Functional beverages can be a valuable component of the human diet with the ability to not only provide essential hydration but to deliver important bioactive compounds that can contribute to chronic disease treatment and prevention. One area of the functional beverage market that has seen an increase in demand in recent years are beverages that promote relaxation and sleep. Sleep is an essential biological process, with optimal sleep being defined as one of adequate duration, quality and timing. It is regulated by a number of neurotransmitters which are, in turn, regulated by dietary intake of essential bioactive compounds.
- sleep
- nutraceuticals
- theanine
- chamomile extract
- tryptophan
- cysteine
- functional beverages
1. Introduction
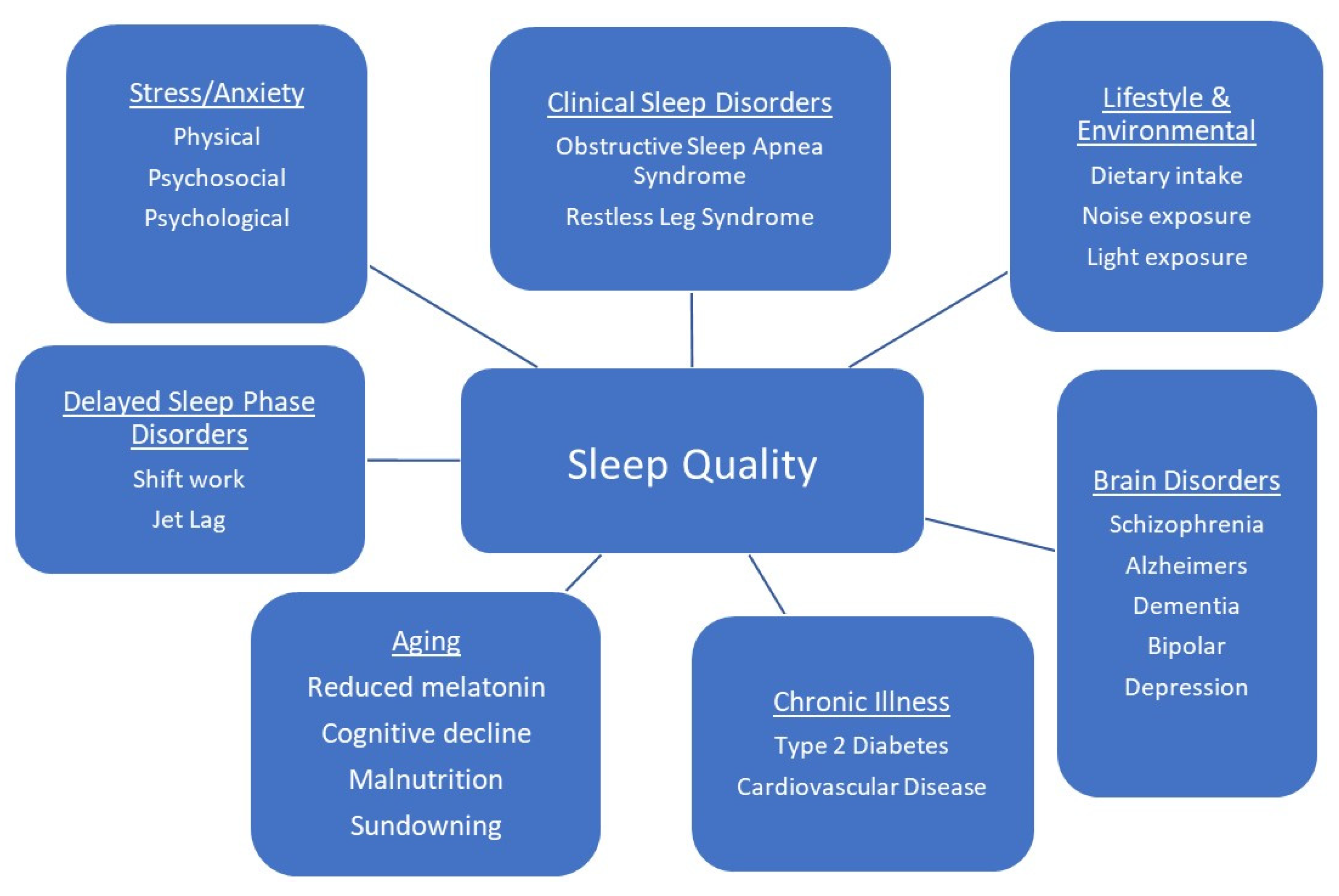
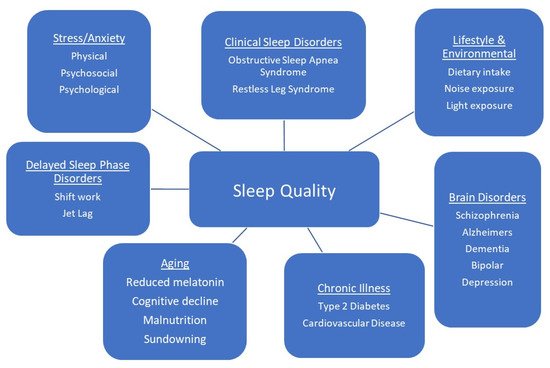
2. Active Compounds
The summary of active compounds included in this review iere is presented in Table 1. The range of compounds is comprised of amino acid, hormone, vitamin and mineral compounds that influence the neurological pathways involved in sleep with potential for development into a functional beverage.Compound | Reference/Country | Participants | Intervention/Duration | Study Design | Outcome | Measures | Effects on Sleep | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Markus et al. (2005) [40] | Netherlands | Adults without sleep complaints | ( n = 14) | Age (22 ± 3 years) | Adults with mild sleep complaint | ( n = 14) | Age (22 ± 2 years) | 20 g L-TRP-enriched | A-LAC protein | (4.8 g L-TRP/100 g amino acids w/w ) | 1 night | Double-blind | Placebo-controlled | Subjective Sleep Quality Measures: | Stanford Sleepiness Scale | Improved morning alertness | ( p = 0.013) and increased attention (p = 0.002) in both groups. | Improved performance in participants with sleep complaints only ( p = 0.05). | ||||||||||||||||||||||||||||||||||||||||||||||||
