth of academic and industrial attention due to their size-dependent electronic, optical, magnetic, and chemical properties, which are significantly different from those of bulk materials as well as from individual atoms or molecules [55,56,57,58,59,60,61,62][55][56][57][58][59][60][61][62]. These nanostructured materials have been extensively applied in nearly every field of science from energy, optics, computing to catalysis, biosciences and medical sciences [48,63,64,65][48][63][64][65]. Undoubtedly, when these novel nanometre-scale structures encounter liquid crystals, a highly interesting and unique synergy will be observed, leading to an abundance of entirely new and potential applications [2,8,42,66,67,68,69,70][2][8][43][66][67][68][69][70].
The addition of nanomaterials to a LC material produces a composite or colloidal dispersion [71,72][71][72]. The new materials are expected to behave differently from their individual components (nanomaterials and LCs) both on the microscopic as well as the macroscopic scale [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77]. There are three basic objectives to making a LC–nanomaterial composite system: (i) to modify the primary physical or chemical properties of the pristine LCs [78,79,80][78][79][80]; (ii) manipulating and ordering nanomaterials in LCs to modify the properties of nanomaterials [81]; and (iii) to obtain additional functionalities that are available from neither the LCs nor the nanomaterials in their intrinsic states [82].
A fundamental investigation of the nanophysics of LCs was published by Brochard and de Gennes, who discussed a theory of magnetic nanoparticles suspended in a nematic LCs in 1970 [83]. Later in the same year, Rault et al. reported the first suspension composed of nematic LCs and small magnetic particles [84]. The main idea of these pioneering studies was to control the director of the LC hosts by a coupling between LC molecules and elongated magnetic particles, producing what has been called magneto-nematics, i.e., nematic liquid crystals with ferromagnetic properties. In fact, it should be mentioned that this conclusion is wrong, as the produced system is not ferromagnetic. As the magnetic field is removed, the magnetization does in fact decrease to zero, while it should retain a finite value for ferromagnetics. Nevertheless, one could call the produced system an anisotropic ferrofluid. Still, this idea led the trend of studies on LC–nanomaterial suspensions for the next few decades. With the rapid development of composite LC materials, it was recognized that long-range orientation and interactions in mesophase can lead to a strong impact of nanomaterials on the properties of the LC hosts [85,86,87][85][86][87] and vice versa; the LC matrix can rearrange the orientational and positional order of nanomaterials [88,89][88][89]. The coupling of long-range ordering in LCs and the unique properties of dopant nanomaterials allows us to change or even impose unique physical properties on the LC–nanomaterial composites by doping different kinds of nanomaterials in LCs [70].
Up to now, there have been four primary groups of nanomaterials used as dopants in LCs, i.e., metal nanoparticles, ferroelectric nanoparticles, semi-conductor nanoparticles, and carbon nanoparticles. Among these dispersions, improved physical characteristics and novel functionalities can be obtained depending on the physical and chemical characteristics of the dopants as well as the interaction between LCs and nanomaterials [90,91,92,93,94,95][90][91][92][93][94][95]. For example, there are abundant reports declaring that doping metallic, semiconducting, oxide and ferroelectric nanoparticles into LCs can efficiently modify the electrical and mechanical (viscoelastic) properties of LC host, leading to large dielectric and optical anisotropy, low threshold voltage, and an improved electro-optical response [87,92,94,96,97][87][92][94][96][97]. In addition, ferroelectric nanoparticles can also increase the order parameter as well as the clearing point of the LC host due to the interaction between the elastic forces of LC molecules and the spontaneous polarization of ferroelectric nanoparticles [86,98,99,100,101][86][98][99][100][101]. Doping nematic LCs with ferromagnetic particles can effectively reorient the director of LCs by magnetic fields induced by the coupling of magnetic particles with LC molecules [83,102,103,104,105,106][83][102][103][104][105][106]. At the same time, there have been reports claiming the exact opposite behaviour: an increase in threshold voltage, a slower optical response, and a decrease in order and in transition temperatures. This indicates that the properties of the composite materials are very much dependent on the dopant size, materials employed, preparation conditions and time. It further indicates that a lot more research is needed to fully understand these systems. With largely varying results, even on a qualitative basis, the field of liquid crystal nanoparticle composites is only in its infancy.
Metal and semiconducting nanoparticles have been used to stabilise the mesophase known as the blue phase, effectively broadening the temperature range of this frustrated phase from 1 K to up to 20 K [107,108][107][108]. Silica, ferroelectric and metal nanoparticles can effectively influence the electro-optical response of nematic LCs and induce a memory effect, i.e., a residual transmittance can be maintained without external electric fields being applied [109,110,111,112][109][110][111][112]. Moreover, a frequency-dependent electro-optical response can be obtained in suspensions of metal nanoparticles and nematic LCs due to the coupling of the dielectric properties of the nanoparticles and the LCs [78,113][78][113]. It was also reported that carbon nanotubes dispersed in nematic LCs would align along the direction of the LC director, therefore largely changing the conductivity depending on the orientation of the tubes [114]. In fact, it could be shown that the nanotubes can be reoriented elastically by reorienting the director through applied electric or magnetic fields. Fullerenes could effectively improve the switching speed of a nematic host LC [95], and improved electro-optic responses could be induced by doping graphene oxides in LCs [115].
