Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 3 by Camila Xu.
Polyvinylidene fluoride (PVDF), the chemical formula is (C2H2F2)n. Its basic building blocks are therefore carbon, hydrogen, and fluorine. These three elements can form several crystalline chain conformations. Conformations are defined by polar and nonpolar phases. Four phases are most commonly found in the literature: α-, β-, γ-, and δ-.
- degradation
- electrospinning
- fabrication
- nanofibers
1. Introduction
Nowadays, there are countless synthetically produced polymers with different properties and functions. Organic polyvinylidene fluoride (PVDF) can be classified as one of the most interesting. The reason that has made it so popular is due to its vast range of applications precisely because of its properties [1]. This semicrystalline fluoropolymer has a considerable chemical resistance, high mechanical strength, extensive operating temperature range (the glass transition temperature is −35 °C and the melting point is 177 °C). Moreover, it is biocompatible and highly hydrophobic. It can be argued that similar properties can be achieved with other types of polymers, which is certainly true. For example, polytetrafluoroethylene (PTFE) is regarded as an ideal alternative, but quite expensive [2]. As another similar, fluorinated ethylene propylene (FEP) can be considered, which is suitable for generators in an outdoor environment [3][4][5]. However, PVDF has one other unique property, and that is the ability to generate a charge, not only using the triboelectric effect but also using piezoelectricity, which is a great advantage over other polymers. Thus, PVDF can rightly be called a nanogenerator. Scientists in many fields are exploring the combination of all these properties and the ability to generate a charge [6][7][8][9][10][11]. This rpapesearchr selects a few of the most widely used and interesting ones. In general, these energy harvesters have taken the name of piezoelectric nanogenerators (PENGs) or triboelectric nanogenerators (TENGs). Although there are many other and not less interesting representatives of both PENGs or TENGs [12], PVDF can also act as a hybrid in both cases [10], and this capability makes it quite distinctive.
First of all, it is necessary to describe PVDF in terms of its chemical structure. The chemical formula is (C2H2F2)n. Its basic building blocks are therefore carbon, hydrogen, and fluorine. These three elements can form several crystalline chain conformations [13]. Conformations are defined by polar and nonpolar phases. Four phases are most commonly found in the literature: α-, β-, γ-, and δ-. Less commonly mentioned is a fifth ϵ-phase [14]. Phases α and ϵ belong to the nonpolar ones. Molecules with antiparallel packing of the dipoles are nonpolar bonds and have no dipole moment [14]. Conversely, polar molecules do not have a full covalent bond, so an imbalance in the electron charge of the molecule is present. The imbalance in the distribution of electrons generates dipoles. The dipoles will try to align themselves when an electric field is provided. The polarity of a molecule affects the attraction between molecular chains. Furthermore, nonpolar polymers are less permeable to water than polar polymers [15]. Thus, it is clear that the polar phases are the most interesting to observe in the case of charge generation. The most associated with charge generation is the β-phase [14]. Furthermore, not neglected is the polar γ-phase, where its polarization effect is weaker. This is because the gauche bond exists in every fourth repeat unit [16]. The often mentioned nonpolar α-phase is usually obtained from the melt by crystallization. The phase conformations of PVDF polymer are illustrated in Figure 1, where their different structures can be clearly seen.
