Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Suhail Ahmad Mir and Version 2 by Conner Chen.
Cancer is a global burden, and as per the latest GLOBOCAN 2020, over 19.3 and 10 million new cases and deaths occurred in 2020, respectively; female breast cancer has surpassed lung cancer and is now the most commonly diagnosed cancer (11.7%), followed by lung cancer (11.4%), colorectal cancer (10%), and prostate cancer (7.3%). Cancer chemoresistance is a growing concern in medical oncology.
- cancer
- chemotherapy
- multidrug resistance
- nanotechnology
1. Introduction
Cancer is a global burden, and as per the latest GLOBOCAN 2020, over 19.3 and 10 million new cases and deaths occurred in 2020, respectively; female breast cancer has surpassed lung cancer and is now the most commonly diagnosed cancer (11.7%), followed by lung cancer (11.4%), colorectal cancer (10%), and prostate cancer (7.3%) [1]. In mortality, lung cancer remains at the top [1]. As per World Health Organization (WHO) statistics 2019, in 112 out of 183 countries in the world, people die of cancer before attaining the age of 70 years [2]. Despite the world having advanced in science and technology, chemotherapy remains a promising option to treat cancer [3]. Conventional chemotherapy has greatly improved the decline in the mortality rate of several dreadful cancers, but its major problem is the killing of cancerous and noncancerous cells causing serious off-target effects such as hair loss, bone marrow depression, and other toxic effects [4]. Therefore, a significant percentage of cancer-related research over the past few decades has focused on creating medications that more precisely target tumor cells rather than normal cells [5]. Precision therapy has greatly advanced thanks to the development of targeted therapy, but there are still numerous unavoidable side effects, and drug resistance has long been an issue [6].
Over 90% of failures in chemotherapy are due to the development of resistance to the already available drugs; this resistance resembles infectious disease treatment resistance and is the most challenging aspect of treating and preventing cancers [7]. This has emerged as a major obstacle and allows cancer to proliferate in presence of a chemotherapeutic agent [8]. Significant resistance develops generally to repeated treatment with one kind of anticancer agent and then develops further towards similar or completely different drugs having a similar mechanism of action. This mechanism, known as multidrug resistance (MDR), can be intrinsic or acquired [9].
To overcome this problem, in recent years, nanotechnology-based drug dosage forms have been explored, which have shown great promise [10]. Most of these nanomedicines are heading toward clinical trials [11]. Nanotechnology has been used in medicine more and more over the past few decades, including applications for safer and more efficient tumor targeting, detection, and treatment [12][13][14][15][16][17][18][19][20][12,13,14,15,16,17,18,19,20]. Drug delivery methods based on nanoparticles (NPs) have demonstrated a number of benefits in the treatment of cancer, including good pharmacokinetics, accurate targeting of tumor cells, a decrease in adverse effects, and reduced drug resistance [21][22][23][24][25][21,22,23,24,25]. Nevertheless, nanomedicine-based formulations have some demerits, such as difficulty in physical handling due to smaller size, particle aggregation, limited drug loading, and burst release [19][20][19,20].
2. Cancer Chemotherapy Resistance and Mechanism
Cancer chemoresistance is a growing concern in medical oncology. Some cancers, including Hodgkin’s lymphomas, acute promyelocytic leukemia, and chronic myeloid leukemia, have been successfully understood and treated despite their complex pathophysiology [26]. The development of anticancer agents against these complex cancers has been achieved by understanding the deep mechanisms, and various drugs have been developed [7]. These mainly include the stimulation of immune response using interferon-alpha (IF-α) and inhibition of oncogenes or oncoproteins [27][28][29][30][31][32][33][34][35][27,28,29,30,31,32,33,34,35]. Many of them are still in practice; however, resistance has developed to the majority of them, which has ultimately affected patient survival [36]. There are various reported resistance mechanisms associated with cancer chemotherapy such as drug efflux, detoxification, stem cells, epithelial-to-mesenchymal transition, inactivation of the drugs before reaching the target, multidrug resistance, inhibiting cell death (apoptosis suppression), augmenting gene amplification and DNA repair of oncogenes, and alteration in the metabolism of drugs. Figure 1 shows the illustration of different possible mechanisms of chemotherapy resistance [37]. Drug resistance in cancer is believed to be due to intrinsic and acquired resistance; however, most cancers in clinical settings have become resistant owing to combinations of these factors [38].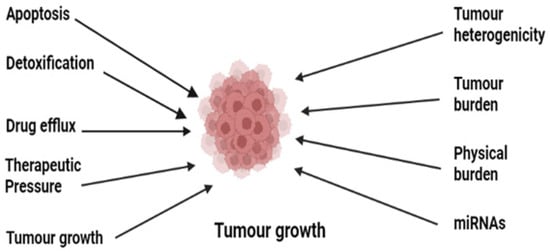
Figure 1.