In addition to changing the intrinsic physical properties of the pristine LC materials mentioned above, intriguing photonic functionalities can also be introduced into the composites by combining nanomaterials with LCs, which is considered a promising method for the building of novel metamaterials [116]. Metamaterials are artificial engineered bulks with regular nanostructures that show exotic optical properties [117]. Combining the emerging field of optical metamaterials with LCs provides an extremely attractive quality, i.e., tunability, which is of the utmost importance in applications such as optically addressed spatial light modulators, tunable photonic materials and dynamic holography [118,119,120][118][119][120]. When integrating LCs with metamaterials, one can modify the director alignment of LCs by applying external stimuli and therefore manipulating the overall optical characteristics of the composite [119]. For instance, upon periodically embedding particular metal nanomaterials, such as gold nanoparticles, into LC matrixes, a localized surface plasmon resonance (LSPR) will be obtained, which can be tuned by adjusting the birefringence of the surrounding LC matrix [77,81,121,122,123,124,125][77][81][121][122][123][124][125]. Apart from the abovementioned technologies, novel photonic properties and applications can be further exploited for nanodopants including semiconducting nanomaterials, dyes, oxides, quantum dots (QDs), etc. [126,127,128,129][126][127][128][129]. Instead of the SPR effect in the metallic nanomaterial–LC colloidal systems, the main property of interest in these cases is photoluminescence. The optical excitation and emission of these nanomaterials could be tuned effectively through the interaction between nanomaterials and the long-range ordered LC molecules, leading to a series of potential applications such as information storage, displays, LC lasers, etc. [130,131,132,133,134][130][131][132][133][134].
At present, one of the central challenges faced by the development of novel LC–nanomaterial composites is reliable assembly of nanoscale building blocks into functional bulk materials by using distorted LCs. It has already been demonstrated that the localisation of a nanoparticle in a distorted LC region allows the decrease of the free energy, and the directional motion of nanomaterials in LCs can be driven by the collective long-range interactions mediated by the LC director field. As a result, the energetic cost of LC defects enables them to entrap and reorient nanomaterials in a reversible manner and with well-manipulated position and orientation [135,136,137,138][135][136][137][138]. In addition, nanomaterials that are suspended in LCs and have a given anchoring energy at the surface will induce a deformation of the orientation in the surrounding LCs, producing topological defects in the vicinity of the nanoparticle. The elastic distortion exerts a force on neighbouring nanomaterials at a range of up to a few micrometres, resulting in defect lines or points around nanomaterials [139,140,141,142,143,144][139][140][141][142][143][144]. This LC-mediated interaction can be either repulsive or attractive and, as a consequence, can be used as an effective method to manipulate the spatial distance between nanoparticles [88,145,146,147,148][88][145][146][147][148]. It is thus anticipated that, by coupling these two LC-mediated effects will result in the assembly of nanomaterials with one-, two- or even three-dimensional (3D) periodic superstructures [143,149,150,151,152,153,154][143][149][150][151][152][153][154]. Due to the great potential in both fundamental science and applications, this top-down assembly approach, where spontaneous or artificially generated LC textures are utilized as templates for organizing nanoparticles on controlled lattices or confining them in designed defects, has been increasingly coming into the spotlight.
Another interesting research field of LC–nanomaterial technology that is worthwhile to discuss is the self-assembly of lyotropic LCs composed of 1- or 2D anisotropic nanomaterials [3,7][3][7]. This topic has been investigated from time to time as people have realized that suspensions of anisodiametric nanomaterials can form LC phases, even at a very low concentration, due to Bernal’'s seminal work on suspensions of tobacco mosaic virus [155] and Onsager’'s theory of the excluded volume mechanism [156]. However, with the boom in the development of nanotechnology and nanoscience in the last decade, various types of novel nanomaterials have emerged. The size dispersion, shape anisotropy, and surface morphology of the nanomaterials are now well controlled. All of these developments will definitely lead to a dramatic increase in the use of nanoparticle-based LCs. Nowadays, it is well known that suspensions of anisotropic nanorods or nanoplates, viruses, nano-cellulose, carbon nanotubes and graphene oxide can form lyotropic LC phases, which are usually accompanied by surprising physical properties [157,158,159,160,161,162,163,164,165][157][158][159][160][161][162][163][164][165].
2. Summary
Here, we summarized the influence of nanoparticle doping on the electro-optical and other physical properties, of liquid crystals, including molecule alignment, viscosity, clearing point, or elastic constants, just to name a few of the properties introduced. Various kinds of nanodopants, such as ferroelectric nanoparticles, noble metallic nanoparticles, semiconductor nanoparticles and carbon nanoparticles, have been presented to induce modifications of the physical properties of LCs. However, these studies create an inconsistent picture of the beneficial effects of nanoparticles on the physical properties of LC hosts. This may be attributed to the effect of different nanoparticles’' size, shape, dispersibility and functionalization. To thoroughly understand the influence of nanoparticle doping on the physical properties of LCs, further systematic investigations are required. We further demonstrated the exciting photonic functionalities, including SPR effect, photothermal effect and lasing, of LC–nanoparticle composites. The intrinsic optical properties of nanoparticles can be effectively modified by a LC medium, leading to a variety of multifunctional photonic applications.
Various nanoparticle mesostructures, obtained either within distorted areas of LCs or topological defects, were discussed. The self-organization-assisted nanoparticle assembly within the disclination lines will effectively eliminate high-energy volume areas and reduce the elastic-free energy density of the LC system. Such an effect can even lead to a stabilization of the 3D blue phase defect lattice.
Additionally, we demonstrated the self-assembly of nanoparticles into lyotropic LC phases. Colloidal suspensions of anisotropic particles, including rods, tubes, disks, flexible chains and wires, can self-organize from the isotropic disordered to the nematic ordered phase as the concentration exceeds a critical value according to Onsager’'s model. These lyotropic LC materials have intriguing physical properties and can be applied to various functional devices.
Finally, we briefly introduce the practical applications of LC–nanoparticle composites in biological systems. The aspects of biological sensors and drug delivery systems are of particular interest, and it is anticipated that this area of novel liquid crystal research will attract much interest in the liquid crystal community and soft matter community in particular.
References
- Tschierske, C. Liquid Crystals: Materials Design and Self-Assembly; Springer Science & Business Media: Berlin, Germany, 2012; Volume 318.
- Bisoyi, H.K.; Kumar, S. Liquid-crystal nanoscience: An emerging avenue of soft self-assembly. Chem. Soc. Rev. 2011, 40, 306–319.
