Iodine is one of the 30 essential elements for life, and its chemical speciation defines its biological activities. Both inorganic and organic chemical species have crucial roles in the biology of most living organisms. The most relevant inorganic species are iodate (IO3−) and iodide (I−), as the major sources of iodine for living beings, together with molecular iodine (I2) and hypoiodous acid (HIO) as the most reactive performing catalytic activities. Conversely, Thyroid Hormones (THs) are the master regulators of the metabolism of vertebrates and the representative organic species. Mainly inorganic species are exploited in the health science industry to produce disinfectants, supplements, medicines, and X-ray contrast media.
- iodine species
- iodide
- molecular iodine
- thyroid hormones
1. Introduction
2. History and Chemical Characteristics
In 1811, the French chemist Bernard Courtois discovered iodine when he tried to obtain saltpeter from seaweed and accidentally added too much sulfuric acid to the mixture, producing a purple vapor. Two years later, iodine was recognized as a new element receiving its name from the Ancient Greek term “οειδής” or “ioeides” which means violet [8][4]. The periodic table represents iodine by the I letter, which has an atomic number of 53, an atomic mass of 126.9045 Da, and belongs to group 17 of halogens [9][5]. There are 37 known isotopes of the element with masses from 108 to 144 (108I – 144I). Most are artificially produced and lose energy via radiation (radiation decay) in days, hours, or less,; except for Iodine-127 ( 127I), the natural and only 100% stable isotope. Iodine 129 (, and 129I), with a half-life of 15.7 million years, can also be which is naturally produced in traces by spontaneous fission and cosmic-ray reactions (see applications of radioisotopes in [10][11] section 6.3 and 6.4) [6,7]. Iodine lacks an electron to fulfill the valence shell; hence, it cannot exist in nature as a free element (neutral atom) because of its high reactivity and electronegativity (2.66 on the Pauling scale). Instead, the diatomic form of I2, formed by a nonpolar covalent bond between two iodine atoms, is recognized as the stable state of the element [9][12][5,8]. Likewise, iodine can combine with most elements (except noble gases and most synthetic elements), yielding broad inorganic and organic chemiocal forms (iodine species. Those chemical species d) with oxidation states of −1, 0, +1, +3, +5, and +7, distributed along the lithosphere, hydrosphere, and atmosphere with diverse oxidation states (−1, 0, +1, +3, +5, and +7) [9].3. Distribution of Iodine
In the Earth’s crust, the richest inorganic source of iodine is oceanic sediments (68.2%) and continental sedimentary rocks (27.7%), followed by igneous and metamorphic rocks (2.7%), seawater (0.81%), and mafic oceanic crust (0.68%) [13][9]. In the atmosphere, sea spray aerosolization, volcanic gases, and human activities contribute to iodine emissions., However,but the highest discharge is given by biological conversion to volatile methyl forms, such as methyl iodide (CH3I). Cycling of iodine involves biotic and abiotic processes through the lithosphere, hydrosphere, and atmosphere. Into seawater, iodine species are cycled during subduction of the oceanic crust (descending of a tectonic plate below another) and via decomposition of marine organisms. In contrast,; whereas the flux from the atmosphere to the lithosphere occurs during rainfall, wet deposition, leaching, and runoff [13][14][15][9,10,11]. Furthermore, many living organisman important amount of iodine is accumulated and use a significant amount of iodined by many living organisms, including algae, plants, corals, sponges, anemones, lobworms, shellfishes, arthropods, and bacteria, that cycle organic and inorganic iodine species in the biosphere (Figure 1) [15][11].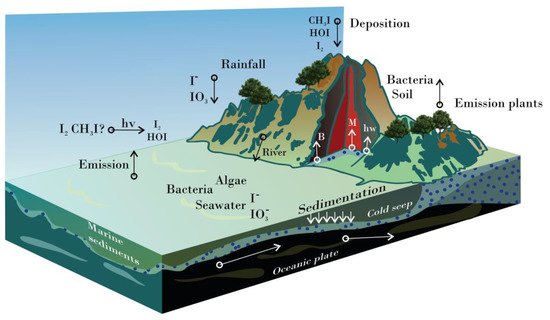
4. Relevant Iodine Species
4.1. Main Inorganic Forms
Dominant iodine species in solid-state are potassium iodate (KIO3) and potassium iodide (KI), whereas IO3- and I- ions lead to aqueous systems [19][15]. I2 and CH3I are the most abundant species in the atmosphere, produced by biotic and abiotic processes [20][16]. Atmospheric photochemistry promotes the speciation of gaseous iodine species (e.g., HI, IO-, HOI, IONO2 INO2, and IO2) [21][22][17,18]. Some of them (mainly I2 and HOI), together with other halogen compounds, are responsible for ozone (O3) depletion in the lower troposphere when interacting with hydrogen-oxygen species and nitrogen oxides [23][19].
