The term “ROS (Reactive Oxygen Species)” refers to radicals and ions that contain an unpaired numbered electron in its outmost electron field which are highly reactive metabolic byproducts that can have both harmful and useful effects within the cell. Quercetin (QC), a plant-derived bioflavonoid, is known for its ROS scavenging properties and was recently discovered to have various antitumor properties in a variety of solid tumors. Adaptive stress responses may be induced by persistent ROS stress, allowing cancer cells to survive with high levels of ROS while maintaining cellular viability. Large amounts of ROS make cancer cells extremely susceptible to quercetin, one of the most available dietary flavonoids. Because of the molecular and metabolic distinctions between malignant and normal cells, targeting ROS metabolism might help overcome medication resistance and achieve therapeutic selectivity while having little or no effect on normal cells. The powerful bioactivity and modulatory role of quercetin has prompted extensive research into the chemical, which has identified a number of pathways that potentially work together to prevent cancer, alongside, QC has a great number of evidences to use as a therapeutic agent in cancer stem cells.
- ROS
- quercetin
- cancer stem cells
1. Introduction
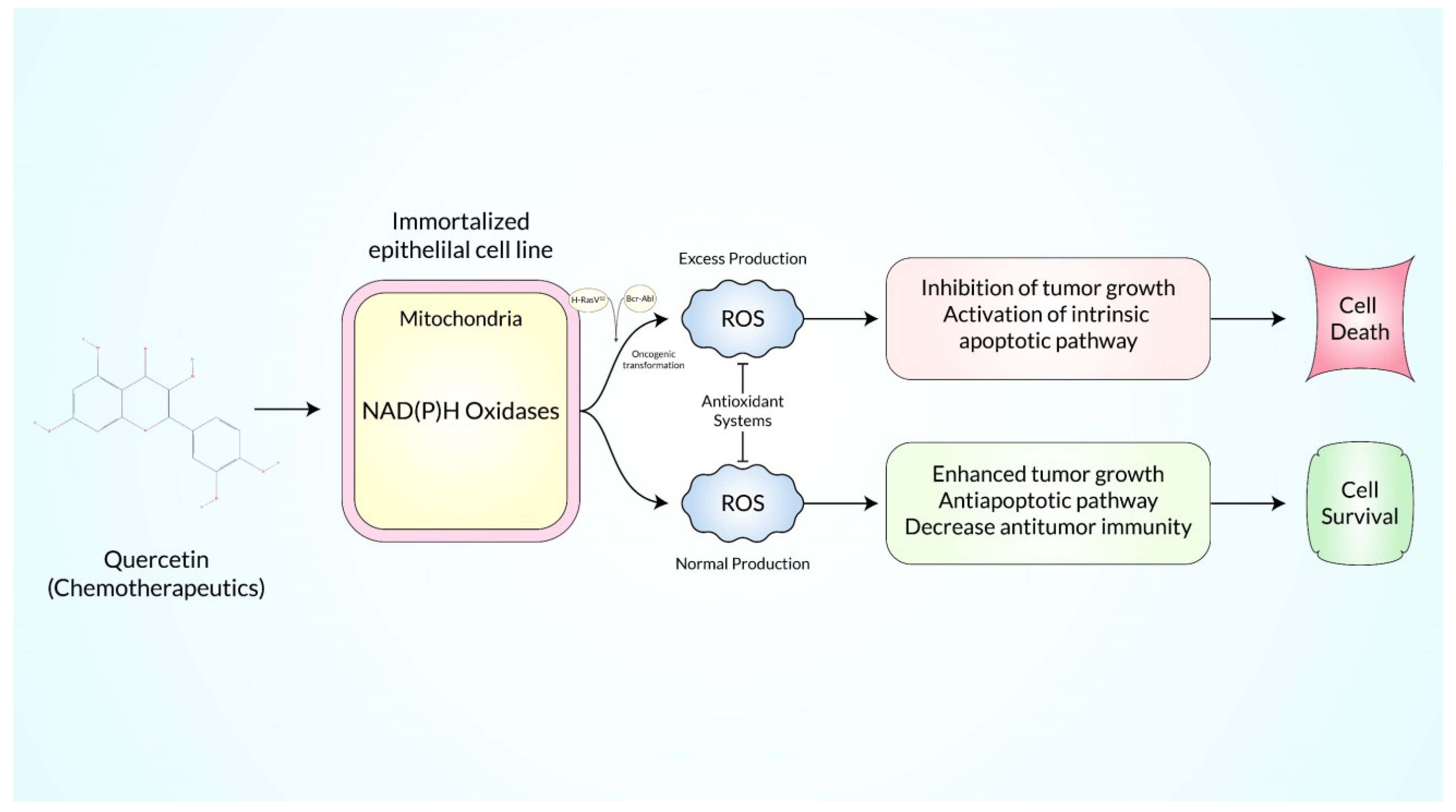
2. Reactive Oxygen Species-Mediated Regulation of the MAPK/ERK1/2 Pathways
| Sort of Cancer | Research/Experiment Type | Specific Cell Line/s | Core Molecular Mechanism | Doses | Final Outcomes | Reference |
|---|---|---|---|---|---|---|
| Colorectal cancer | In vitro | HT29 cells | Induced G2/M arrest | 75 µM | Enhanced the efficacy of low concentration of doxorubicin chemotherapy in inhibiting cell proliferation, enhance cytotoxicity and apoptosis | [35] |
| Breast cancer | In vitro | MDA-MB-231 | Lowered the expression levels of proteins such as aldehyde dehydrogenase 1A1, C-X-C chemokine receptor type 4, mucin 1 and epithelial cell adhesion molecules responsible for tumorigenesis | 50 μM | Suppressed breast cancer stem cell proliferation, self-renewal, and invasiveness | [36] |
| Prostate cancer | In vitro | PC-3 and LNCaP cells | Activated capase-3/7 and inhibit the expression of Bcl-2, surviving and XIAP in CSCs. Furthermore, inhibits epithelial-mesenchymal transition by inhibiting the expression of vimentin, slug, snail and nuclear β-catenin, and the activity of LEF-1/TCF responsive reporter | 20 μM | Quercetin synergized with epigallocatechin gallate inhibited the self-renewal properties of prostate CSCs, inducing apoptosis, and blocking CSC’s migration and invasion | [37] |