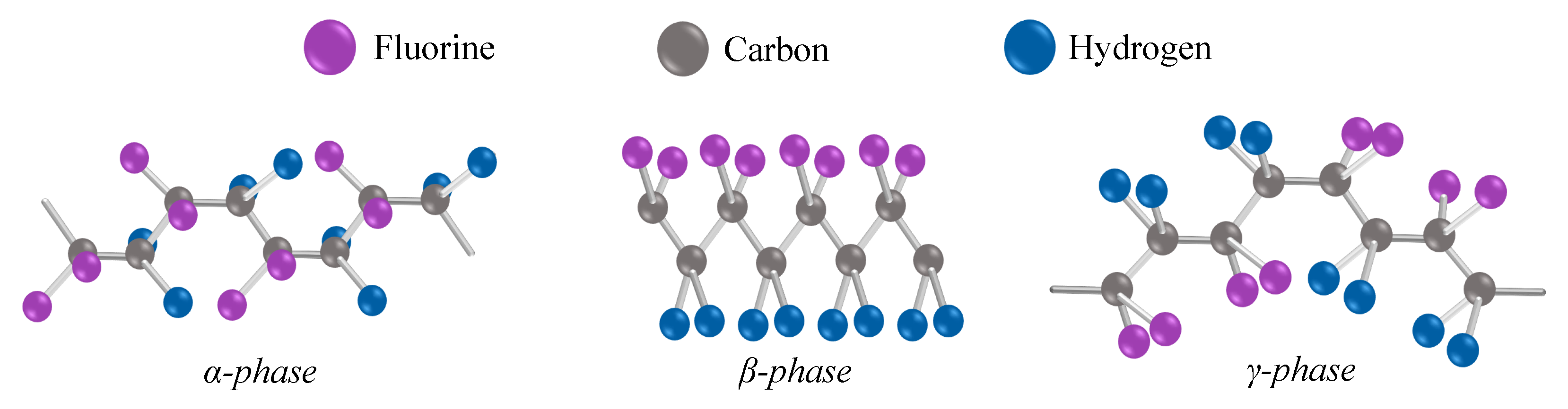
Figure 1. The chain conformation of the most observed phases in PVDF [17]. Because the fluorine atoms in the β-phase are situated on the same side of the molecular chains, which are arranged parallel to one another in a specific direction, with the same dipole orientation and enhanced polarity, the β-phase exhibits spontaneous polarization strength as well as pyro- and piezoelectric properties [18].
Popular methods for phase characteristics are Fourier transform infrared spectroscopy (FTIR), Raman Spectroscopy, X-ray diffraction (XRD), and differential scanning calorimetry (DSC).
2. Material Reactions and Degradation
As already mentioned, PVDF is a nonreactive polymer and has a high toxic resistance. Its resistance to degradation can be considered higher compared to other polymers [19]. Changes occur mainly with higher temperatures and standardly may be combinations of two or more effects (mechanical properties, crystallinity, color, etc.) [20]. It is commonly able to resist basic solutions, chlorine solution, alcohols, several acids, halogens, and aliphatic or aromatic compounds [21]. PVDF weakens when various alkaline solutions are used [22][23]. Lactic acid C3H6O3, nitric acid HNO3, sulfuric acid H2SO4, and tetrahydrofuran C4H8O are mentioned as having little resistance to acids. Its color may change from pure white to yellow, and during the dehydrochlorination process (loss of hydro fluoride units from the polymer chain), it may darken to black [24]. Furthermore, less resistant are glucose and wine vinegar, which is essentially a concentrate of acetic acid. When exposed to higher temperatures, the damage caused by these substances increases. Regarding thermal degradation, temperature can affect charge generation mainly because of the weakening of mechanical properties at very low temperatures, i.e., the glass transition temperature of −35 °C, when the material hardens and becomes more brittle. Below this critical value, the material may degenerate as it loses its elasticity and flexibility, which naturally limits the development of piezoelectric phenomena. This temperature is relatively low compared to commonly known polymers. For example, PTFE has Tg = 115 °C. On the other hand, at the melting temperature of 177 °C, the crystallization process is affected. For PTFE, Tm = 327 °C. Exceeding any of these threshold temperatures can lead to a different phase transition of the material, which is addressed, for example, by the previously mentioned DSC [25]. Despite all the mentioned drawbacks, PVDF is very stable within these values and it is often called a thermoplastic polymer. The temperatures mentioned may vary slightly in units of degrees depending on the manufacturer of the commercial polymer type. For PVDF, which has flexible fibers, the critical issue is mechanical strength which is, of course, very different from solid layers. In fact, a good tensile strength is required for a piezoelectric generator. If the nanofibers do not have additional support, they can be damaged more quickly. Microcracks and point defects can already occur with imperfect fabrication, which can be, for example, a contamination of the microparticles. However, it can still be argued that cracks may not be spread throughout the material in the case of single fibers as opposed to solid layers. The tensile strength, elongation at break, and Young’s modulus of the PVDF fiber were measured by Hasim, Liu, and Li [26] in sodium hydroxide NaOH solutions. The results in degradation changes were classified as significant. Here again, the increased temperature also accelerated the aging of the samples.3. Utilization in Real Applications