In general terms, all iodine species formed in aqueous solutions are relevant for the biological systems. Up to 10 different iodine species can be formed when I2 and I- combine in an aqueous solution (reactions 1-9), where the chemical speciation is highly influenced by iodide amount [c(I-)], pH, and redox potential (Eh) [24][25][26](Fig. 2A) [22,23,24]. An excellent example is Lugol’s solution (typically made of 5% I2 and 10% KI), used as a disinfectant and for other commercial purposes (see sections 5 and 6.1). Under those conditions, I2, I-, triiodide (I3–), pentaiodide (I5–), and hexaiodide (I62–) coexist. I2 is the most reactive of those, showing several biological activities, I3– is responsible for staining, and I5–/I62– barely represents ~8% of the oxidizing potential [26][24]. However, only I-, I2, and I3- are formed and available in significant amounts under physiological conditions [26][27][24,25].I
(1) I
2
+ H
2
O ↔ HIO + I
−
+ H
+. (1)
.
HIO ↔ OI
(2) HIO ↔ OI
−
+ H
+. (2)
.
I
(3) I
2
+ I
−
↔ I
3−. (3)
.
HIO + H
(4) HIO + H
+
↔ H
2
OI
+. (4)
.
I
(5) I
3−
+ I
2
↔ I
5-. (5)
.
2I
(6) 2I
3−
↔ I
62−. (6)
.
OI
(7) OI
−
+ I
-
+H
2
O ↔ HIO
2-
+ OH
-. (7)
.
HIO
(8) HIO
2-
↔ I
2
O
-
+ H
+. (8)
.
3HIO ↔ IO
(9) 3HIO ↔ IO
3-
+ 2I
-
+ H
+. (9)
.
4.2. Representative Organic Species
4.2. Representative organic species
Thyroid hormones (THs) are the protagonists of organic iodine species because of their hormonal role in vertebrate animals. THs comprise i) 3-iodotyrosine or monoiodotyrosine (MIT or T1) and 3,5-diiodotyrosine (DIT) as precursors; ii) 3’,5,3-triiodothyronine (T3) and thyroxine (T4), typically considered as the active hormones; and iii) 3’,5’,3 triiodothyronine or reverse T3 (rT3), 3’,5’ diiodotyrosine (3’,5’-DIT), and 3’- monoiodotyrosine (3’-MIT), the T4 derivatives [28][29][40]. Other THs derivatives include thyronamines (TAMs) and thyroacetic acids like 3,3',5-triiodothyroacetic acid (Triac), and 3,3′,5,5′-tetraiodothyroacetic acid (Tetrac) produced by deiodination and decarboxylation [29][41].