| Breast cancer | In vitro | MCF-7 and MCF-7/dox cell lines | Downregulation of P-gp expression and eliminate BCSCs mediated by YB-1 nuclear translocation | 0.7 μm | Enhanced the antitumor activity of doxorubicin, paclitaxel and vincristine by reversing multidrug resistance | [38] |
| Prostate cancer | In vitro | PC3, LNCaP and ARPE-19 cells | Down-regulated the expression of PI3K/PTEN, MAPK and NF-κB signaling pathways | 40 μM | Quercetin inhibited PC3 and CD44+/CD133+ stem cell proliferation in a time- and dose-dependent manner. | [39] |
| Pancreatic cancer | In vitro | Human pancreatic CSCs (CD133þ/CD44þ/CD24þ/ESAþ) | Inhibited the expression of Bcl-2 and XIAP and activate caspase-3, attenuate transcriptional activities of Gli and TCF/LEF | 20μM | Epigallocatechin-3-gallate with quercetin had synergistic inhibitory effects on self-renewal capacity of pancreatic CSCs | [40] |
| Pancreatic cancer | In vitro | PANC-1 | Affected IL-1b, TNF-α, vimentin, N-cadherin, and ACTA-2 expressions | 10μM | Quercetin could prevent Epithelial Mesenchymal Transition by reducing expression of N-cadherin | [41] |
| Breast cancer | In vitro | MCF-7 and MDA-MB-231 cells | Suppressed EGFR signaling and inhibited PI3K/Akt/mTOR/GSK-3β | 50μM(MCF-7), 100μM (MDA-MB-231) | Gold nanoparticles-conjugated quercetin reduce cell proliferation through induction of apoptosis of breast cancer cell | [42] |
| Pancreatic cancer | In vitro | IA Paca-2, BxPC3, AsPC-1, HPAC and PANC1 | Silencing RAGE expression by suppressing the PI3K/AKT/mTOR axis | Quercetin increased gemcitabine drug chemosensitivity in pancreatic cancer cells | [43] | |
| Colon cancer | In vitro | HT-29, SW-620, and Caco-2 cells | Redistributed the TRAIL receptors and other components of the DISC complex into lipid rafts, which facilitates the formation of the DISC and the downstream signaling pathway, contributing to Bax conformational changes, release of cytochrome c, and apoptosis. | 30 μmol/L | Quercetin enhanced tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-mediated apoptosis | [44] |
| Breast cancer | In vivo | NOD/SCID mice | Inhibited the overexpression of Hsp27 through the regulation of epithelial mesenchymal transition and nuclear factor-kB | (50, 25 or 12.5 μM) | Effectively suppressed the overexpression of Hsp27 and inhibit the breast cancer stem cells | [45] |
| Cancer Type | Research/Experiment Type | Research Models/Cell Lines | Mechanism of Action | Outcomes | Reference | |
| Leukemia | In vitro | U937 cell | Cell cycle arrest at G2/M, decrease in cyclin D, cyclin E and E2F, increase in the level of the cyclin B | Apoptosis and growth inhibition in The human leukemia cells | [14] | |
| Leukemia | In vitro and In vivo | HL-60 AML cells | Induced caspase-8, caspase-9, and caspase-3 activation, PARP cleavage, mitochondrial membrane depolarization, induced intratumoral oxidative stress | Anticancer effects in acute myeloid leukemia (AML) cells | [15] | |
