Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nagaraju Kerru and Version 2 by Camila Xu.
Heterogeneous catalysis (HC) has become a valuable means for designing sustainable production protocols to obtain superior intermediates, synthons, and potent bioactive heterocycles. The heterogeneous catalysts proved economical and eco-friendly due to the effectiveness of non-hazardous metals for constructing nanomaterials for atom-economical organic reactions. Heterogeneous catalysts significantly affected scientific and industrial innovations due to their tuneable textural properties, flexible acid-base characteristics, higher thermal stability, and improved reusability, with their excellent performance over their enormous counterparts.
- green synthesis
- single-step synthesis
- pyrans
1. Introduction
Heterogeneous catalysis (HC) has become a valuable means for designing sustainable production protocols to obtain superior intermediates, synthons, and potent bioactive heterocycles [1][2][3][1,2,3]. The heterogeneous catalysts proved economical and eco-friendly due to the effectiveness of non-hazardous metals for the construction of nanomaterials for atom-economical organic reactions [4][5][4,5]. Heterogeneous catalysts significantly affected scientific and industrial innovations due to their tuneable textural properties, flexible acid-base characteristics, higher thermal stability, and improved reusability, with their excellent performance over their enormous counterparts [6][7][8][9][10][11][6,7,8,9,10,11]. Due to their enhanced utility in academia and industries, these materials have received considerable attention in recent years as reusable catalysts to improve the rate of chemical transformation with excellent yields and create substantial routes [12][13][14][15][12,13,14,15]. Over the years, diverse transition metal oxide nanomaterials have been investigated as sustainable, recyclable catalysts for developing bioactive promising small organic molecules [16][17][18][16,17,18]. Thus, the research outcomes have showcased the path for designing novel nanomaterials and investigating the mechanism and reactivity to allow the new ideal routes of organic synthesis [19][20][21][19,20,21].
The prominence of viable heterogeneous catalysts and economic advantages is being revived through efficient green protocols, which are vital in improved organic synthesis [21][22][23][21,22,23]. In particular, one-pot synthetic methodology protocols under green conditions of heterocyclic analogues have become a sizable alternative in modern organic chemistry to conventional multistep synthesis [24][25][26][27][24,25,26,27]. Because of the improved productivity and substantial time saving, multicomponent reactions (MCRs) are recognised as reliable synthetic green methodologies [21][28][29][30][31][21,28,29,30,31]. Several advantages of MCRs driven by green credentials are crucial in organic synthesis and drug discovery programs [32][33][34][35][36][37][38][39][32,33,34,35,36,37,38,39]. Hence, chemists focused on eco-friendly methods to build heterocyclic molecules under sustainable green protocols.
Oxygen-based heterocyclic frameworks have prominence in organic synthesis due to their significant pharmacological applications [40][41][42][43][44][45][40,41,42,43,44,45]. Among the various oxygen-heterocycles, pyran ring moieties have gotten immense consideration for a broad spectrum of medicinal properties. Antidiabetic, anti-HIV, antituberculosis, anticancer, antiproliferative, and antimicrobial activities are some of the prime assets [46][47][48][49][50][51][46,47,48,49,50,51]. Currently, various drugs involving the pyran skeleton are accessible in the market for biological applications (Figure 1). Based on the different therapeutic applications of the pyran ring skeleton, synthesising pyran analogues through MCR strategies by employing diverse heterogeneous catalysts received considerable attention [52][53][52,53]. This monograph summarises the advances in the one-pot construction of molecules with pyran ring derivatives via MCR using sustainable heterogeneous catalysts under green protocols.
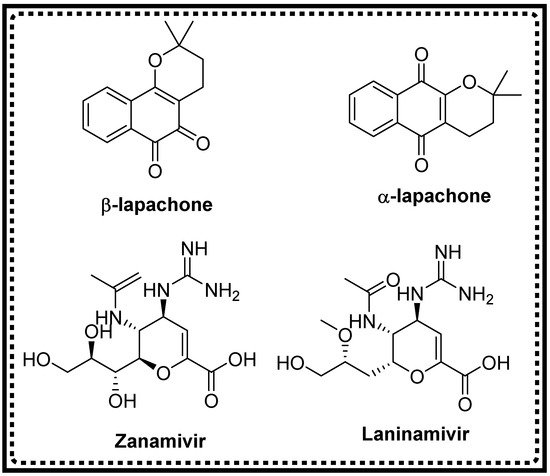
Figure 1.