Thyroid Peroxidase (TPO) is the enzyme responsible for synthesizing THs using thyroglobulin (TG) as a substrate., TG is the main iodinated compound in the thyroid gland [30][31][32][42,43,44]. The sequence forllowed in TH production is TPO incorporatesing one iodine atom into Tyr residues at position 3 of the ring to produce MIT (reaction 10) and then at position 5 to form DIT (reaction 11). Subsequently, T3 is constructed by an oxidative coupling reaction between one DIT and one MIT (reaction 12), still incorporated into the TG. At the same time, T4 is formed by coupling two adjacent DITs (reaction 13). At the end of the process, only 37 of the ~70 Tyr residues in the TG are known iodination targets [30][31][42,43]. Most MITs and DITs produced are kept in the thyroid follicellles and are eventually degraded by iodotyrosine deiodinases (IYD) enzymes into MIT and Tyr (T0), respectively (reactions 14 and 15) [33][99]. Meainwhile, T3 and T4 are transported into the bloodstream, mostly bound to plasma proteins. Just a fraction of T3 (21.8%) is synthesized and released in the same fashion as T4; most of it is generated by the deiodination of T4 out of the thyroid by two kinds of iodothyronine deiodinases (ID) (Reaction (16)) [34][35][90,101]. A different iodothyronine deiodinase transforms T4 into rT3 (Reaction (17)), an inactive TH produced to maintain hormone homeostasis [36][102].
Tyr residue + I
(10) Tyr residue + I
−
+ H
2
O
2
+ H
+
+ TPO → MIT + 2H
2O + TPO. (10)
O + TPO.
MIT + I− + H
(11) MIT + I− + H
2
O
2
+ H
+ + TPO → DIT + 2H2O + TPO. (11)
+ TPO → DIT + 2H2O + TPO.
MIT + DIT + H
(12) MIT + DIT + H
2
O
2
+ H
+
+ TPO → T3 + 2H
2O + TG (dehydroalanine). (12)
O + TG (dehydroalanine).
2DIT + I
(13) 2DIT + I
−
+ H
2
O
2
+ H
+
+ TPO → T4 + 2H
2O + TG (dehydroalanine). (13)
O + TG (dehydroalanine).
DIT + NADPH + IYD → MIT + NADP
(14) DIT + NADPH + IYD → MIT + NADP
+
+ I
− + IYD. (14)
+ IYD.
MIT + NADPH + IYD → Tyr + NADP
(15) MIT + NADPH + IYD → Tyr + NADP
+
+ I
− + IYD. (15)
+ IYD.
T4 + NADPH + ID→ T3 + NADP
(16) T4 + NADPH + ID→ T3 + NADP
+
+ I
− + ID. (16)
+ ID.
T4 + NADPH + ID→ rT3 + NADP
(17) T4 + NADPH + ID→ rT3 + NADP
+
+ I
− + ID. (17)
+ ID.
TPO is also responsible for the iodination of lipids (iodolipids) as α-IHDA and 6-IL. Noticeably, 6-IL was recently related to the antioxidant, anti-inflammatory, and anticarcinogenic activities [37][38] [45,46]. Other peroxidase enzymes like Lactoperoxidase (LPO) are responsible for the synthesis of iodolactones (e.g., δ-lactone of 6-iodo-4-hydroxy-eicosa-8,11,14-trienoic acid, and ε-lactone of 5-iodo-4-hydroxy-7-docosapentaenoic acid) using docosahexaenoic acid (DHA) as a substrate [39][32].