Illustration of the various possible underlying mechanisms in the development of drug resistance in cancer.
2.1. Role of Drug Efflux Pumps in Cancer Drug Resistance
The human genome encodes 48 members of drug efflux proteins called ATP-binding cassettes which are further classified into seven subgroups (ABCA, ABCB ABCC, ABCD, ABCE, ABCF, and ABCG) [39]. These proteins have a significant role in the development of drug resistance [40]. These proteins expel the drug out of the cell, thereby reducing the therapeutic concentration of the drug inside the cell [41]. Enough evidence suggests the overexpression of these proteins, especially multiple drug resistance protein 1 (MDR 1) known as P-glycoprotein, multiple drug resistance-associated protein (MDRA), and breast cancer resistant protein (BCRP), on cellular surfaces [42]. Normally these transporters help in pumping out toxins and foreign substances [43]. These transporters in general and P-pg in particular transport a range of substances including anticancer agents out of the cell, causing depletion of therapeutic concentration. Overexpression of P-pg in patients causes efflux of paclitaxel and doxorubicin and leads to resistance to these drugs [44]. This is evidenced by a study conducted on a genetically engineered mouse model (GEMM), where the tumor recurred due to upregulation of ABCB1a and b and was found to be cross-resistant to docetaxel also [39][45][39,45]. Another study shows the non-responsiveness of the tumor to olaparib, a PARP inhibitor, due to the overexpression of ABC1 a/b [46][47][46,47], thus confirming the overexpression of these efflux proteins in drug-resistant cancers.2.2. Suppression of Apoptosis
Although apoptosis and autophagy are altogether different, they ultimately contribute to cell death [48]. Two different mechanisms in apoptosis contribute to cell death: (a) intrinsic, which involves the mitochondrial-mediated bcl2 proteins, Akt, and caspase-9, and (b) extrinsic, which involves death receptors on the cellular surface [49]. Ample evidence supports the initiation of human cancer from cancer stem cells (CSCs), and it is believed that these apoptotic pathways become dysregulated and lead to cancer chemotherapy resistance and tumor recurrence [50]. High levels of antiapoptotic proteins which are considered the hallmarks of cancer have been seen in drug-resistant cancers [51]. The antiapoptotic protein family which includes Bcl-2, Mcl-1, and Bcl-xL has been seen at raised levels compared to proapoptotic proteins Bax, Puma, Noxa, Bak, Bil, and Bid, causing an imbalance between the pro- and antiapoptotic proteins which ultimately leads to cancer drug resistance [52]. The formation of the mitochondrial apoptosis-induced channel (MAC), which is formed by binding of tBid with Bax and Bak through activated caspase-8, is hindered by the downregulation of proapoptotic and upregulation of antiapoptotic proteins, which leads to the formation of resistant cancers by inhibiting the release of cytochrome C, a key protein for electron transfer in mitochondria [53]. This overexpression of antiapoptotic proteins is responsible for drug resistance in multiple cancers [54]. Additionally, overexpression of Nf-kB, P53, and PI3/AKT cell death-related receptors is also involved in chemoresistance [55]. In addition, apoptosis evasion through aberrant autophagy is another factor in the development of multiple drug resistance [56].2.3. Drug Inactivation