| Leukemia | In vitro | Acute leukemia cell line, HL-60 | Induces apoptosis in a caspase-3-dependent pathway by inhibiting Cox-2 expression and regulates the expression of downstream apoptotic components, including Bcl-2 and Bax | Inhibited cell proliferation and induced apoptosis in a time- and dose-dependent manner | [16] | |
| Liver cancer | In vivo | Hyperplastic nodules in rat liver | Prevented DEN-mediated development of hepatocarcinoma and oxidative damage in rat liver | Potent therapeutic formulation against DEN-induced hepatocarcinoma | [17] | |
| Liver cancer | In vivo | HepG2 cells | Induced apoptosis, alter cell cycle in hepg2 cells, decreased the gene expression of cyclin D1 | Significantly inhibit the growth and proliferation of liver cancer cell. | [18] | |
| Colorectal cancer | In vivo | ApcMin mice, and HCT116 tumors | Decreased tumor proliferation and development, increased apoptosis and p53 expression | Chemical modification of quercetin generates safe and efficacious agents for colorectal Cancer | [19] | |
| Colon cancer | In vitro | HT-29 colon cancer cells | Induced caspase-3 cleavage, increased PARP cleavage, decreased the expression of Sp1, Sp3, Sp4 mrna, and survivin, decreased microrna-27a, and induced ZBTB10 | Cytotoxic effects in colon cancer cells, Resulting in apoptosis. | [20] | |
| Colon cancer | In vitro | CX-1, SW480, HT-29, HCT116 | Downregulation of transcriptional activity of β-catenin/Tcf signal pathway, and cyclin D1 and the survivin gene | Inhibited proliferation in colon cancer cells | [21] | |
| Colon cancer | In vivo | Male F344 rats | Decreased β-catenin accumulation in BCA-C; decreased number of ACF | Suppressed tumor growth and at high dose reduced colorectal carcinogenesis | [22] | |
| Lung cancer | In vitro | H460, A549 | Induction of DR5 and suppression of survivin expression | TRAIL-induced cytotoxicity in lung cancer cells | [23] | |
| Lung cancer | In vitro | H460 | Increased the expression of TRAILR, caspase-10, DFF45, TNFR 1, FAS, and decreased the expression of NF-κb, ikkα | Useful in the prevention and therapy of NSCLC | [24] | |
| Lung cancer | In vitro | Human A549 lung cancer cells | Downregulation of the expression of cdk1 and cyclin B, increased PPAR-γ expression | Inhibiton of human A549 lung cancer cell growth | [25] | |
| Ovarian cancer | In vitro and in vivo | A2780S ovarian cancer cells | Activated caspase-3 and caspase-9. MCL-1 downregulation, Bcl-2 downregulation, Bax upregulation, inhibited angiogenesis in vivo | Novel nano-formulation of quercetin with a potential clinical application in ovarian cancer therapy | [26] | |