5. Iodine in the Industry
Molecular iodine is one the most exploited chemicals in the industry, mainly as a catalyst for organic synthesis (including many heterocyclic compounds used in pharmacy), because of its low cost, efficiency, and high selectivity [40][203]. I− is considered a mild reducing agent and , and it is a much less reactive species than I2. IO3− is less reactive and has low toxicology; together with I−, it can also be used as a chemical reagent or serve as an ingredient for various products (e.g., supplements and iodophors) [12][41][8,204]. Hydrogen iodide (HI), iodine pentoxide (I2O5), iodic acid (HIO3), and sodium periodate (NaIO4) are other frequent chemicals used in the pharmaceutical industry for organic synthesis [42][43][205,206]. In this resviearchw, the chemical synthesis is not addressed, but it is recommended to consult Küpper et al. (2011) and Wang et al. (2021). Historically, Lugol’s solution has been one of the most used iodine-based products in medicine and the industry. It was first used in the early 20th century by J.G.A. Lugol as a treatment for tuberculosis and is still considered an essential component of basic healthcare systems [44][45][207,208]. Lugol’s solution is used as an antiseptic, disinfectant, supplement, and laboratory reagent, among the most important applications [2][46][2,29]. Currently, many iodine-based products are being developed, and the global demand for iodine has increased in rthecen last decades. In 2017, the iodine market value was 833 million USD, and it is forecasted to be ~1.14 billion USD by 2024 [47][209]. Chile and Japan lead the global production of iodine (having also the biggest reserves worldwide), followed by Turkmenistan, Azerbaijan, Indonesia, and Russia (the US has been excluded from statistics in recent years) [48][49][210,211]. In the case of radioisotopes (used in nuclear medicine), ithey must be produced in nuclear reactors or cyclotrons from tellurium or xenon gases [50][51][212,213]. The major industrial applications of iodine include production of X-ray contrast media (XCM) (22%), pharmaceuticals (13%), polarization films (12%), animal feed (8%), iodophors (7%), fluorochemicals (7%), biocides (4%), nylon (4%), and human nutrition supplements (3%) [52][214]. The section below describes the relevant iodine applications of iodine in biomedicine and some of the representative iodine-based products.6. Applications in the Health Sciences
6.1. Disinfection, Asepsis, and Wound Care
Iodine has a broad spectrum against several microorganisms, including resistant forms (e.g., conidia, endospores, cysts, biofilms) [53][54][55][84,85,86]. Of all inorganic iodine species with catalytic activity, I2 is the most abundant and the leading antimicrobial agent in iodine-based disinfectants [26][24]. Other iodine species recognized as antimicrobials are HOI and H2OI+. However, they are restricted to extreme pH ranges, and the amount of I- [56][57][27,28]. Within solvents like alcohol, the solvated forms I2⋅H2O or I2⋅ROH are proposed as the anti-microbial species [58][89]. Lugol’s solution became popular as a disinfectant but had some drawbacks, such as staining of the skin and materials, local pain, and irritation. In addition, a proportion of I2 is lost by volatilization. These problems were solved in the early 1950s by the development of iodophors. The advantages of iodophoric preparations are reduced staining, increased I2 solubility, broad pH range (2.5–7.0), and slow release to prolong the antimicrobial action [59][60][25,218]. Iodophors are made with solubilizing agents such as natural or synthetic polymers or surfactants. They can be anionic, cationic, or nonionic [61][62][24,219]. Natural polymers are mostly starch and cellulose derivatives (e.g., amylose, amylopectin, Cadexomer, carboxymethyl cellulose, and methyl hydroxypropyl cellulose). Chitosan and lecithin are used in more complex iodophoric formulations (e.g., carboxymethyl chitosan and hydroxylated lecithin). Regarding synthetic polymers, PVP/I is the most used, followed by polyvinyl alcohol with iodine (PVAI). PVP/I is routinely used as a milk and teat disinfectant and is, in fact, an important contributor