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. Int. Ed. 2005, 45, 38–68.
- Ohzono, T.; Fukuda, J.I. Zigzag line defects and manipulation of colloids in a nematic liquid crystal in microwrinkle grooves. Nat. Commun. 2012, 3, 701.
- Chuang, I.; Durrer, R.; Turok, N.; Yurke, B. Cosmology in the laboratory: Defect dynamics in liquid crystals. Science 1991, 251, 1336–1342.
- Pieranski, P.; Yang, B.; Burtz, L.-J.; Camu, A.; Simonetti, F. Generation of umbilics by magnets and flows. Liq. Cryst. 2013, 40, 1593–1608.
- Dierking, I.; Al-Zangana, S. Lyotropic liquid crystal phases from anisotropic nanomaterials. Nanomaterials 2017, 7, 305.
- Saliba, S.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Liquid crystalline thermotropic and lyotropic nanohybrids. Nanoscale 2013, 5, 6641–6661.
- Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; et al. Discotic liquid crystals: From tailor-made synthesis to plastic electronics. Angew. Chem. Int. Ed. 2007, 46, 4832–4887.
- Hegmann, T.; Qi, H.; Marx, V.M. Nanoparticles in liquid crystals: Synthesis, self-assembly, defect formation and potential applications. J. Inorg. Organomet. Polym. Mater. 2007, 17, 483–508.
- Dierking, I. Textures of Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2003.
- Tschierske, C. Non-conventional liquid crystals—the importance of micro-segregation for self-organisation. J. Mater. Chem. 1998, 8, 1485–1508.
- Takezoe, H.; Eremin, A. Bent-Shaped Liquid Crystals: Structures and Physical Properties; CRC Press: Boca Raton, FL, USA, 2017.
- Coleman, D.; Fernsler, J.; Chattham, N.; Nakata, M.; Takanishi, Y.; Körblova, E.; Link, D.; Shao, R.-F.; Jang, W.; Maclennan, J. Polarization-modulated smectic liquid crystal phases. Science 2003, 301, 1204–1211.
- Lagerwall, J.P.F.; Scalia, G. A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412.
- Park, H.-S.; Lavrentovich, O.D. Lyotropic chromonic liquid crystals: Emerging applications. Liq. Cryst. Beyond Disp. 2012, doi:10.1002/9781118259993.
- Oldenbourg, R.; Wen, X.; Meyer, R.B.; Caspar, D.L.D. Orientational distribution function in nematic tobacco-mosaic-virus liquid crystals measured by X-ray diffraction. Phys. Rev. Lett. 1988, 61, 1851–1854.
- Zheng, Z.G.; Li, Y.; Bisoyi, H.K.; Wang, L.; Bunning, T.J.; Li, Q. Three-dimensional control of the helical axis of a chiral nematic liquid crystal by light. Nature 2016, 531, 352–356.
- Li, S.-S.; Shen, Y.; Chang, Z.-N.; Li, W.-S.; Xu, Y.-C.; Fan, X.-Y.; Chen, L.-J. Dynamic cholesteric liquid crystal superstructures photoaligned by one-step polarization holography. Appl. Phys. Lett. 2017, 111, 231109.
- Zheng, Z.G.; Zola, R.S.; Bisoyi, H.K.; Wang, L.; Li, Y.; Bunning, T.J.; Li, Q. Controllable dynamic zigzag pattern formation in a soft helical superstructure. Adv. Mater. 2017, 29, 1701903.
- Bisoyi, H.K.; Bunning, T.J.; Li, Q. Stimuli-driven control of the helical axis of self-organized soft helical superstructures. Adv. Mater. 2018, 30, e1706512.
- Shen, Y.; Xu, Y.C.; Ge, Y.H.; Jiang, R.G.; Wang, X.Z.; Li, S.S.; Chen, L.J. Photoalignment of dye-doped cholesteric liquid crystals for electrically tunable patterns with fingerprint textures. Opt. Express 2018, 26, 1422–1432.
- Subacius, D.; Bos, P.J.; Lavrentovich, O.D. Switchable diffractive cholesteric gratings. Appl. Phys. Lett. 1997, 71, 1350–1352.
- Bisoyi, H.K.; Urbas, A.M.; Li, Q. Soft materials driven by photothermal effect and their applications. Adv. Opt. Mater. 2018, 6, 1800458.
- Ma, L.-L.; Li, S.-S.; Li, W.-S.; Ji, W.; Luo, B.; Zheng, Z.-G.; Cai, Z.-P.; Chigrinov, V.; Lu, Y.-Q.; Hu, W.; et al. Rationally designed dynamic superstructures enabled by photoaligning cholesteric liquid crystals. Adv. Opt. Mater. 2015, 3, 1691–1696.
- Lin, Y.; Yang, Y.; Shan, Y.; Gong, L.; Chen, J.; Li, S.; Chen, L. Magnetic nanoparticle-assisted tunable optical patterns from spherical cholesteric liquid crystal bragg reflectors. Nanomaterials 2017, 7, 376.
- O’Neill, M.; Kelly, S.M. Ordered materials for organic electronics and photonics. Adv. Mater. 2010, 23, 566–584.
- Sano, S.; Miyama, T.; Takatoh, K.; Kobayashi, S. Enhancement of the characteristics of lcds by doping nanoparticles: Reduction of the operating voltage, viscosity, and response times. In Proceedings of the Integrated Optoelectronic Devices 2006, San Jose, CA, USA, 2006; SPIE: Bellingham, WA, USA, 2006; p.613501.
- Stannarius, R. Liquid crystals: More than display fillings. Nat. Mater. 2009, 8, 617–618.
- Ryabchun, A.; Bobrovsky, A. Cholesteric liquid crystal materials for tunable diffractive optics. Adv. Opt. Mater. 2018, 6, 1800335.