Before a drug reaches the gastrointestinal tract or systemic circulation, some drugs that are in prodrug form interact with certain proteins which partially degrade, modify, and form complexes with other endogenous substances, leading to the activation of a drug [57]. Certain cancers have developed resistance due to decreased activation of prodrugs to active drugs [58]; the most prominent example is the mutation and downregulation of phosphorylation events in the conversion of AraC into AraC-triphosphate which is used in the treatment of acute myelogenous leukemia [8][59][8,59]. Several drugs metabolizing enzymes such as uridine diphosphate-glucuronosyltransferase, the glutathione-S-transferase family, and cytochrome P450 are muted one way or another and ultimately lead to resistance to already available drugs [60]. The overactivity of cytochrome p450 has been reported to lead to its resistance in breast cancer [61]. Detoxification of drugs by overproduction of glutathione has led to the development of resistance to many platinum compounds and alkylating agents such as cisplatin and doxorubicin [62]. Thus, mutations in phase I and phase II reactions either reduce the activity of drugs by increasing their detoxification or lead to the development of drug resistance by inactivating certain drugs.2.4. Role of miRNAs in Cancer Drug Resistance
miRNAs are processed from RNA hairpin structures, which regulate genes in cancer, especially in resistant ones [63]. They are involved in apoptosis, cellular proliferation, stress tolerance, the cell cycle, and immune response [64]. Around 30% of human genes are regulated by miRNAs and have a role in tumor development [63]. Some act as protumor genes, some act as suppressor genes, and some act as both [65]. Studies conducted by various researchers provide evidence of miRNAs being involved in cancer drug resistance by either enhancing tumor cancer genes or having involvement in genes that are related to apoptosis, cellular proliferation, and the cell cycle [66]. Due to their tissue specificity, one kind of microRNA could be targeted by multiple microRNAs; hence the same miRNA can either promote or inhibit resistance to chemotherapy [67][68][67,68]. In breast cancer, upregulation of miRNA-21 downregulates phosphatase tensin homolog (PTEN) and thereby decreases the susceptibility of doxorubicin to cancer cells, while overexpression of PTEN inhibits miRNA-21 and reduces the resistance of breast cancer cells to doxorubicin [69]. Table 1 reports some of the miRNAs that regulate cancer chemotherapeutic drug resistance [70][71][72][73][74][75][76][77][78][79][80][70,71,72,73,74,75,76,77,78,79,80].Table 1.
List of some miRNAs that regulate cancer chemoresistance.
| miRNA | Target | Cancer Type | Drug Target | Reference |
|---|---|---|---|---|
| miR-7 | MDR1 | SCLC | Anthracyclines | [70] |
| miR-21 | PTEN | Breast | Trastuzumab | [71] |
| miR-20a | MAPK1 | Colorectal | 5-Fluorouracil | [72] |
| miR-103/107 | P-gp | Gastric | Doxorubicin | [73] |
| miR-196a | MDR1/MRP1 | NSCLC | Cisplatin | [74] |
| miR-17-5p | PHIPP2 | MCL | Topotecan | [75] |
| microRNA-34a | SIRT1, Bcl-2 | Prostate | Paclitaxel | [76] |
| miR-96 | XIAP | Colorectal | 5-Fluorouracil | [77] |
| miR-499a | UBE2V2 | Cervical | 5-Fluorouracil | [78] |
| miR-RNA-449 | NOTCH1 | Ovarian | Doxorubicin | [79] |
| miR-320c | SMARCC1 | Pancreatic | Gemcitabine | [80] |
Abbreviations: MDR1: multidrug resistance mutation 1; PTEN: phosphatase tensin homolog; MAPK1: mitogen-activated protein kinase 1; P-gp: P-glycoprotein; MRP1: multidrug resistance-associated protein 1; PHIPP2: phage phi-PP2; SIRT1: sirtuin 1; XIAP: X-linked inhibitor of apoptosis protein; Bcl-2: B-cell lymphoma-2; UBE2V2: ubiquitin conjugated enzyme E2V2; NOTCH1: human gene; SMARCC1: protein; SCLC: small cell lung carcinoma; NSCLC: non-small-cell lung carcinoma; MCL: mantle cell lymphoma.