Although PVDF can be used as a nanogenerator of energy, it is a relatively broad concept. Therefore, it is appropriate to mention several interesting works that are experimentally devoted to specific applications. Hence, this section does not primarily serve to describe the various fillers and other composite combinations that can improve PVDF properties, but to describe the direct applications of PVDF itself. A widespread and intended use of PVDF as a nanofabric is directly for the human body. There can be several uses. The simplest uses can be as a wearable shirt, generating charge when walking and moving [27], during inhaling and exhaling [28], or blood flow [29][30][31], or as gloves [32]. It can also serve as a shoe insole [33][34][35][36], or while typing on the keyboard [37]. For these applications, PVDF can operate just as a common sensor (safety monitoring, medical diagnostics), or as a nanogenerator depending on its performance [38][39]. In these cases, the connection to the IoT is also assumed [40]. In addition to such practical uses, PVDF can also be used purely as a green energy harvester, for example, for wind energy harvesting [3][5][41], or for energy harvesting from ocean waves and applications [42][43]. Among the less common but emerging uses may include the use of PVDF as scaffolds in tissue engineering due to its biocompatibility. For example, for osteoblasts (bone cells), where their electromechanical stimulation can accelerate cell spreading [44][45][46]. For solar cells, PVDF can serve as an enhancer of the crystallinity of currently very popular perovskites [47], when there are already attempts to create a hybrid inducing the piezophototronic effect [48]. Another unique use of the PVDF nanogenerator also appears to be energy harvesting from sound waves [49][50].References
- Centa, U.G.; Mihelčič, M.; Bobnar, V.; Remškar, M.; Perše, L.S. The Effect of PVP on Thermal, Mechanical, and Dielectric Properties in PVDF-HFP/PVP Thin Film. Coatings 2022, 12, 1241.
- Zhang, S.; Shen, J.; Qiu, X.; Weng, D.; Zhu, W. ESR and vibrational spectroscopy study on poly(vinylidene fluoride) membranes with alkaline treatment. J. Power Sources 2006, 153, 234–238.
- Ren, Z.; Wang, Z.; Liu, Z.; Wang, L.; Guo, H.; Li, L.; Li, S.; Chen, X.; Tang, W.; Wang, Z.L. Energy Harvesting from Breeze Wind (0.7–6 m s−1) Using Ultra-Stretchable Triboelectric Nanogenerator. Adv. Energy Mater. 2020, 10, 2001770.
- Ren, Z.; Ding, Y.; Nie, J.; Wang, F.; Xu, L.; Lin, S.; Chen, X.; Wang, Z.L. Environmental Energy Harvesting Adapting to Different Weather Conditions and Self-Powered Vapor Sensor Based on Humidity-Responsive Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2019, 11, 6143–6153.
- Ren, Z.; Wang, Z.; Wang, F.; Li, S.; Wang, Z.L. Vibration behavior and excitation mechanism of ultra-stretchable triboelectric nanogenerator for wind energy harvesting. Extrem. Mech. Lett. 2021, 45, 101285.
- Tofel, P.; Částková, K.; Říha, D.; Sobola, D.; Papež, N.; Kaštyl, J.; Ţălu, Ş.; Hadaš, Z. Triboelectric Response of Electrospun Stratified PVDF and PA Structures. Nanomaterials 2022, 12, 349.