Commercially used pyran moiety drugs.
2. Synthesis of Pyran Derivatives
2.1. Iron Base Catalysts
Shabani and co-workers [54] concocted an iron-based chlorosilane hetero-nanocomposite and used them synthesised 4H-pyran analogues via green approaches. The prepared magnetic nanocatalyst was subjected to various spectral characterisations such as VSM, TGA, FT-IR, XRD, TEM, SEM, and AFM. VSM established its super magnetic properties, and its particle size was on an average of 23 nm in diameter, as suggested by SEM, TEM and AFM analysis. Its catalytic efficiency in preparing 4H-pyran derivatives (4) via Knoevenagel condensation of substituted aldehydes (2), active methylene group (3) and methyl acetoacetate (1) in ethanol was assessed. The catalytic amount of 10 mg was sufficient to gain a pyran yield of 97% in 15 min of reaction time (Scheme 1).
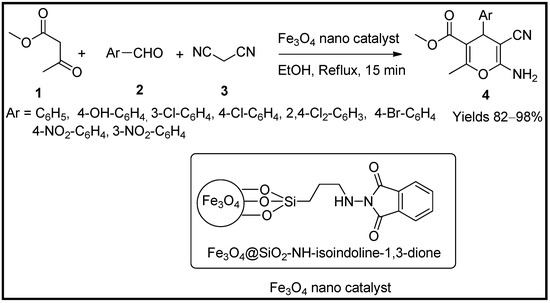
Scheme 1.
Pyran derivatives synthesised by Fe
3
O
4
/SiO
2
/NH-isoindoline-1,3-dione catalyst.
Heravi and co-workers [55] have developed and studied the efficiency of magnetic nanocatalysts embedded with palladium (Fe3O4@SiO2-NH2@Pd). To formulate the catalyst, Fe3O4 nanoparticles were initially prepared by the co-precipitation method of iron sulphates in a 1:2 (Fe2+:Fe3+) ratio in the aqueous medium. The obtained Fe3O4@SiO2@PrNH2 were given an alcoholic wash and vacuum dried at 60 °C for one whole day. Palladium was embedded in the resulting magnetic nanocatalyst by mixing it with Pd(OAc)2. The magnetic nanocatalyst with palladium collected by magnetic decantation was washed with ethanol and vacuum dried. The prepared nanocatalysts were characterised using SEM, TEM, FTIR, TGA and EDS, which revealed a uniform spherical shape of average particle size of 22.7 nm with a core diameter of 21 nm. The EDS data also collected the palladium embedment data, i.e., 2.74 × 10−3 mol/g, with certainty. Its catalytic activity was measured using a model reaction between substituted benzaldehyde (4), active methylene group (3) and dimedone (5)/ethyl acetoacetate (1) in a single-pot system. The reaction followed the Knoevenagel condensation mechanism to gain pyran (7) and teterahydro-chromene (6) derivatives (Scheme 2). The catalyst was quite efficient, with a small quantity of 10 mg to achieve 98% product yield. The catalyst maintained its worth with any considerable degeneration for ten consecutive cycles.
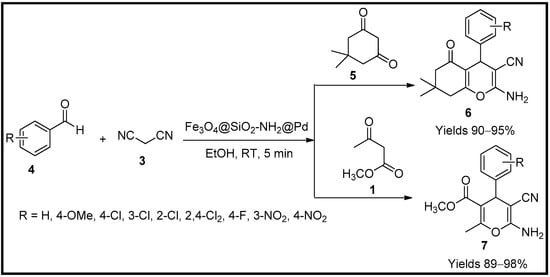
Scheme 2.
Pyran derivatives are synthesised by iron nanocatalysts.