- Li, W.-S.; Ma, L.-L.; Gong, L.-L.; Li, S.-S.; Yang, C.; Luo, B.; Hu, W.; Chen, L.-J. Interlaced cholesteric liquid crystal fingerprint textures via sequential uv-induced polymer-stabilization. Opt. Mater. Express 2016, 6, 19–28.
- Li, W.-S.; Shen, Y.; Chen, Z.-J.; Cui, Q.; Li, S.-S.; Chen, L.-J. Demonstration of patterned polymer-stabilized cholesteric liquid crystal textures for anti-counterfeiting two-dimensional barcodes. Appl. Opt. 2017, 56, 601–606.
- Huang, W.; Yuan, C.L.; Shen, D.; Zheng, Z.G. Dynamically manipulated lasing enabled by a reconfigured fingerprint texture of a cholesteric self-organized superstructure. J. Mater. Chem. C 2017, 5, 6923–6928.
- Price, A.D.; Schwartz, D.K. DNA hybridization-induced reorientation of liquid crystal anchoring at the nematic liquid crystal/aqueous interface. J. Am. Chem. Soc. 2008, 130, 8188–8194.
- Sivakumar, S.; Wark, K.L.; Gupta, J.K.; Abbott, N.L.; Caruso, F. Liquid crystal emulsions as the basis of biological sensors for the optical detection of bacteria and viruses. Adv. Funct. Mater. 2009, 19, 2260–2265.
- Chen, C.-H.; Yang, K.-L. Detection and quantification of DNA adsorbed on solid surfaces by using liquid crystals. Langmuir 2010, 26, 1427–1430.
- Spicer, P.T. Progress in liquid crystalline dispersions: Cubosomes. Curr. Opin. Colloid Interface Sci. 2005, 10, 274–279.
- Sagalowicz, L.; Leser, M.E.; Watzke, H.J.; Michel, M. Monoglyceride self-assembly structures as delivery vehicles. Trends Food Sci. Technol. 2006, 17, 204–214.
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147-148, 333-342.
- Mushenheim, P.C.; Trivedi, R.R.; Weibel, D.B.; Abbott, N.L. Using liquid crystals to reveal how mechanical anisotropy changes interfacial behaviors of motile bacteria. Biophys. J. 2014, 107, 255–265.
- Zhou, S.; Sokolov, A.; Lavrentovich, O.D.; Aranson, I.S. Living liquid crystals. Proc. Natl. Acad. Sci. USA 2014, 111, 1265.
- Kim, Y.H.; Yoon, D.K.; Jeong, H.S.; Lavrentovich, O.D.; Jung, H.-T. Smectic liquid crystal defects for self-assembling of building blocks and their lithographic applications. Adv. Funct. Mater. 2011, 21, 610–627.
- Tschierske, C. Liquid crystal engineering—new complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970.
- Zhou, S. Living liquid crystals. In Lyotropic Chromonic Liquid Crystals: From Viscoelastic Properties to Living Liquid Crystals; Zhou, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp 77–92.
- Wang, L.; Ge, S.; Hu, W.; Nakajima, M.; Lu, Y. Tunable reflective liquid crystal terahertz waveplates. Opt. Mater. Express 2017, 7, 2023.
- Kularatne, R.S.; Kim, H.; Boothby, J.M.; Ware, T.H. Liquid crystal elastomer actuators: Synthesis, alignment, and applications. J. Polym. Sci. B Polym. Phys. 2017, 55, 395–411.
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346.
- Kim, F.S.; Ren, G.; Jenekhe, S.A. One-dimensional nanostructures of π-conjugated molecular systems: Assembly, properties, and applications from photovoltaics, sensors, and nanophotonics to nanoelectronics. Chem. Mater. 2010, 23, 682–732.
- Hamley, I.W. Nanotechnologie mit weichen Materialien. Angew. Chem. 2003, 115, 1730–1752.
- Anisa, M.; Abdallah, S.D.; Peter, A.S. ‘Mind the gap’: Science and ethics in nanotechnology. Nanotechnology 2003, 14, R9.
- Gupta, S.M.; Tripathi, M. A review of TO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639.
- Koch, U.; Fojtik, A.; Weller, H.; Henglein, A. Photochemistry of semiconductor colloids. Preparation of extremely small ZnO particles, fluorescence phenomena and size quantization effects. Chem. Phys. Lett. 1985, 122, 507–510.
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301.
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Jiang, H.; Shao, W.; Zhang, L.; He, Y. Synthesis and optical properties of TiO2 nanoparticles. Mater. Lett. 2007, 61, 79–83.
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102.
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent advances of plasmonic nanoparticles and their applications. Materials 2018, 11, 1833.
- Zhang, S.; Pelligra, C.I.; Feng, X.; Osuji, C.O. Directed assembly of hybrid nanomaterials and nanocomposites. Adv. Mater. 2018, 30, 1705794.
- Meng, Q.B.; Fu, C.H.; Einaga, Y.; Gu, Z.Z.; Fujishima, A.; Sato, O. Assembly of highly ordered three-dimensional porous structure with nanocrystalline TiO2 semiconductors. Chem. Mater. 2002, 14, 83–88.
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853.
- Robel, I.; Kuno, M.; Kamat, P.V. Size-dependent electron injection from excited CdSe quantum dots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 4136–4137.
- Hyun, B.-R.; Zhong, Y.-W.; Bartnik, A.C.; Sun, L.; Abruña, H.D.; Wise, F.W.; Goodreau, J.D.; Matthews, J.R.; Leslie, T.M.; Borrelli, N.F. Electron injection from colloidal PbS quantum dots into titanium dioxide nanoparticles. ACS Nano 2008, 2, 2206–2212.
- Tripathi, A.K.; Singh, M.K.; Mathpal, M.C.; Mishra, S.K.; Agarwal, A. Study of structural transformation in TiO2 nanoparticles and its optical properties. J. Alloy Compd. 2013, 549, 114–120.