- Singh, H.H.; Khare, N. Flexible ZnO-PVDF/PTFE based piezo-tribo hybrid nanogenerator. Nano Energy 2018, 51, 216–222.
- Mariello, M. Recent Advances on Hybrid Piezo-Triboelectric Bio-Nanogenerators: Materials, Architectures and Circuitry. Nanoenergy Adv. 2022, 2, 4.
- Wang, X.; Yang, B.; Liu, J.; Zhu, Y.; Yang, C.; He, Q. A flexible triboelectric-piezoelectric hybrid nanogenerator based on P(VDF-TrFE) nanofibers and PDMS/MWCNT for wearable devices. Sci. Rep. 2016, 6, 36409.
- Jung, W.S.; Kang, M.G.; Moon, H.G.; Baek, S.H.; Yoon, S.J.; Wang, Z.L.; Kim, S.W.; Kang, C.Y. High Output Piezo/Triboelectric Hybrid Generator. Sci. Rep. 2015, 5, 9309.
- Lapčinskis, L.; Mā Lnieks, K.; Linarts, A.; Blū Ms, J.; Šmits, K.N.; Järvekülg, M.; Knite, M.R.; Šutka, A. Hybrid Tribo-Piezo-Electric Nanogenerator with Unprecedented Performance Based on Ferroelectric Composite Contacting Layers. ACS Appl. Energy Mater. 2019, 2, 4027–4032.
- Ren, Z.; Nie, J.; Shao, J.; Lai, Q.; Wang, L.; Chen, J.; Chen, X.; Lin Wang, Z.; Ren, Z.; Nie, J.; et al. Fully Elastic and Metal-Free Tactile Sensors for Detecting both Normal and Tangential Forces Based on Triboelectric Nanogenerators. Adv. Funct. Mater. 2018, 28, 1802989.
- Bohlén, M.; Bolton, K. Conformational studies of poly(vinylidene fluoride), poly(trifluoroethylene) and poly(vinylidene fluoride-co-trifluoroethylene) using density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 12929–12939.
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly(vinylidene fluoride). Polymers 2018, 10, 228.
- McKeen, L. Film Properties of Plastics and Elastomers, 3rd ed.; William Andrew Publishing: Boston, MA, USA, 2012; p. 408.
- Song, R.; Yang, D.; He, L. Effect of surface modification of nanosilica on crystallization, thermal and mechanical properties of poly(vinylidene fluoride). J. Mater. Sci. 2007, 42, 8408–8417.
- Pisarenko, T.; Papež, N.; Sobola, D.; Ţălu, Ş.; Částková, K.; Škarvada, P.; Macků, R.; Ščasnovič, E.; Kaštyl, J. Comprehensive Characterization of PVDF Nanofibers at Macro- and Nanolevel. Polymers 2022, 14, 593.
- Lin, Y.; Zhang, Y.; Zhang, F.; Zhang, M.; Li, D.; Deng, G.; Guan, L.; Dong, M. Studies on the electrostatic effects of stretched PVDF films and nanofibers. Nanoscale Res. Lett. 2021, 16, 79.
- Wang, H.; Klosterhalfen, B.; Müllen, A.; Otto, T.; Dievernich, A.; Jockenhövel, S. Degradation resistance of PVDF mesh in vivo in comparison to PP mesh. J. Mech. Behav. Biomed. Mater. 2021, 119, 104490.
- de Jesus Silva, A.J.; Contreras, M.M.; Nascimento, C.R.; da Costa, M.F. Kinetics of thermal degradation and lifetime study of poly(vinylidene fluoride) (PVDF) subjected to bioethanol fuel accelerated aging. Heliyon 2020, 6, e04573.
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26.
- Hoa, S.V.; Ouellette, P. Stress corrosion cracking of poly(vinylidene fluoride) in sodium hydroxide. Polym. Eng. Sci. 1983, 23, 202–205.