Esmaeili and co-workers [56] formulated a magnetic biopolymer nanocatalyst using xanthan gum to synthesise amino cyano-pyran analogues. The catalyst was prepared by dispersing iron chloride with an ionic ratio of 1:2 for Fe2+:Fe3+ in a solution containing xanthan gum (Fe3O4@Xanthan gum). The prepared catalyst was characterised via FT-IR, FE-SEM, EDX, VSM, TGA, and XRD. The analysis revealed that Fe3O4/xanthan gum had a magnetic concentration of 20 emu/g with monodispersed nanospheres of 14–38 nm with crosslinking among biopolymer matrixes. Its catalytic efficiency on the Knoevenagel condensation between substituted aldehydes (8), malononitrile (3) and dimedone (5) in ethanol was evaluated. The transient intermediate, α-cyanocinnamonitrile, further undergoes addition and tautomerisation to yield 2-amino-3-cyano-4H-pyran analogues (9) (Scheme 3). The biopolymeric nanocatalyst, Fe3O4/xanthan gum, was successful in producing a yield of 96% within 4 min of reaction time at room temperature. The material did not lose its activity even after nine reaction cycles at a stretch.
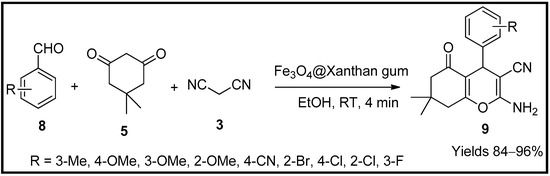
Scheme 3.
Pyran derivatives synthesised by Fe
3
O
4
@Xanthan gum catalyst.
Maleki and co-workers [57] have developed magnetic nanoparticles functionalised with SO3H dendrimers, which can be used as a hetero-catalyst (Fe3O4@-D-NH-(CH2)4-SO3H) for the synthesis of pyrans (11) and polyhydroquinolines (14). Structures established using TEM, FT-IR, SEM, TGA, EDX, and XRD suggested well-established dendrimeric bondage with Fe3O4, uniform spherical formats with a dark magnetic core, and a grey matrix of dendrimer units. It proved an ideal catalyst for the reaction between aldehyde (10), melanonitrile (3) and dimedone (5) and cyclohexanedione (13) (Scheme 4). The proposed mechanism was via Knoevenagel condensation of malononitrile to aldehyde leading to the formation of benzylidenemalononitrile. The product further undergoes addition with cyclohexanedione via Michael addition and cyclisation, resulting in 4H-pyran scaffolds. The prepared nanocomposites offered excellent yields, up to 92% within 10 min, using 0.5 g of the catalyst. The catalyst also retained its capability for five consecutive cycles without any degradation.
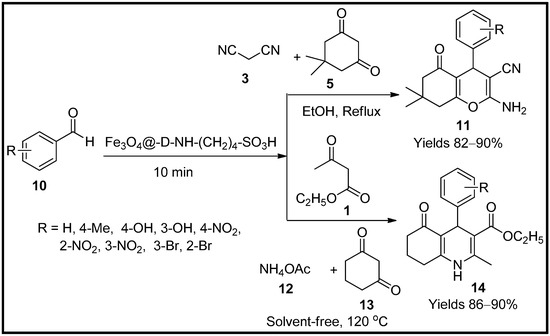
Scheme 4.
Pyran derivatives synthesised by Fe
3
O
4
@-D-NH-(CH
2
)
4
-SO
3
H catalyst.
Mohammadi and Sheibani [58] described the magnetic nanocomposites of guanidine poly acrylic acid functionalised with SO2 (Fe3O4@SiO2-guanidine-PAA). The prepared magnetic nanoparticles were characterised using VSM, TGA, FTIR, TEM, SEM and EDS analysis which suggest a central amorphous shell of silica encapsulating Fe3O4 with a diameter of 14 nm. The morphological size of Fe3O4/SiO2-guanidine@Poly acrylic acid was 20 nm in diameter. Its catalytic activity in preparing dihydro pyranochromenes (17) and tetrahydro benzopyran (18) moieties were evaluated. The one-pot system at 70 °C involved malononitrile (3) and aromatic aldehyde (15). In addition, hydrocoumarin (16) in the former reaction and dimedone for the second (Scheme 5). The catalyst produced excellent yields of 98% in 20 min of reaction time with a minimalist utilise of 50 mg of the catalytic load.