to the iodine content in dairy products [63][221]. Other excellent polymeric carriers include poly(4-vinyl pyridine), poly(3-vinyl-10-methylphenothiazine), poly(2-ethyl-2-oxazoline), and poly(tetramethylene ether) glycol [62][64][219,220]. Iodophors like PVP/I have shown biocidal effects on different species of bacteria (e.g., Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Chlamydia trachomatis), fungi (e.g., Candida spp. and Trichophyton spp.), protozoa (e.g., Trichomonas and Acanthamoeba spp.), and viruses (e.g., herpes simplex virus type I, adenovirus, influenza A viruses, rotavirus, poliovirus, and human immunodeficiency virus) [65][66][67][68][69][70][71][72][73][57,225,227,228,229,230,231,232,233]. Enveloped viruses are susceptible to iodine, and PVP-I’s efficacy (0.2–5%) was more recently proven in reducing viral loads of SARS-CoV-2 down to undetectable levels. Currently, skin, ophthalmic, nasal, and oral PVP-I-based antiseptic products are recommended to prevent transmissions during medical consultation [74][75][76][234,235,236]. Iodophors have also been exploited in novel wound healing and anti-inflammatory products because of their biocompatibility. PVAI and PVP/I-based hydrogels are good examples; they are constituted by flexible matrices that maintain asepsis and moisture. Moreover, they also absorb exudates, allow wound oxygenation, and have low toxicity and skin irritation [77][78][54,237]. Together with other components, hydrogels can serve as scaffolds for tissue regeneration, regulate medication released, or display unique properties to respond to stimuli, such as color change by temperature (thermochromism) or pH/thermal sensitivity [79][80][238,239]. It is important to mention that using iodine-based disinfectants in drinking water is not recommended because of the risk of undesirable byproducts. There are more than 600 peptides in water [81][215] reacting with chloramine (NH2Cl), H2OI+, and HIO to form highly toxic compounds of concern for people and the environment [82][216]. Reactions of HIO, I2, OI− species, and chloramines also affect pyridine nucleotides and glutathione (GSH), a potent and essential cellular antioxidant molecule. HIO (resulting from I2 and OH−) is a more selective downgrader of reduced dihydro-nicotinamide mononucleotide (NMNH) and nicotinamide adenine dinucleotide phosphate (NADH) than HOCl, and it can directly oxidize GSH or decrease its production by reducing the availability of NADPH (needed in GSH cycling) [83][217].6.2. Supplementation
When iodine ingestion is insufficient, there is a need to consume supplements to maintain normal thyroid function (euthyroidism). Iodine intake by groups of age is recommended as follows: 90 µg/day for infants and children 0–59 months, 120 µg/day for school children (6 to 12 years), 150 µg/day for adults, and 200 µg/day for pregnant and breastfeeding women [84][240]. Upper limits are much more difficult to establish because they are influenced by previous long-term iodine status [85][84] and even the kind of supplementation [86][166]. Some studies reported normal thyroid function at high doses of iodine from the diet (mainly from seaweed, 1.2 mg/day) [87][241], supplements (1–3 mg/day) [88][242], Lugol’s solution, and PVP-based water disinfectants (4–8 mg/L water) [89][243]. However, the recommended safe iodine intake for euthyroid people by the WHO is up to 1 mg/day, except for pregnant and lactating women that have no extra benefit receiving >500 µg/day and children of <2 years that should not exceed >180 µg/day [90][244]. The most common and suitable indicator to evaluate the iodine status in a population is urinary iodine, given that iodine is mostly excreted in urine (~90%). In this sense, the best method is urinary iodine excretion (UIE), which is determined from the whole urine collected for 24 h. Urinary iodine concentration (UIC) from one urine sample (preferably collected in the morning) is enough and more practical to define the general status according to WHO [2013]. For most people (including lactating women), >100 µg/L UIC is considered adequate, but <100 µg/L UIC reflects insufficient intake (<20 µg/L UIC suggests severe iodine