- Zhao, X.; Song, L.; Fu, J.; Tang, P.; Liu, F. Experimental and DFT investigation of surface degradation of polyvinylidene fluoride membrane in alkaline solution. Surf. Sci. 2011, 605, 1005–1015.
- Shinohara, H. Fluorination of polyhydrofluoroethylenes. II. Formation of perfluoroalkyl carboxylic acids on the surface region of poly(vinylidene fluoride) film by oxyfluorination, fluorination, and hydrolysis. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 1543–1556.
- Ross, G.J.; Watts, J.F.; Hill, M.P.; Morrissey, P. Surface modification of poly(vinylidene fluoride) by alkaline treatment1. The degradation mechanism. Polymer 2000, 41, 1685–1696.
- Awanis Hashim, N.; Liu, Y.; Li, K. Stability of PVDF hollow fibre membranes in sodium hydroxide aqueous solution. Chem. Eng. Sci. 2011, 66, 1565–1575.
- Tao, X.; Zhou, Y.; Qi, K.; Guo, C.; Dai, Y.; He, J.; Dai, Z. Wearable textile triboelectric generator based on nanofiber core-spun yarn coupled with electret effect. J. Colloid Interface Sci. 2022, 608, 2339–2346.
- Mhetre, M.R.; Abhyankar, H.K. Human exhaled air energy harvesting with specific reference to PVDF film. Eng. Sci. Technol. Int. J. 2017, 20, 332–339.
- Wang, Y.J.; Sue, C.Y. Noninvasive Blood Pressure Measurement Using PVDF Fibers Fabricated by NFES and A Photoplethysmography Sensor. J. Autom. Control. Eng. 2017, 7, 34–38.
- Bifulco, P.; Gargiulo, G.D.; D’Angelo, G.; Liccardo, A.; Romano, M.; Clemente, F.; Cesarelli, M. Monitoring of respiration, seismocardiogram and heart sounds by a PVDF piezo film sensor. In Proceedings of the 20th IMEKO TC4 Symposium on Measurements of Electrical Quantities: Research on Electrical and Electronic Measurement for the Economic Upturn, Together with 18th TC4 International Workshop on ADC and DCA Modeling and Testing, IWADC 2014, Benevento, Italy, 15–17 September 2014; pp. 786–789.
- Lee, W.K.; Chung, G.S.; Baek, H.J.; Park, K.S. Heart sounds measurement using PVDF film sensor and their comparison with RR intervals of ECG signals. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics: Global Grand Challenge of Health Informatics, BHI 2012, Hong Kong, China, 5–7 January 2012; pp. 864–866.
- Åkerfeldt, M.; Lund, A.; Walkenström, P. Textile sensing glove with piezoelectric PVDF fibers and printed electrodes of PEDOT:PSS. Text. Res. J. 2015, 85, 1789–1799.
- Yu, L.; Zhou, P.; Wu, D.; Wang, L.; Lin, L.; Sun, D. Shoepad nanogenerator based on electrospun PVDF nanofibers. Microsyst. Technol. 2018, 25, 3151–3156.
- Xin, Y.; Li, X.; Tian, H.; Guo, C.; Sun, H.; Wang, S.; Wang, C. A shoe-equipped piezoelectric transducer system based on PVDF film. Integr. Ferroelectr. 2016, 176, 140–149.
- Klimiec, E.; Zaraska, W.; Piekarski, J.; Jasiewicz, B. PVDF Sensors—Research on Foot Pressure Distribution in Dynamic Conditions. Adv. Sci. Technol. 2013, 79, 94–99.
- Lee, D.W.; Jeong, D.G.; Kim, J.H.; Kim, H.S.; Murillo, G.; Lee, G.H.; Song, H.C.; Jung, J.H. Polarization-controlled PVDF-based hybrid nanogenerator for an effective vibrational energy harvesting from human foot. Nano Energy 2020, 76, 105066.