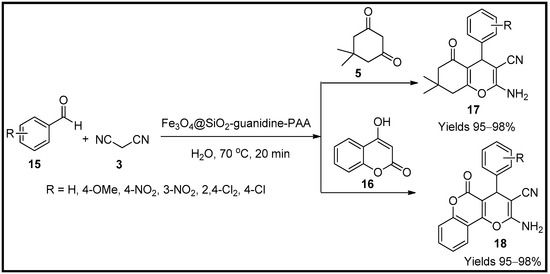
Scheme 5.
Pyran derivatives synthesised by Fe
3
O
4
@SiO
2
-guanidine-PAA catalyst.
Jamshidi et al. [59] have tailored magnetic nanoparticles adhered with HPA dendrimer, a hybrid catalyst material for synthesising pyran analogues (20 and 21), via a green protocol. The prepared nanocomposites were characterised using various analytical techniques such as FTIR, TGA, SEM, XRD and VSM, which suggest a sphere-shaped nanoparticle ranging from 23–25 nm in diameter. A single-pot system involving malononitrile (3), dimedone (5) or ethyl acetoacetate (1) and various benzaldehydes (19) (with electron-donating and withdrawing groups) in the ethanolic medium under reflux conditions was examined (Scheme 6). The catalyst produced a 92% yield within 5 min of reaction time. The reaction mechanism was assumed to proceed via Knoevenagel condensation, Michael addition and cyclisation in sequence. The catalytic activity originated from the conjugated nanocomplex, Fe3O4@Dendrimer-NH2-HPA, by a leaching arrangement. The catalyst excels in various aspects such as easy separation, environmentally supportive and reusability for several reaction cycles without any degradation.
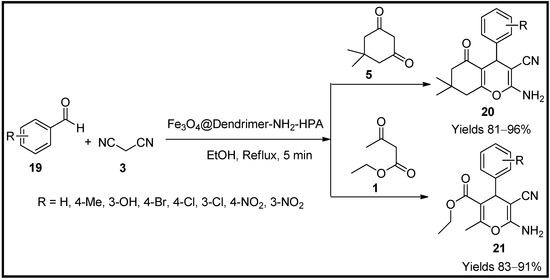
Scheme 6.
Pyran derivatives synthesised by Fe
3
O
4
@Dendrimer-NH
2
-HPA catalyst.
Elhamifar and co-workers [60] prepared phenylsulphonic acid-affiliated magnetite nanoparticles. They employed them as reusable catalysts for preparing tetrahydropyran (23) analogues. The collected nanoparticles of Fe3O4@PhSO3H were subjected to acidic determination through ion-exchange pH assessment, which was reflected in 1.6 mmol/g of sulphonic acid. It was characterised by different analytical instruments such as PXRD, TGA, FTIR, SEM, TEM, VSM and EDX. The thermal stability of the catalyst was below 100 °C. Its sulphonyl and phenyl groups were removed at 400 °C and 700 °C, respectively. The average particle size was 25 nm of spherical particles with a magnetic core and a matrix of phenylsulphonic acid. The catalytic performance was evaluated using an MCR consisting of benzaldehyde (22), malononitrile (3) and dimedone (5) in the presence of 0.2 mg of Fe3O4@PhSO3H nanoparticles under ultrasonication (Scheme 7). The catalyst produced a product of 95% within 25 min of the reaction time. The catalyst was stable and durable for nine consecutive cycles with the same affinity.
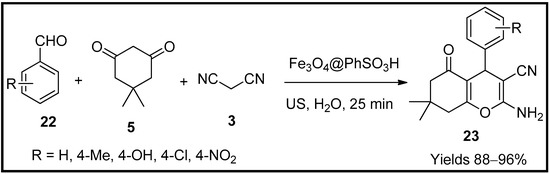
Scheme 7.