| Ovarian cancer | In vitro | SKOV3 | Reduction in cyclin D1 level | Inhibited cell growth in ovarian carcinoma | [27] | |
| Breast cancer | In vitro | MCF-7, HCC1937, SK-Br3, 4T1, MDA-MB-231 | Decreased Bcl-2 expression, increasedBax expression, inhibition of PI3K-Akt pathway | Decreases proliferationand increases apoptosis in MCF-7 human breast cancer cells | [28] | |
| Breast cancer | In vitro | MDA-MB-231 | Induced the expression of E-cadherin and downregulated vimentin levels, modulation of β-catenin target genes such as cyclin D1 and c-Myc | Inhibited TNBC metastasis and also improve the therapeutic efficacy of existing chemotherapeutic drug | [29] | |
| Breast cancer | In vitro | MCF-7 | Suppressed the epithelial–mesenchymal transition process, upregulated E-cadherin expression, downregulated vimentin and MMP-2 expression, decreased Notch1 expression and induced PI3K and Akt phosphorylation | Potential therapeutic for the treatment of triple negative and hormone-sensitive breast cancer | [30] | |
| Breast cancer | In vivo | MCF-7/DO X | Overcoming the drug efflux by ABC transporters and promoting PCD with the arrest of cell cycle, counteracted P-gp and BCRPPumps | Reverses multidrug resistance and restores chemosensitivity to human breast cancer cells | [31] | |
| Gastric cancer | In vitro | GCSCs | Activation of caspase-3 and -9, downregulation of Bcl-2, upregulation of Bax and cytochrome c (Cyt-c) | Potential agent for the treatment of gastric cancer. | [32] | |
| Pancreatic cancer | In vivo | PANC-1, PATU-8988 | Decreased the secretion of MMP and MMP7, blocked the STAT3 signaling pathway | New therapeutic strategy for the treatment of pancreatic cancer cells That targets emt, invasion, and metastasis. |
[33] | |
| Prostate cancer | In vitro and in vivo | PC-3, HUVECs | Reduced angiogenesis, increased TSP-1 protein and mrna expression | Good foundation for applying quercetin to clinical for human prostate cancer in the near future | [34] |
3. Reactive Oxygen Species-Mediated Regulation of the p53 Pathway
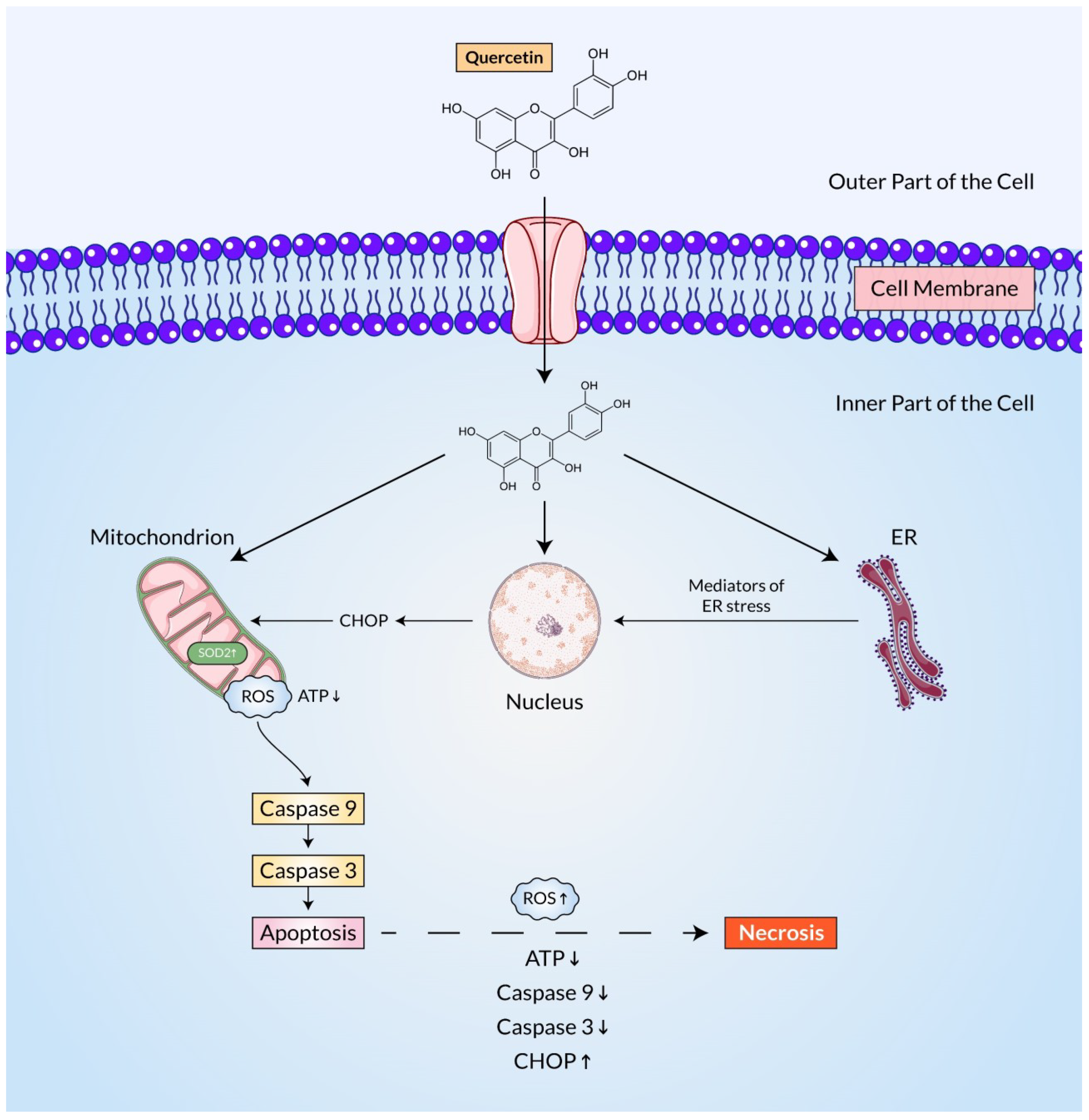
4. Reactive Oxygen Species-Mediated Regulation of the JAK/STAT and TRAIL Pathways
5. Reactive Oxygen Species-Mediated Regulation of the AMPKα1/ASK1/p38 Pathways
6. Reactive Oxygen Species-Mediated Regulation of the RAGE/PI3K/AKT/mTOR Axis
7. Reactive Oxygen Species-Mediated Regulation of the HMGB1 and NF-κB Pathways
8. Reactive Oxygen Species -Mediated Regulation of the Nrf2-Induced Phase II Enzyme and Signaling Pathways
References
- Boots, A.W.; Haenen, G.R.; Hartog, G.J.D.; Bast, A. Oxidative damage shifts from lipid peroxidation to thiol arylation by catechol-containing antioxidants. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2002, 1583, 279–284.
- Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010, 6, 135.
- Chien, S.Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Wood, W.G.; Kuo, S.J.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp. Toxicol. 2009, 28, 493–503.
- Kashyap, D.; Mittal, S.; Sak, K.; Singhal, P.; Tuli, H.S. Molecular mechanisms of action of quercetin in cancer: Recent advances. Tumor Biol. 2016, 37, 12927–12939.
- Kumar, S.; Pandey, A.K.J. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750.
- Lautraite, S.; Musonda, A.; Doehmer, J.; Edwards, G.; Chipman, J.J.M. Flavonoids inhibit genetic toxicity produced by carcinogens in cells expressing CYP1A2 and CYP1A1. Mutagenesis 2002, 17, 45–53.
- Ong, C.S.; Tran, E.; Nguyen, T.T.; Ong, C.K.; Lee, S.K.; Lee, J.J.; Ng, C.P.; Leong, C.; Huynh, H.J. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol. Rep. 2004, 11, 727–733.
- Tan, W.-f.; Lin, L.-p.; Li, M.-h.; Zhang, Y.-X.; Tong, Y.-g.; Xiao, D.; Ding, J. Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur. J. Pharmacol. 2003, 459, 255–262.
- Vijayababu, M.; Arunkumar, A.; Kanagaraj, P.; Venkataraman, P.; Krishnamoorthy, G.; Arunakaran, J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol. Cell. Biochem. 2006, 287, 109–116.
- Nguyen, T.; Tran, E.; Nguyen, T.; Do, P.; Huynh, T.; Huynh, H.J.C. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis 2004, 25, 647–659.
- Cho, S.-Y.; Park, S.-J.; Kwon, M.-J.; Jeong, T.-S.; Bok, S.-H.; Choi, W.-Y.; Jeong, W.-I.; Ryu, S.-Y.; Do, S.-H.; Lee, C.-S.J.M.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160.
- Raman, M.; Chen, W.; Cobb, M.J.O. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112.
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529.
- Lee, T.-J.; Kim, O.H.; Kim, Y.H.; Lim, J.H.; Kim, S.; Park, J.-W.; Kwon, T.K. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006, 240, 234–242.
- Lee, W.J.; Hsiao, M.; Chang, J.L.; Yang, S.F.; Tseng, T.H.; Cheng, C.W.; Chow, J.M.; Lin, K.H.; Lin, Y.W.; Liu, C.C.; et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch. Toxicol. 2015, 89, 1103–1117.
- Niu, G.; Yin, S.; Xie, S.; Li, Y.; Nie, D.; Ma, L.; Wang, X.; Wu, Y. Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells. Acta Biochim. Biophys. Sin. 2011, 43, 30–37.
- Mandal, A.K.; Das, S.; Mitra, M.; Chakrabarti, R.N.; Chatterjee, M.; Das, N. Vesicular flavonoid in combating diethylnitrosamine induced hepatocarcinoma in rat model. J. Exp. Ther. Oncol. 2008, 7, 123–133.
- Zhou, J.; Fang, L.; Liao, J.; Li, L.; Yao, W.; Xiong, Z.; Zhou, X. Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS ONE 2017, 12, e0172838.
- Howells, L.M.; Britton, R.G.; Mazzoletti, M.; Greaves, P.; Broggini, M.; Brown, K.; Steward, W.P.; Gescher, A.J.; Sale, S. Preclinical colorectal cancer chemopreventive efficacy and p53-modulating activity of 3′,4′,5′-trimethoxyflavonol, a quercetin analogue. Cancer Prev. Res. 2010, 3, 929–939.
- Del Follo-Martinez, A.; Banerjee, N.; Li, X.; Safe, S.; Mertens-Talcott, S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr. Cancer 2013, 65, 494–504.
- Shan, B.E.; Wang, M.X.; Li, R.Q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Investig. 2009, 27, 604–612.
- Dihal, A.A.; de Boer, V.C.; van der Woude, H.; Tilburgs, C.; Bruijntjes, J.P.; Alink, G.M.; Rietjens, I.M.; Woutersen, R.A.; Stierum, R.H. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J. Nutr. 2006, 136, 2862–2867.
- Chen, W.; Wang, X.; Zhuang, J.; Zhang, L.; Lin, Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis 2007, 28, 2114–2121.
- Youn, H.; Jeong, J.C.; Jeong, Y.S.; Kim, E.J.; Um, S.J. Quercetin potentiates apoptosis by inhibiting nuclear factor-kappaB signaling in H460 lung cancer cells. Biol. Pharm. Bull. 2013, 36, 944–951.
- Yeh, S.L.; Yeh, C.L.; Chan, S.T.; Chuang, C.H. Plasma rich in quercetin metabolites induces G2/M arrest by upregulating PPAR-γ expression in human A549 lung cancer cells. Planta Med. 2011, 77, 992–998.