- Chik, M.W.; Hussain, Z.; Zulkefeli, M.; Tripathy, M.; Kumar, S.; Majeed, A.B.A.; Byrappa, K. Polymer-wrapped single-walled carbon nanotubes: A transformation toward better applications in healthcare. Drug Deliv. Transl. Res. 2018, 9, 578–594.
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20.
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed self-assembly of nanoparticles. ACS Nano 2010, 4, 3591–3605.
- Goodby, J.W.; Saez, I.M.; Cowling, S.J.; Görtz, V.; Draper, M.; Hall, A.W.; Sia, S.; Cosquer, G.; Lee, S.-E.; Raynes, E.P. Transmission and amplification of information and properties in nanostructured liquid crystals. Angew. Chem. Int. Ed. 2008, 47, 2754–2787.
- Qi, H.; Hegmann, T. Impact of nanoscale particles and carbon nanotubes on current and future generations of liquid crystal displays. J. Mater. Chem. 2008, 18, 3288–3294.
- Blanc, C.; Coursault, D.; Lacaze, E. Ordering nano- and microparticles assemblies with liquid crystals. Liq. Cryst. Rev. 2013, 1, 83–109.
- Shivakumar, U.; Mirzaei, J.; Feng, X.; Sharma, A.; Moreira, P.; Hegmann, T. Nanoparticles: Complex and multifaceted additives for liquid crystals. Liq. Cryst. 2011, 38, 1495–1514.
- Dierking, I. Nanomaterials in liquid crystals. Nanomaterials 2018, 8, 453.
- Garbovskiy, Y.A.; Glushchenko, A.V. Liquid crystalline colloids of nanoparticles. In Solid State Physics; Academic Press: Cambridge, MA, USA, 2010; Volume 62, pp. 1–74.
- Poulin, P.; Stark, H.; Lubensky, T.C.; Weitz, D.A. Novel colloidal interactions in anisotropic fluids. Science 1997, 275, 1770.
- Khoo, I.; Chen, K.; Williams, Y.Z. Orientational photorefractive effect in undoped and cdse nanorods-doped nematic liquid crystal—Bulk and interface contributions. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 443–450.
- Khoo, I.C.; Williams, Y.Z.; Lewis, B.; Mallouk, T. + − Photorefractive CdSe and gold nanowire-doped liquid crystals and polymer-dispersed-liquid-crystal photonic crystals. Mol. Cryst. Liq. Cryst. 2006, 446, 233–244.
- Kinkead, B.; Hegmann, T. Effects of size, capping agent, and concentration of CdSe and CdTe quantum dots doped into a nematic liquid crystal on the optical and electro-optic properties of the final colloidal liquid crystal mixture. J. Mater. Chem. 2010, 20, 448–458.
- Bartkiewicz, S.; Matczyszyn, K.; Miniewicz, A.; Kajzar, F. High gain of light in photoconducting polymer–nematic liquid crystal hybrid structures. Opt. Commun. 2001, 187, 257–261.
- Müller, J.; Sönnichsen, C.; von Poschinger, H.; von Plessen, G.; Klar, T.A.; Feldmann, J. Electrically controlled light scattering with single metal nanoparticles. Appl. Phys. Lett. 2002, 81, 171–173.
- Tomohiro, M.; Jirakorn, T.; Hiroyuki, S.; Yoshio, S.; Yukihide, S.; Naoki, T.; Shunsuke, K. Fast switching of frequency modulation twisted nematic liquid crystal display fabricated by doping nanoparticles and its mechanism. Jpn. J. Appl. Phys. 2004, 43, 2580.
- Rajiv, M.; Sat Prakash, Y.; Abhishek Kumar, S.; Abhishek Kumar, M.; Kamal Kumar, P.; Prashant, K.S.; Avinash Chand, P. Zinc oxide (1% Cu) nanoparticle in nematic liquid crystal: Dielectric and electro-optical study. Jpn. J. Appl. Phys. 2009, 48, 101501.
- Li, X.; Yang, C.; Wang, Q.; Jia, D.; Hu, L.; Peng, Z.; Xuan, L. Enhanced birefringence for metallic nanoparticle doped liquid crystals. Opt. Commun. 2013, 286, 224–227.
- Park, S.Y.; Stroud, D. Surface-enhanced plasmon splitting in a liquid-crystal-coated gold nanoparticle. Phys. Rev. Lett. 2005, 94, 217401.
- Kossyrev, P.A.; Yin, A.; Cloutier, S.G.; Cardimona, D.A.; Huang, D.; Alsing, P.M.; Xu, J.M. Electric field tuning of plasmonic response of nanodot array in liquid crystal matrix. Nano Lett. 2005, 5, 1978–1981.
- Brochard, F.; De Gennes, P. Theory of magnetic suspensions in liquid crystals. J. Phys. 1970, 31, 691–708.
- Rault, J.; Cladis, P.E.; Burger, J.P. Ferronematics. Phys. Lett. A 1970, 32, 199–200.
- Kaczmarek, M.; Buchnev, O.; Nandhakumar, I. Ferroelectric nanoparticles in low refractive index liquid crystals for strong electro-optic response. Appl. Phys. Lett. 2008, 92, 103307.
- Li, F.; Buchnev, O.; Cheon, C.I.; Glushchenko, A.; Reshetnyak, V.; Reznikov, Y.; Sluckin, T.J.; West, J.L. Orientational coupling amplification in ferroelectric nematic colloids. Phys. Rev. Lett. 2006, 97, 147801.
- Kaur, S.; Singh, S.P.; Biradar, A.M.; Choudhary, A.; Sreenivas, K. Enhanced electro-optical properties in gold nanoparticles doped ferroelectric liquid crystals. Appl. Phys. Lett. 2007, 91, 023120.