Pyran derivatives synthesised by Fe
3
O
4
@PhSO
3
H catalyst.
Aghbash and co-workers [61] have tailored magnetic silica functionalised with MCM-41 adhered to DABCO (Fe3O4@silica-MCM-41@DABCO) and used as a catalyst material for synthesising amino dihydropyrano analogues (26). The catalyst was characterised using different analytical methods such as VAM, XRD, FTIR, SEM, EDX and TEM. A mesoporous framework with a regular spherical morphology of a particle size 19–67 nm and magnetisation of 12.5 emu/g was suggested. The catalytic affinity of the prepared nanocatalyst was evaluated in the preparation of amino dihydropyrano cyanoparan moieties using a multicomponent system of benzaldehyde (25), azido-KA (24) and malononitrile (3) in DMF solvent medium. The catalyst produced excellent yields of up to 98% with a catalytic load of 3 mg under reflux within half an hour (Scheme 8). The mechanism of the product formation was presumed to proceed through Knoevenagel condensation leading to Michael’s addition. This method has an advantage in getting high yields at shorter reaction spans.
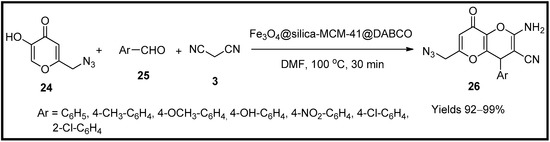
Scheme 8.
Pyran derivatives synthesised by Fe
3
O
4
@silica-MCM-41@DABCO catalyst.
Mofrad and co-workers [62] have developed silica-adhered magnetite nanoparticles composed of ferrocene-infused ionic liquid, which can be used as green catalytic media for the preparation of amino cyano pyran (29) scaffolds. The researcheuthors established the catalyst characteristics using FTIR, SEM, XRD, TEM and EDX techniques. The magnetisation of the prepared particles was 28 emu/g, and a spherical shape with a magnetic core enveloped in a silica shell with a matric of imidoferrocene having a crystalline structure of 50.39 nm in diameter. To assess the catalytic efficiency of [Fe3O4/SiO2/Im-Fc][OAc], a single-pot system consisting of β-naphthol (27), malononitrile (3) along with an aldehyde (28) was used. In the absence of solvent, the mixture was continuously stirred at 90 °C to obtain the desired amino cyano pyran moieties (Scheme 9). The feasible reaction mechanism proceeded via Knoevenagel, Michael and tautomeric cyclisations. The catalyst with a load of 10 mg generated the product of 94% yield within 15 min. This catalyst offers high yields with a short reaction time with minimal usage.
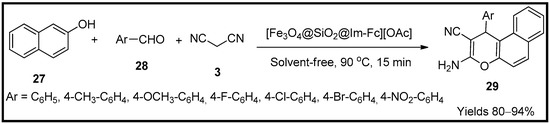
Scheme 9.
Pyran derivatives synthesised by [Fe
3
O
4
/SiO
2
/Im-Fc][OAc catalyst.
Manesh and co-workers [63] have developed magnetic nanoparticles of Fe3O4/SiO2/CPS encapsulated with spiro[indoline-3,4′-[1][3][1,3][dithiine]-Ni-composite, which can be used as green heterogeneous catalyst for the preparation of dihydropyran (32) scaffolds. The magnetic nanoparticles were characterised using FTIR, XRD, EDX, VSM, BET, SEM and TEM. The material possessed a mesoporous particle size of 16 nm and a surface area of 16.92 m2/g with 55.1 emu/g of magnetisation. The catalytic properties of the catalyst were confirmed using a single-pot reaction system consisting of oxirane (31) and isocyanide (30) in ethanol followed by malononitrile (3) with continuous stirring at 60 °C (Scheme 10). The reaction was carried out in a basic medium from which the desired pyran product was separated from the catalyst using an external magnetic field. The prepared pyran moieties were washed and dried over MgSO4. The pyran moieties were characterised using various spectral analyses such as FTIR, 1HNMR and 13CNMR. The catalyst resulted in good yields of 88% within 8 h of reaction time and a catalytic load of 15 min under reflux. The proposed mechanism leads via Knoevenagel, Michael addition followed by cyclisation.