- Gao, X.; Wang, B.; Wei, X.; Men, K.; Zheng, F.; Zhou, Y.; Zheng, Y.; Gou, M.; Huang, M.; Guo, G.; et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 2012, 4, 7021–7030.
- Catanzaro, D.; Ragazzi, E.; Vianello, C.; Caparrotta, L.; Montopoli, M. Effect of Quercetin on Cell Cycle and Cyclin Expression in Ovarian Carcinoma and Osteosarcoma Cell Lines. Nat. Prod. Commun. 2015, 10, 1365–1368.
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456.
- Srinivasan, A.; Thangavel, C.; Liu, Y.; Shoyele, S.; Den, R.B.; Selvakumar, P.; Lakshmikuttyamma, A. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol. Carcinog. 2016, 55, 743–756.
- Cao, L.; Yang, Y.; Ye, Z.; Lin, B.; Zeng, J.; Li, C.; Liang, T.; Zhou, K.; Li, J. Quercetin-3-methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int. J. Mol. Med. 2018, 42, 1625–1636.
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother. Res. 2017, 31, 1529–1538.
- Shen, X.; Si, Y.; Wang, Z.; Wang, J.; Guo, Y.; Zhang, X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int. J. Mol. Med. 2016, 38, 619–626.
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. OncoTargets Ther. 2017, 10, 4719–4729.
- Yang, F.; Jiang, X.; Song, L.; Wang, H.; Mei, Z.; Xu, Z.; Xing, N. Quercetin inhibits angiogenesis through thrombospondin-1 upregulation to antagonize human prostate cancer PC-3 cell growth in vitro and in vivo. Oncol. Rep. 2016, 35, 1602–1610.
- Atashpour, S.; Fouladdel, S.; Movahhed, T.K.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 2015, 18, 635–643.
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med. Sci. Monit. 2018, 24, 412–420.
- Tang, S.N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14.
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Health benefits of vanadium and its potential as an anticancer agent. Met. Ions Life Sci. 2018, 18, 251–279.
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, O.; Doganlar, Z.B.; Bilir, A.; Oktem, G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018, 107, 793–805.
- Tang, S.N.; Fu, J.; Nall, D.; Rodova, M.; Shankar, S.; Srivastava, R.K. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int. J. Cancer 2012, 131, 30–40.
- Hoca, M.; Becer, E.; Kabadayı, H.; Yücecan, S.; Vatansever, H.S. The Effect of Resveratrol and Quercetin on Epithelial-Mesenchymal Transition in Pancreatic Cancer Stem Cell. Nutr. Cancer 2020, 72, 1231–1242.
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles-conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt-mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2017, 35, 217–231.
- Lan, C.Y.; Chen, S.Y.; Kuo, C.W.; Lu, C.C.; Yen, G.C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 2019, 27, 887–896.
- Psahoulia, F.H.; Drosopoulos, K.G.; Doubravska, L.; Andera, L.; Pintzas, A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol. Cancer Ther. 2007, 6, 2591–2599.
- Wei, L.; Liu, T.T.; Wang, H.H.; Hong, H.M.; Yu, A.L.; Feng, H.P.; Chang, W.W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res. BCR 2011, 13, R101.
- Qin, J.J.; Li, X.; Hunt, C.; Wang, W.; Wang, H.; Zhang, R. Natural products targeting the p53-MDM2 pathway and mutant p53: Recent advances and implications in cancer medicine. Genes Dis 2018, 5, 204–219.
- Liu, B.; Chen, Y.; Clair, D.K.S. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535.
- Kuo, P.C.; Liu, H.F.; Chao, J.I. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004, 279, 55875–55885.
- Vidya Priyadarsini, R.; Senthil Murugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91.
- Chan, S.T.; Yang, N.C.; Huang, C.S.; Liao, J.W.; Yeh, S.L. Quercetin enhances the antitumor activity of trichostatin A through upregulation of p53 protein expression in vitro and in vivo. PLoS ONE 2013, 8, e54255.
- Tanigawa, S.; Fujii, M.; Hou, D.X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci. Biotechnol. Biochem. 2008, 72, 797–804.
- Lee, Y.-J.; Lee, Y.-J.; Park, I.-S.; Song, J.-H.; Oh, M.-H.; Nam, H.-S.; Cho, M.-K.; Woo, K.-M.; Lee, S.-H. Quercetin exerts preferential cytotoxic effects on malignant mesothelioma cells by inducing p53 expression, caspase-3 activation, and apoptosis. Mol. Cell. Toxicol. 2015, 11, 295–305.
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345.
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAK-STAT 2013, 2, e24137.
- Horr, B.; Borck, H.; Thurmond, R.; Grösch, S.; Diel, F. STAT1 phosphorylation and cleavage is regulated by the histamine (H4) receptor in human atopic and non-atopic lymphocytes. Int. Immunopharmacol. 2006, 6, 1577–1585.