- Yada, M.; Yamamoto, J.; Yokoyama, H. Direct observation of anisotropic interparticle forces in nematic colloids with optical tweezers. Phys. Rev. Lett. 2004, 92, 185501.
- Smalyukh, I.I.; Kuzmin, A.N.; Kachynski, A.V.; Prasad, P.N.; Lavrentovich, O.D. Optical trapping of colloidal particles and measurement of the defect line tension and colloidal forces in a thermotropic nematic liquid crystal. Appl. Phys. Lett. 2005, 86, 021913.
- Lapanik, A.; Rudzki, A.; Kinkead, B.; Qi, H.; Hegmann, T.; Haase, W. Electrooptical and dielectric properties of alkylthiol-capped gold nanoparticle–ferroelectric liquid crystal nanocomposites: Influence of chain length and tethered liquid crystal functional groups. Soft Matter 2012, 8, 8722–8728.
- Shukla, R.K.; Feng, X.; Umadevi, S.; Hegmann, T.; Haase, W. Influence of different amount of functionalized bulky gold nanorods dopant on the electrooptical, dielectric and optical properties of the flc host. Chem. Phys. Lett. 2014, 599, 80–85.
- Joshi, T.; Kumar, A.; Prakash, J.; Biradar, A.M. Low power operation of ferroelectric liquid crystal system dispersed with zinc oxide nanoparticles. Appl. Phys. Lett. 2010, 96, 253109.
- Lisetski, L.N.; Minenko, S.S.; Zhukov, A.V.; Shtifanyuk, P.P.; Lebovka, N.I. Dispersions of carbon nanotubes in cholesteric liquid crystals. Mol. Cryst. Liq. Cryst. 2009, 510, 43–50.
- Singh, U.B.; Dhar, R.; Dabrowski, R.; Pandey, M.B. Enhanced electro-optical properties of a nematic liquid crystals in presence of BaTiO3 nanoparticles. Liq. Cryst. 2014, 41, 953–959.
- San, S.E.; Okutan, M.; Köysal, O.; Yerli, Y. Carbon nanoparticles in nematic liquid crystals. Chin. Phys. Lett. 2008, 25, 212.
- Zhao, D.; Xu, L.; Shang, Y.; Li, X.; Guo, L. Facet-dependent electro-optical properties of cholesteric liquid crystals doped with Cu2O nanocrystals. Nano Res. 2018, 11, 4836–4845.
- Williams, Y.; Chan, K.; Park, J.H.; Khoo, I.C.; Lewis, B.; Mallouk, T.E. Electro-Optical and Nonlinear Optical Properties of Semiconductor Nanorod Doped Liquid Crystals, In Proceedings of the Optics and Photonics 2005, San Diego, CA, USA, 22 July 2005; SPIE: Bellingham, WA, USA, 2005, p. 593613.
- Garbovskiy, Y.; Glushchenko, A. Ferroelectric nanoparticles in liquid crystals: Recent progress and current challenges. Nanomaterials 2017, 7, 361.
- Shelestiuk, S.M.; Reshetnyak, V.Y.; Sluckin, T.J. Frederiks transition in ferroelectric liquid-crystal nanosuspensions. Phys. Rev. E 2011, 83, 041705.
- Kurochkin, O.; Buchnev, O.; Iljin, A.; Park, S.K.; Kwon, S.B.; Grabar, O.; Yu, R. A colloid of ferroelectric nanoparticles in a cholesteric liquid crystal. J. Opt. A Pure Appl. Opt. 2009, 11, 024003.
- Lopatina, L.M.; Selinger, J.V. Maier-saupe-type theory of ferroelectric nanoparticles in nematic liquid crystals. Phys. Rev. E 2011, 84, 041703.
- Chen, S.-H.; Amer, N.M. Observation of macroscopic collective behavior and new texture in magnetically doped liquid crystals. Phys. Rev. Lett. 1983, 51, 2298–2301.
- Martínez-Miranda, L.J.; McCarthy, K.; Kurihara, L.K.; Harry, J.J.; Noel, A. Effect of the surface coating on the magnetic nanoparticle smectic-a liquid crystal interaction. Appl. Phys. Lett. 2006, 89, 161917.
- Kopčanský, P.; Tomašovičová, N.; Koneracká, M.; Závišová, V.; Timko, M.; Džarová, A.; Šprincová, A.; Éber, N.; Fodor-Csorba, K.; Tóth-Katona, T.; et al. Structural changes in the 6chbt liquid crystal doped with spherical, rodlike, and chainlike magnetic particles. Phys. Rev. E 2008, 78, 011702.
- Mertelj, A.; Lisjak, D.; Drofenik, M.; Čopič, M. Ferromagnetism in suspensions of magnetic platelets in liquid crystal. Nature 2013, 504, 237.
- Mertelj, A.; Osterman, N.; Lisjak, D.; Čopič, M. Magneto-optic and converse magnetoelectric effects in a ferromagnetic liquid crystal. Soft Matter 2014, 10, 9065–9072.
- Hiroyuki, Y.; Yuma, T.; Kosuke, K.; Hitoshi, K.; Tetsuya, T.; Akihiko, F.; Susumu, K.; Hirotsugu, K.; Masanori, O. Nanoparticle-stabilized cholesteric blue phases. Appl. Phys. Express 2009, 2, 121501.
- Karatairi, E.; Rožič, B.; Kutnjak, Z.; Tzitzios, V.; Nounesis, G.; Cordoyiannis, G.; Thoen, J.; Glorieux, C.; Kralj, S. Nanoparticle-induced widening of the temperature range of liquid-crystalline blue phases. Phys. Rev. E 2010, 81, 041703.
- Prakash, J.; Choudhary, A.; Kumar, A.; Mehta, D.S.; Biradar, A.M. Nonvolatile memory effect based on gold nanoparticles doped ferroelectric liquid crystal. Appl. Phys. Lett. 2008, 93, 112904.