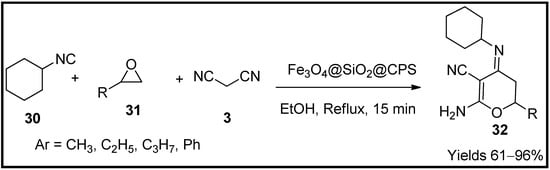
Scheme 10.
Pyran derivatives synthesised by Fe3O4/SiO2/CPS catalyst.
Maleki and co-workers [64] synthesised tetrahydro pyran (34) scaffolds using a Schiff-nickel complex and infused them with magnetite nanoparticles (Fe3O4@SiO2@NiSB). The gathered catalytic nanoparticles were characterised using FTIR, SEM, EDX, XRD, TEM, TGA and VSM. The nanoparticles were spherical with 35 nm in diameter and a surface area of 61.606 m2/g. The catalytic capacity was evaluated through an MCR consisting of benzaldehyde (33), malononitrile (3) and dimedone (5) to prepare tetrahydro pyrans in a solvent-free environment (Scheme 11). The catalyst yielded excellent products of up to 98% with a catalytic load of 10 mg within a reaction time of 5 min at room temperature. The reaction occurs via Knoevenagel condensation and Michael’s addition. The catalyst produced excellent yields and considerably decreased the reaction time under green optimisation.
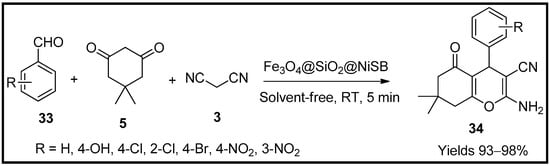
Scheme 11.
Pyran derivatives synthesised by Fe
3
O
4
@SiO
2
@NiSB catalyst.
Dazmiri and co-workers [65] have extracted magnetic zinc oxide nanoparticles (Fe3O4/ZnO MCNPs) from the water extract of Petasites hybridus rhizome to be used as an eco-friendly catalyst for the synthesis of pyran scaffolds. They also studied the antimicrobial and antioxidative properties of the prepared pyran scaffolds (36). The gathered nanoparticles were characterised using FTIR, EDX, XRD, SEM and TEM, which determined their particle size to be 40 nm in diameter with a uniform distribution of elements. The catalytic activity was evaluated using a single-pot reaction consisting of aromatic aldehyde (35), dimedone (5), malononitrile (3) and catalyst composite in an ethanolic medium. The obtained pyran yield was excellent, up to 90% within 10 min at room temperature (Scheme 12). The reaction mechanism proceeds via Knoevenagel and Michael’s reactions. The antimicrobial properties were tested against E. Coli bacteria, whereas the antioxidant and reducing properties were evaluated using DPPH and Fe3+, respectively.
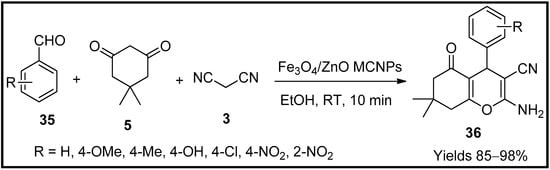
Scheme 12.
Pyran derivatives synthesised by Fe
3
O
4
/ZnO MCNPs catalyst.