deficiency), except for pregnant women whose optimal UIC is considered 150–249 µg/L, with <100 µg/L considered insufficient [91][245]. Nonetheless, urinary concentrations might vary in short periods (days–hours). Thus, to determine the iodine status and thyroid function of individuals, it is recommended to screen the levels of TSH and free T4 as a minimum [92][93][66,246]. Other parameters are evaluated to monitor thyroid function, such as goiter rate (%), levels of T3, rT3, TG, antibodies for TG (TgAb) or TPO (TPOAb), and thyroid-stimulating immunoglobulin (TSI) [88][242]. Optimal consumption of iodine can be obtained with an appropriate diet, consuming seafood (e.g., seaweed, fish, crabs, and oyster), dairy products (e.g., milk, yogurt, and cheese), tubers (e.g., turnip and saffron), beef kidney, green beans, eggs, yeast, some condiments (e.g., chili powder and mint), and iodized salt [94][95][39,247]. However, some geographical areas might have iodine-deficient water and soils; consequently, the iodine content in food is lower. For example, iodine content in plants can be up to 1 mg/kg or down to 10 μg/kg (dry weight), depending on the soil [92][66]. Geographic iodine-deficient areas include mountainous and inland regions of South America (e.g., the Andes and inland Brazil), the Midwestern United States, Europe (e.g., Alps, Pyrenees, England, Wales, Greece, and the Netherlands), Asia (China, India, Bangladesh, Himalayan hillsides, and Indonesia), Africa (e.g., Atlas Mountains, Nigeria, Cameroon, the Central African Republic Democratic Republic of Congo, Uganda, and Ethiopia), Southern Australia, and Highlands of New Guinea [96][65]. The additional intake of iodine from supplements is recommended to prevent IDD in iodine-deficient areas (especially among women of reproductive age). Currently, the best strategy to face IDD in the world has been the universal iodization of table salt (with KI, NaI, or KIO3), implemented in 1994 by the International Council for the Control of Iodine Deficiency Disorders (ICCIDD). The advantages of this product lie in the global consumption of salt, cheap cost production, long shelf-life, accessibility for remote communities, and imperceptible taste modification [4][248]. IDD persists even in developed countries due to the unequal consumption and distribution of iodized salt among low- and high-income households [97][98][249,250]. Moreover, it has been demonstrated that iodine can be partially or entirely lost during cooking depending on cookware materials and ingredients such as ascorbic acid, glucose, and some condiments, as well as because of impurities and storage conditions, mainly the kind of package and humidity [99][5][251,252]. In the market, there is a wide variety of supplements that can also be taken with one or more of the following components in their formulations: KI, NaI, KIO3, I2, iodized oil, natural products based on seaweed (from species Laminaria spp., Fucus vesiculosus, Alaria esculenta, Ascophyllum nodosum, Ecklonia maxima, Eisenia bicyclis, Hizikia fusiforme, Palmaria palmata, Porphyra tenera, Postelsia palmaeformis, and Sargassum sp.) or plants (e.g., Commiphora mukul and Iris versicolor) [94][100][101][39,253,254]. Among all supplements, natural formulations have the most variability or declare the highest iodine contents and should be avoided for pregnant women or those planning a pregnancy [101][102][254,255]. Natural or synthetic thyroid hormones are used not as supplements but under prescription to treat hypothyroidism. Currently, levothyroxine (or l-thyroxine) is the most recommended by endocrine societies (and produced worldwide) for thyroid hormone replacement therapy [103][256]. Probiotic consumption might improve iodine status, given that the gut microbiome participates in nutrient absorption and cycling of THs. Autoimmune thyroid dysfunctions and thyroid carcinoma are linked to dysbiosis (imbalance in the microbiota). Hypothyroidism and hyperthyroidism are correlated with the reduction in intestinal bacteria from Lactobacillaceae and Bifidobacteriaceae families [104][257]. Vitamin D and other nutrients such as iron, copper, selenium, and zinc are often altered in thyroid