- Dolgov, L.O.; Yaroshchuk, O.V. Electrooptic properties of liquid crystals filled with silica nanoparticles of different sorts. Colloid Polym. Sci. 2004, 282, 1403–1408.
- Basu, R. Soft memory in a ferroelectric nanoparticle-doped liquid crystal. Phys. Rev. E 2014, 89, 022508.
- Kempaiah, R.; Liu, Y.; Nie, Z.; Basu, R. Giant soft-memory in liquid crystal nanocomposites. Appl. Phys. Lett. 2016, 108, 083105.
- Shiraishi, Y.; Toshima, N.; Maeda, K.; Yoshikawa, H.; Xu, J.; Kobayashi, S. Frequency modulation response of a liquid-crystal electro-optic device doped with nanoparticles. Appl. Phys. Lett. 2002, 81, 2845–2847.
- Dierking, I.; Scalia, G.; Morales, P.; LeClere, D. Aligning and reorienting carbon nanotubes with nematic liquid crystals. Adv. Mater. 2004, 16, 865–869.
- Özgan, Ş.; Eskalen, H.; Tapkıranlı, Y. Thermal and electro-optic properties of graphene oxide-doped hexylcyanobiphenyl liquid crystal. J. Theor. Appl. Phys. 2018, 12, 169–176.
- Gardner, D.F.; Evans, J.S.; Smalyukh, I.I. Towards reconfigurable optical metamaterials: Colloidal nanoparticle self-assembly and self-alignment in liquid crystals. Mol. Cryst. Liq. Cryst. 2011, 545, 1227–1245.
- Valentine, J.; Zhang, S.; Zentgraf, T.; Ulin-Avila, E.; Genov, D.A.; Bartal, G.; Zhang, X. Three-dimensional optical metamaterial with a negative refractive index. Nature 2008, 455, 376.
- Zheludev, N.I.; Kivshar, Y.S. From metamaterials to metadevices. Nat. Mater. 2012, 11, 917–924.
- Si, G.; Leong, E.S.P.; Jiang, X.; Lv, J.; Lin, J.; Dai, H.; Liu, Y.J. All-optical, polarization-insensitive light tuning properties in silver nanorod arrays covered with photoresponsive liquid crystals. Phys. Chem. Chem. Phys. 2015, 17, 13223–13227.
- Nemati, A.; Wang, Q.; Hong, M.; Teng, J. Tunable and reconfigurable metasurfaces and metadevices. Opto Electron. Adv. 2018, 1, 180009.
- Liu, Y.J.; Hao, Q.; Smalley, J.S.T.; Liou, J.; Khoo, I.C.; Huang, T.J. A frequency-addressed plasmonic switch based on dual-frequency liquid crystals. Appl. Phys. Lett. 2010, 97, 091101.
- Link, S.; El-Sayed, M.A. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366.
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706.
- Chu, K.C.; Chao, C.Y.; Chen, Y.F.; Wu, Y.C.; Chen, C.C. Electrically controlled surface plasmon resonance frequency of gold nanorods. Appl. Phys. Lett. 2006, 89, 103107.
- Evans, P.R.; Wurtz, G.A.; Hendren, W.R.; Atkinson, R.; Dickson, W.; Zayats, A.V.; Pollard, R.J. Electrically switchable nonreciprocal transmission of plasmonic nanorods with liquid crystal. Appl. Phys. Lett. 2007, 91, 043101.
- Singh, D.P.; Gupta, S.K.; Srivastava, A.; Manohar, R. The phenomenon of induced photoluminescence in ferroelectric mesophase. J. Lumin. 2013, 139, 60–63.
- Tong, X.; Zhao, Y. Liquid-crystal gel-dispersed quantum dots: Reversible modulation of photoluminescence intensity using an electric field. J. Am. Chem. Soc. 2007, 129, 6372–6373.
- Rodarte, L.A.; Cisneros, F.; Hein, E.J.; Ghosh, S.; Hirst, S.L. Quantum dot/liquid crystal nanocomposites in photonic devices. Photonics 2015, 2, 855–864.
- Danilov, V.V.; Artem’ev, M.V.; Baranov, A.V.; Ermolaeva, G.M.; Utkina, N.A.; Khrebtov, A.I. Fluorescence of semiconductor nanorods in liquid-crystal composites. Opt. Spectrosc. 2008, 105, 306–309.
- Ozaki, M.; Kasano, M.; Ganzke, D.; Haase, W.; Yoshino, K. Mirrorless lasing in a dye-doped ferroelectric liquid crystal. Adv. Mater. 2002, 14, 306–309.
- Chen, L.-J.; Lee, C.-R.; Chu, C.-L. Surface passivation assisted lasing emission in the quantum dots doped cholesteric liquid crystal resonating cavity with polymer template. RSC Adv. 2014, 4, 52804–52807.
- Lee, C.-R.; Lin, S.-H.; Guo, J.-W.; Lin, J.-D.; Lin, H.-L.; Zheng, Y.-C.; Ma, C.-L.; Horng, C.-T.; Sun, H.-Y.; Huang, S.-Y. Electrically and thermally controllable nanoparticle random laser in a well-aligned dye-doped liquid crystal cell. Opt. Mater. Express 2015, 5, 1469–1481.
- Rodarte, A.L.; Gray, C.; Hirst, L.S.; Ghosh, S. Spectral and polarization modulation of quantum dot emission in a one-dimensional liquid crystal photonic cavity. Phys. Rev. B 2012, 85, 035430.
- Wu, K.-J.; Chu, K.-C.; Chao, C.-Y.; Chen, Y.-F.; Lai, C.-W.; Kang, C.-C.; Chen, C.-Y.; Chou, P.-T. CdS nanorods imbedded in liquid crystal cells for smart optoelectronic devices. Nano Lett. 2007, 7, 1908–1913.
- Ruhwandl, R.W.; Terentjev, E.M. Long-range forces and aggregation of colloid particles in a nematic liquid crystal. Phys. Rev. E 1997, 55, 2958–2961.