Afruzi and co-workers [66] have developed a polymer of magnetised acrylonitrile in combination with melamine (PAN@melamine/Fe3O4) that can be used in the preparation of pyrano pyrazoles (40) and amino cyano pyrans (39). The gathered magnetic polymeric chains were characterised using FTIR, XRD, EDX, TGA, SEM, TEM and VSM, which suggest a spherical shape with 40 nm in diameter and magnetisation of 29.2 emu/g. A single-pot reaction consisting of benzaldehyde (37), malononitrile (3), hydrazine hydrate (38) and acetoacetate (1) in an ethanolic medium under the influence of the polymer material (Scheme 13) was evaluated. The reaction was proposed as a combination of Knoevenagel and Michael and tautomeric cyclisations. The catalyst load of 10 mg was efficient in generating 97% of yield in 10 min. The catalyst did not show any considerable degradation even after five consecutive cycles and performed almost as a freshly prepared catalyst for every use. This method’s assets are eco-friendly conditions and easily recyclable catalysts.
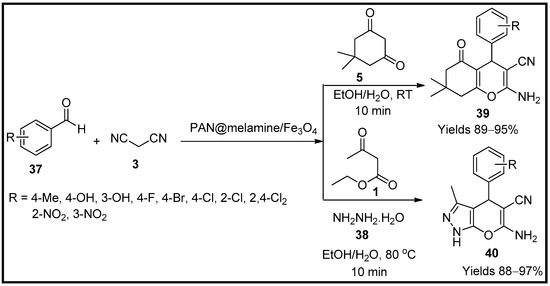
Scheme 13.
Pyran derivatives synthesised by PAN@melamine/Fe
3
O
4
catalyst.
Heravi et al. [67] synthesised pyran (44) and pyrimidine (45) analogues using magnetite nanoparticles (nano-Fe3O4) as a heterogeneous catalyst. The prepared magnetic nanoparticles were characterised using FTIR, SEM, TEM, and VSM, which suggest a spherical shape of the catalyst particles. A mixture consisting of benzaldehyde (41), malononitrile (3) and pyruvic acid (42) was used as a model reaction in the presence of magnetite nanoparticles (Scheme 14). They also compared various other catalysts under the same reaction conditions. The freshly prepared magnetite nanoparticles produced a 91% yield within a reaction span of one hour under reflux with a catalytic load of 20 mg. The target compounds were analysed using FTIR, 1H and 13C NMR. This catalyst was rotated for four consecutive cycles without considerable degradation. The probable mechanism involves the Knoevenagel reaction and magnetite acting as Lewis acid to facilitate the Michael addition and cyclisation.
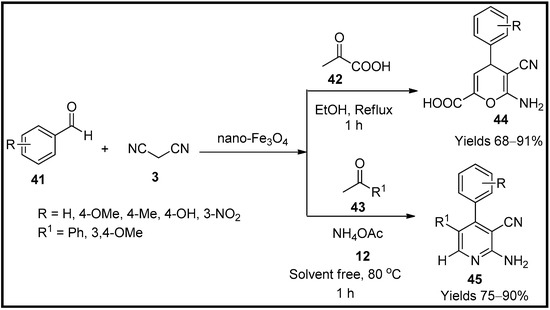
Scheme 14.
Pyran derivatives synthesised by nano-Fe
3
O
4
catalyst.
Sandaroos et al. [68] have synthesised tetrahydro furopyran (48) scaffolds using iron triflate nanocatalyst (Fe(OTf)3). It was explored as a catalyst for an MCR system consisting of aromatic aldehyde (46), tetronic acid (47) and malononitrile (3) in the presence of acetonitrile. The reaction mixture was refluxed at ambient temperature with continuous stirring to obtain the desired furopyran moieties separated from the solvent through evaporation and then diluted with CH2Cl2 and water (Scheme 15). The reaction mechanism was expected to proceed through Knoevenagel and Michael reaction within a reaction time of 7 h to produce a yield of 92% with a catalytic load of 15 mg. This method has the advantage of excellent harvests with an easy workup plan.
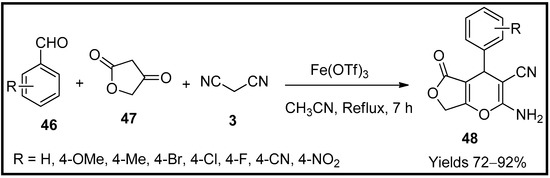
Scheme 15.
Pyran derivatives synthesised by Fe(OTf)
3
catalyst.