- Liu, S.; Chen, L.; Zhu, X.; Dai, L.; Xin, Y. Digital piano keyboard based on PVDF piezoelectric film. In Proceedings of the ICALIP 2010—2010 International Conference on Audio, Language and Image Processing, Shanghai, China, 23–25 November 2010; pp. 378–382.
- Mokhtari, F.; Shamshirsaz, M.; Latifi, M.; Foroughi, J. Nanofibers-Based Piezoelectric Energy Harvester for Self-Powered Wearable Technologies. Polymers 2020, 12, 2697.
- Szperlich, P. Piezoelectric A15B16C17 Compounds and Their Nanocomposites for Energy Harvesting and Sensors: A Review. Materials 2021, 14, 6973.
- Dai, Y.; Chen, J.; Tian, W.; Xu, L.; Gao, S. A PVDF/Au/PEN Multifunctional Flexible Human–Machine Interface for Multidimensional Sensing and Energy Harvesting for the Internet of Things. IEEE Sens. J. 2020, 20, 7556–7568.
- Wen, Q.; He, X.; Lu, Z.; Streiter, R.; Otto, T. A comprehensive review of miniatured wind energy harvesters. Nano Mater. Sci. 2021, 3, 170–185.
- Viet, N.V.; Wu, N.; Wang, Q. A review on energy harvesting from ocean waves by piezoelectric technology. J. Model. Mech. Mater. 2017, 1.
- Jbaily, A.; Yeung, R.W. Piezoelectric devices for ocean energy: A brief survey. J. Ocean. Eng. Mar. Energy 2015, 1, 101–118.
- Gong, T.; Li, T.; Meng, L.; Chen, Y.; Wu, T.; Zhou, J.; Lu, G.; Wang, Z. Fabrication of piezoelectric Ca-P-Si-doped PVDF scaffold by phase-separation-hydration: Material characterization, in vitro biocompatibility and osteoblast redifferentiation. Ceram. Int. 2022, 48, 6461–6469.
- Dumitrescu, L.N.; Neacsu, P.; Necula, M.G.; Bonciu, A.; Marascu, V.; Cimpean, A.; Moldovan, A.; Rotaru, A.; Dinca, V.; Dinescu, M. Induced Hydrophilicity and In Vitro Preliminary Osteoblast Response of Polyvinylidene Fluoride (PVDF) Coatings Obtained via MAPLE Deposition and Subsequent Thermal Treatment. Molecules 2020, 25, 582.
- Kitsara, M.; Blanquer, A.; Murillo, G.; Humblot, V.; De Bragança Vieira, S.; Nogués, C.; Ibáñez, E.; Esteve, J.; Barrios, L. Permanently hydrophilic, piezoelectric PVDF nanofibrous scaffolds promoting unaided electromechanical stimulation on osteoblasts. Nanoscale 2019, 11, 8906–8917.
- Sun, C.; Guo, Y.; Fang, B.; Yang, J.; Qin, B.; Duan, H.; Chen, Y.; Li, H.; Liu, H. Enhanced Photovoltaic Performance of Perovskite Solar Cells Using Polymer P(VDF-TrFE) as a Processed Additive. J. Phys. Chem. C 2016, 120, 12980–12988.
- Nie, J.; Zhang, Y.; Dan, M.; Wang, J.; Li, L.; Zhang, Y. Piezophototronic Effect Enhanced Perovskite Solar Cell Based on P(VDF-TrFE). Solar RRL 2021, 5, 2100692.
- Park, S.; Kim, Y.; Jung, H.; Park, J.Y.; Lee, N.; Seo, Y. Energy harvesting efficiency of piezoelectric polymer film with graphene and metal electrodes. Sci. Rep. 2017, 7, 17290.
- Kargar, S.M.; Hao, G. An Atlas of Piezoelectric Energy Harvesters in Oceanic Applications. Sensors 2022, 22, 1949.
More