- Stark, H. Physics of colloidal dispersions in nematic liquid crystals. Phys. Rep. 2001, 351, 387–474.
- Pires, D.; Fleury, J.-B.; Galerne, Y. Colloid particles in the interaction field of a disclination line in a nematic phase. Phys. Rev. Lett. 2007, 98, 247801.
- Samitsu, S.; Takanishi, Y.; Yamamoto, J. Molecular manipulator driven by spatial variation of liquid-crystalline order. Nat. Mater. 2010, 9, 816.
- Kuksenok, O.V.; Ruhwandl, R.W.; Shiyanovskii, S.V.; Terentjev, E.M. Director structure around a colloid particle suspended in a nematic liquid crystal. Phys. Rev. E 1996, 54, 5198–5203.
- Mondain-Monval, O.; Dedieu, J.C.; Gulik-Krzywicki, T.; Poulin, P. Weak surface energy in nematic dispersions: Saturn ring defects and quadrupolar interactions. Eur. Phys. J. B Condens. Matter Complex. Syst. 1999, 12, 167–170.
- Stark, H. Director field configurations around a spherical particle in a nematic liquid crystal. Eur. Phys. J. B Condens. Matter Complex. Syst. 1999, 10, 311–321.
- Koenig, G.M.; de Pablo, J.J.; Abbott, N.L. Characterization of the reversible interaction of pairs of nanoparticles dispersed in nematic liquid crystals. Langmuir 2009, 25, 13318–13321.
- Škarabot, M.; Muševič, I. Direct observation of interaction of nanoparticles in a nematic liquid crystal. Soft Matter 2010, 6, 5476–5481.
- Tomar, V.; Roberts, T.F.; Abbott, N.L.; Hernández-Ortiz, J.P.; de Pablo, J.J. Liquid crystal mediated interactions between nanoparticles in a nematic phase. Langmuir 2012, 28, 6124–6131.
- Kotar, J.; Vilfan, M.; Osterman, N.; Babič, D.; Čopič, M.; Poberaj, I. Interparticle potential and drag coefficient in nematic colloids. Phys. Rev. Lett. 2006, 96, 207801.
- Škarabot, M.; Ravnik, M.; Žumer, S.; Tkalec, U.; Poberaj, I.; Babič, D.; Osterman, N.; Muševič, I. Interactions of quadrupolar nematic colloids. Phys. Rev. E 2008, 77, 031705.
- Vilfan, M.; Osterman, N.; Čopič, M.; Ravnik, M.; Žumer, S.; Kotar, J.; Babič, D.; Poberaj, I. Confinement effect on interparticle potential in nematic colloids. Phys. Rev. Lett. 2008, 101, 237801.
- Chernyshuk, S.B.; Lev, B.I. Elastic interaction between colloidal particles in confined nematic liquid crystals. Phys. Rev. E 2010, 81, 041701.
- Muševič, I.; Škarabot, M.; Tkalec, U.; Ravnik, M.; Žumer, S. Two-dimensional nematic colloidal crystals self-assembled by topological defects. Science 2006, 313, 954.
- Škarabot, M.; Ravnik, M.; Žumer, S.; Tkalec, U.; Poberaj, I.; Babič, D.; Osterman, N.; Muševič, I. Two-dimensional dipolar nematic colloidal crystals. Phys. Rev. E 2007, 76, 051406.
- Muševič, I.; Škarabot, M. Self-assembly of nematic colloids. Soft Matter 2008, 4, 195–199.
- Ognysta, U.; Nych, A.; Nazarenko, V.; Škarabot, M.; Muševič, I. Design of 2d binary colloidal crystals in a nematic liquid crystal. Langmuir 2009, 25, 12092–12100.
- Prathap Chandran, S.; Mondiot, F.; Mondain-Monval, O.; Loudet, J.C. Photonic control of surface anchoring on solid colloids dispersed in liquid crystals. Langmuir 2011, 27, 15185–15198.
- Nych, A.; Ognysta, U.; Škarabot, M.; Ravnik, M.; Žumer, S.; Muševič, I. Assembly and control of 3d nematic dipolar colloidal crystals. Nat. Commun. 2013, 4, 1489.
- Bawden, F.C.; Pirie, N.W.; Bernal, J.D.; Fankuchen, I. Liquid crystalline substances from virus-infected plants. Nature 1936, 138, 1051.
- Onsager, L. The effects of shape on the interaction of colloidal particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659.
- Li, L.S.; Walda, J.; Manna, L.; Alivisatos, A.P. Semiconductor nanorod liquid crystals. Nano Lett. 2002, 2, 557–560.
- Li, L.S.; Alivisatos, A.P. Semiconductor nanorod liquid crystals and their assembly on a substrate. Adv. Mater. 2003, 15, 408–411.
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172.
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80.
- Shimoda, H.; Oh, S.J.; Geng, H.Z.; Walker, R.J.; Zhang, X.B.; Mcneil, L.E.; Zhou, O. Self-assembly of carbon nanotubes. Adv. Mater. 2002, 14, 899–901.
- Song, W.; Kinloch, I.A.; Windle, A.H. Nematic liquid crystallinity of multiwall carbon nanotubes. Science 2003, 302, 1363–1363.
- Behabtu, N.; Lomeda, J.R.; Green, M.J.; Higginbotham, A.L.; Sinitskii, A.; Kosynkin, D.V.; Tsentalovich, D.E.; Parravasquez, A.N.G.; Schmidt, J.; Kesselman, E. Spontaneous high-concentration dispersions and liquid crystals of graphene. Nat. Nanotechnol. 2010, 5, 406–411.
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene oxide liquid crystals. Angew. Chem. 2011, 50, 3043–3047.
- Xu, Z.; Gao, C. Aqueous liquid crystals of graphene oxide. ACS Nano 2011, 5, 2908–2915.