disorders. They can be considered in supplementation and the avoidance of goitrogens [96][104][65,257]. Goitrogens decrease iodine absorption, primarily by inhibiting cellular iodine transporters, interfering with TH synthesis, or reducing the conversion of T3 from T4 [96][105][106][65,258,259]. They comprise cyanide (from smoking), nitrates, fluoride, some contaminants (e.g., perchlorate and disulfides), foods rich in glucosides (e.g., cassava, lima beans, linseed, sorghum, and sweet potato), glucosinolates (e.g., broccoli, kale, cauliflower, cabbage, and turnips), and flavonoids (e.g., onions, lettuce, tomatoes, and grapes). Even nutrient deficiencies of iron, selenium, and vitamin A are considered goitrogens [106][107][259,260].6.3. Contrast Agents
X-rays allow us to see structures of high density because the X-ray absorption coefficient (µ) is directly proportional to the atomic mass of the compound. Bones and some organs in the body can be directly distinguished by X-ray, but other less dense structures need contrast agents to visualize them [108][261]. X-ray contrast media (XCM) are substances that allow visualization of those biological structures, and, depending on the difference in µ, they can be classified as positive or negative. Negative XCM are gases of lower absorption coefficient (e.g., O2 and CO2), and positive XCM contain elements of high atomic number, such as iodine or barium, that stand out in X-ray imaging [108][109][261,262]. Positive XCM can be classified as targeted (or specific) and extracellular (or unspecific), depending on whether the contrasting reagent is metabolized in the body [108][110][15,261]. Extracellular XCM are water-soluble agents mainly used to visualize the circulatory system. The pharmacokinetics is very similar among extracellular XCM, taking 3–10 min for distribution and typically 1–2 h in elimination (via glomerular filtration). Under renal impairment, the excretion takes up to 10 h with a higher risk of nephrotoxicity [108][110][15,261]. On the contrary, targeted XCM (alsor biliary contrast agents) have good lipophilicity and different distribution and elimination times. The disadvantages of XCM with low hydrophilicity are less tolerability, hepatic or renal toxicity, and higher allergic reactions [108][111][261,263]. For those reasons, research on targeted XCM is now developing into noniodine compounds, although promising advances have been accomplished with liposomes and polymers as carriers [111][263]. The simplest contrast agent is NaI, the first iodinated XCM in the market (in 1918), with an excellent µ value but not as good tolerability at high doses. Currently, most XCM are based on molecules of one or more triiodobenzene rings. Triiodobenzene derivatives are the extracellular XCM of choice because of their stability, tolerability, low toxicity, and wide range of compounds that can be produced. The synthesis is achieved by substituting the hydrogens at positions 1, 3, and 5 of the benzene ring with I atoms. Some good examples are iohexol, iopamidol, ioversol, ioxilan, iopromide, ioxaglic acid, iotrolan, iodixanol, and diatrizoate meglumine [112][264]. Examples of approved targeted agents are iodipamide meglumine to visualize the bile duct and the gallbladder and lipiodol for imaging hepatocellular carcinoma [113][114][115][265,266,267].6.4. Applications in Nuclear Medicine
All kinds of radiation can be dangerous for life, but the extent of damage depends on the decay mode and half-life of the radioactive atom. Alpha (α) and beta (β) radiation are weaker but significantly dangerous when particles are inhaled, swallowed, or absorbed in the body. Gamma (γ) and X-rays are much more powerful and penetrating, causing immediate severe cellular damage from external sources [116][268]. In a controlled fashion, nuclear medicine takes advantage of radioisotopes that can be detected in small amounts and represent a lesser danger to lifefor life, to diagnose and treat several diseases. Diagnostics in nuclear medicine differ from radiology in the studying of the functionality of tissues and organs, not only examining anatomic structures. For this purpose, contrast reagentradiopharmaceuticals are injected (most of the time), inhaled, or orally administered to follow their circulation, accumulation, or metabolization in the body [108][117] [261,269]. For imaging, developed technologies are scintigraphy, single-photon emission computed tomography (SPECT), positron emission tomography (PET), or combinations with computed tomography (CT) such as SPECT/CT and PET/CT [117][118][119][269,270,271]. Radiotherapy also uses radioisotopes to target specific abnormal cells or tissues and destroy them via internal radiation (e.g., hyperthyroidism caused by Graves’ disease toxic nodular goiter) [120][272]. The most used radioisotope for both diagnostics and therapy is technetium (99Tc), followed by iodine, because of their high intensity and short half-life [117][120][269,272]. The main iodine radioisotopes of iodine exploited in medicine and for research are 123I, 124I, 125I, and 131I [121][273]; their physicochemical properties are described in Table 1.| Isotope | Abundance | Atomic Mass | Half-Life | Decay Mode (%) | keV | Production | Application | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 123 | I | Synthetic | 122.9056 | 13.2232 h | β | + | (100%) | 159 | Cyclotron | Diagnosis (SPECT) and therapy |
| 124 | I | Synthetic | 123.9062 | 4.176 d | β | + | (100%) | 603 | Cyclotron | Research and diagnosis (PET) |
| 125 | I | Synthetic | 124.9046 | 59.392 d | ε (100%) | 27.5 | Nuclear reactor and cyclotron | Therapy and radioimmunoassay | ||
| 127 | I | Natural (1) | 126.9045 | Stable | Natural | Diagnosis (X-rays and CT) and therapy | ||||
| 131 | I | Synthetic | 130.9061 | 8.0249 d | β | − | (100) | 364.5 | Nuclear reactor | Diagnosis, therapy, and RIA |
6.5. Prophylaxis
Radioactive contamination after a nuclear explosion or an accident can bring severe consequences for people and the environment in the short and long term, as occurred with the atomic bombings of Hiroshima and Nagasaki in 1945 or the Chernobyl (former Soviet Union) and Fukushima (Japan) nuclear accidents in 1986 and 2011, respectively. Beyond the immediate damage of radiation, iodine radioisotopes are of particular concern because of their accumulation in the body and potential incorporation into the food chain following regular iodine cycling [131][132][133][283,284,285]. High radiation levels can induce hypothyroidism or acute thyroiditis. Lower exposure to radiation increases the risk of thyroid cancer and benign thyroid nodules in the long term, especially in infants, children, and adolescents [134][69]. HTo prevent the absorption of radioactive isotopes (prophylaxis), high doses of stable iodine (127I) can be promptly distributed in the population to prevent the absorption of radioactive isotopes (prophylaxis) [88][242]. Prophylaxis is not used to restore iodine status; instead, it takes advantage of the Wolff–Chaikoff effect, a transient inhibition of TH production induced by excess iodine. In most cases, the thyroid function is restored in 1–2 weeks. However,but vulnerable groups (e.g., fetuses, neonates, and patients with autoimmune thyroiditis and Graves'’ disease or treated with antithyroid drugs) may not "“escape"” from this effect [135] [286]. Prophylaxis is recommended when exposure to vulnerable groups (neonates, infants, and children) is ≥10 mGy (1 Gy is equal to 1 J of radiation absorbed per kg) [136][287]. Older adults (>40 years) have a minimal thyroid cancer risk and more side-effects with excessive iodine supplementation; therefore, prophylaxis is recommended at ≥100 mGy. According to WHO, a single dose of I2, KI, or KIO3 is sufficient for all age groups of age, as indicated in Table 2. Repetition doses are only prescribed for pregnant women from iodine-deficient areas and vulnerable people with prolonged exposure (for example, infants inhaling radioactive material) [136][287].| Age Group | I | 2 | (mg) |
KI (mg) |
KIO | 3 | (mg) | Fraction of a Tablet (100 mg) |
|---|---|---|---|---|---|---|---|---|
| Adults and adolescents, including lactating women (>12 years) | 100 | 130 | 170 | 1 | ||||
| Children (3–12 years) | 50 | 65 | 85 | 1/2 | ||||
| Infants (1 month–3 years) | 25 | 32 | 42 | 1/4 | ||||
| Neonates (birth–1 month) | 12.5 | 16 | 21 | 1/8 |