- Muthian, G.; Bright, J.J. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J. Clin. Immunol. 2004, 24, 542–552.
- Liao, Y.-R.; Lin, J.-Y.J. Quercetin, but not its metabolite quercetin-3-glucuronide, exerts prophylactic immunostimulatory activity and therapeutic antiinflammatory effects on lipopolysaccharide-treated mouse peritoneal macrophages ex vivo. J. Agric. Food Chem. 2014, 62, 2872–2880.
- Senggunprai, L.; Kukongviriyapan, V.; Prawan, A.; Kukongviriyapan, U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phyther. Res. 2014, 28, 841–848.
- Matsuzawa, A.; Ichijo, H.J.B. Redox control of cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 1325–1336.
- Lee, Y.-K.; Hwang, J.-T.; Kwon, D.Y.; Surh, Y.-J.; Park, O.J. Induction of apoptosis by quercetin is mediated through AMPKα1/ASK1/p38 pathway. Cancer Lett. 2010, 292, 228–236.
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869.
- Dutta, S.; Sadhukhan, P.; Saha, S.; Sil, P.C. Regulation of oxidative stress by different naturally occurring polyphenolic compounds: An emerging anticancer therapeutic approach. React. Oxyg. Species 2017, 3, 81–95.
- Arzuman, L.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Synergism from combinations of tris(benzimidazole) monochloroplatinum(II) chloride with capsaicin, quercetin, curcumin and cisplatin in human ovarian cancer cell lines. Anticancer. Res. 2014, 34, 5453–5464.
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers 2010, 2, 1288–1311.
- Matsuzawa, A.; Nishitoh, H.; Tobiume, K.; Takeda, K.; Ichijo, H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: Advanced findings from ASK1 knockout mice. Antioxid. Redox Signal. 2002, 4, 415–425.
- Watanabe, T.; Sekine, S.; Naguro, I.; Sekine, Y.; Ichijo, H. Apoptosis Signal-regulating Kinase 1 (ASK1)-p38 Pathway-dependent Cytoplasmic Translocation of the Orphan Nuclear Receptor NR4A2 Is Required for Oxidative Stress-induced Necrosis. J. Biol. Chem. 2015, 290, 10791–10803.
- Maurya, A.K.; Vinayak, M. Quercetin Attenuates Cell Survival, Inflammation, and Angiogenesis via Modulation of AKT Signaling in Murine T-Cell Lymphoma. Nutr. Cancer 2017, 69, 470–480.
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Xu, M.; et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE 2012, 7, e47516.
- Xiao, Y.; Zhou, L.; Zhang, T.; Qin, C.; Wei, P.; Luo, L.; Luo, L.; Huang, G.; Chen, A.; Liu, G. Anti-fibrosis activity of quercetin attenuates rabbit tracheal stenosis via the TGF-β/AKT/mTOR signaling pathway. Life Sci. 2020, 250, 117552.
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136.
- Rivera Rivera, A.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251.
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(-)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62.
- Hamidullah; Kumar, R.; Saini, K.S.; Kumar, A.; Kumar, S.; Ramakrishna, E.; Maurya, R.; Konwar, R.; Chattopadhyay, N. Quercetin-6-C-β-D-glucopyranoside, natural analog of quercetin exhibits anti-prostate cancer activity by inhibiting Akt-mTOR pathway via aryl hydrocarbon receptor. Biochimie 2015, 119, 68–79.
- He, K.; Yu, X.; Wang, X.; Tang, L.; Cao, Y.; Xia, J.; Cheng, J. Baicalein and Ly294002 induces liver cancer cells apoptosis via regulating phosphatidyl inositol 3-kinase/Akt signaling pathway. J. Cancer Res. Ther. 2018, 14, 519.
- Zhao, S.; Jiang, Y.; Zhao, J.; Li, H.; Yin, X.; Wang, Y.; Xie, Y.; Chen, X.; Lu, J.; Dong, Z.; et al. Quercetin-3-methyl ether inhibits esophageal carcinogenesis by targeting the AKT/mTOR/p70S6K and MAPK pathways. Mol. Carcinog. 2018, 57, 1540–1552.
- Roy, S.; Banerjee, S.; Chakraborty, T. Vanadium quercetin complex attenuates mammary cancer by regulating the P53, Akt/mTOR pathway and downregulates cellular proliferation correlated with increased apoptotic events. Biometals 2018, 31, 647–671.
- Granato, M.; Rizzello, C.; Romeo, M.A.; Yadav, S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Concomitant reduction of c-Myc expression and PI3K/AKT/mTOR signaling by quercetin induces a strong cytotoxic effect against Burkitt’s lymphoma. Int. J. Biochem. Cell Biol. 2016, 79, 393–400.
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130.
- Wu, H.; Pan, L.; Gao, C.; Xu, H.; Li, Y.; Zhang, L.; Ma, L.; Meng, L.; Sun, X.; Qin, H. Quercetin Inhibits the Proliferation of Glycolysis-Addicted HCC Cells by Reducing Hexokinase 2 and Akt-mTOR Pathway. Molecules 2019, 24, 1993.
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L.; et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy 2011, 7, 966–978.
- Meng, F.D.; Li, Y.; Tian, X.; Ma, P.; Sui, C.G.; Fu, L.Y.; Jiang, Y.H. Synergistic effects of snail and quercetin on renal cell carcinoma Caki-2 by altering AKT/mTOR/ERK1/2 signaling pathways. Int. J. Clin. Exp. Pathol. 2015, 8, 6157–6168.
- Lou, M.; Zhang, L.N.; Ji, P.G.; Feng, F.Q.; Liu, J.H.; Yang, C.; Li, B.F.; Wang, L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharm. 2016, 84, 1–9.
- Rendon-Mitchell, B.; Ochani, M.; Li, J.; Han, J.; Wang, H.; Yang, H.; Susarla, S.; Czura, C.; Mitchell, R.A.; Chen, G. IFN-γ induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 2003, 170, 3890–3897.
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.-C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570.
- Degryse, B.; Bonaldi, T.; Scaffidi, P.; Müller, S.; Resnati, M.; Sanvito, F.; Arrigoni, G.; Bianchi, M.E. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol. 2001, 152, 1197–1206.
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxidative Med. Cell. Longev. 2013, 2013, 596496.
- Han, Y.; Yu, H.; Wang, J.; Ren, Y.; Su, X.; Shi, Y.J.H. Quercetin alleviates myocyte toxic and sensitizes anti-leukemic effect of adriamycin. Hematology 2015, 20, 276–283.
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.-Y.; Strassheim, D.; Ishizaka, A.; Abraham, E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004, 279, 7370–7377.
- Kokkola, R.; Andersson, Å.; Mullins, G.; Östberg, T.; Treutiger, C.J.; Arnold, B.; Nawroth, P.; Andersson, U.; Harris, R.; Harris, H. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 2005, 61, 1–9.
- Luo, J.-L.; Kamata, H.; Karin, M. IKK/NF-κB signaling: Balancing life and death–a new approach to cancer therapy. J. Clin. Investig. 2005, 115, 2625–2632.
- Kwak, M.-K.; Itoh, K.; Yamamoto, M.; Sutter, T.R.; Kensler, T.W. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3 H-1, 2-dithiole-3-thione. Mol. Med. 2001, 7, 135–145.
- Tan, X.-L.; Spivack, S.D. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: A review. Lung Cancer 2009, 65, 129–137.
- Kansanen, E.; Jyrkkänen, H.-K.; Volger, O.L.; Leinonen, H.; Kivelä, A.M.; Häkkinen, S.-K.; Woodcock, S.R.; Schopfer, F.J.; Horrevoets, A.J.; Ylä-Herttuala, S.; et al. Nrf2-dependent and-independent responses to nitro-fatty acids in human endothelial cells. J. Biol. Chem. 2009, 284, 33233–33241.
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140.
- Kansanen, E.; Jyrkkänen, H.-K.; Levonen, A.-L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012, 52, 973–982.
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913.
- Tanigawa, S.; Fujii, M.; Hou, D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free. Radic. Biol. Med. 2007, 42, 1690–1703.
- Odenthal, J.; van Heumen, B.W.; Roelofs, H.M.; te Morsche, R.H.; Marian, B.; Nagengast, F.M.; Peters, W.H. The influence of curcumin, quercetin, and eicosapentaenoic acid on the expression of phase II detoxification enzymes in the intestinal cell lines HT-29, Caco-2, HuTu 80, and LT97. Nutr. Cancer 2012, 64, 856–863.
- Ramyaa, P.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 681–692.
- Shi, Y.; Liang, X.-c.; Zhang, H.; Wu, Q.-l.; Qu, L.; Sun, Q. Quercetin protects rat dorsal root ganglion neurons against high glucose-induced injury in vitro through Nrf-2/HO-1 activation and NF-κB inhibition. Acta Pharmacol. Sin. 2013, 34, 1140–1148.
- Lee, Y.-J.; Song, J.-H.; Oh, M.-H.; Lee, Y.-J.; Kim, Y.-B.; Im, J.-H.; Lee, S.-H. ERK1/2 activation in quercetin-treated BEAS-2B cell plays a role in Nrf2-driven HO-1 expression. Mol. Cell. Toxicol. 2011, 7, 347–355.
- Chow, J.-M.; Shen, S.-C.; Huan, S.K.; Lin, H.-Y.; Chen, Y.-C. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem. Pharmacol. 2005, 69, 1839–1851.
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261.