L-Tryptophan | Ong et al. (2017) [41] | Australia | Healthy males without sleep complaint | ( n = 10) | Age (26.9 ± 5.3 years) | 20 g L-TRP-enriched | A-LAC protein | (4.8 g L-TRP/100 g amino acids w/w ) of A-LAC protein | 2 nights | Double-blind | Placebo-controlled | Randomized | Crossover | Objective Sleep Quality Measures (Actigraphy): | Total sleep time | Sleep onset latency | Sleep efficiency (%) | Wake time after sleep onset | Subjective Sleep Measures (Sleep Log): | Bedtime | Time taken to fall asleep | Frequency of awakenings | Time taken to return to sleep | Waking time | Rising time | Total sleep time | Increased objective and subjective total sleep time by 12.8% (p = 0.037) and 10.8% | ( p = 0.013), respectively; increased objective sleep efficiency by 7.0% | ( p = 0.028). | |||||||||||||||||||||||||||||||||||||
Cubero et al. (2007) [42] | Spain | Pre-weaning infants | ( n = 30) | Age (4–20 weeks) | Diet A: Standard formula Diet B: Standard formula during the day and night formula (3.4 g L-TRP/100 g protein) | Diet C: Day formula during the day (1.5 g L-TRP/100 g protein) + night formula (3.4 g L-TRP/100 g protein) in the evening | 1 week per formula | Double-blind | Randomized | Objective Sleep Quality Measures (Actigraphy): | Time of nocturnal sleep | Minutes of immobility | Sleep latency | Nocturnal awakenings | Sleep efficiency (%) | Sleep Diary: | Sleep over 24 h | Number of bottle feeds | Observations or incidences that would influence the infants rest | Diet C improved objective total sleep time (p < 0.05) and subjective (parent) sleep improvement; Diet B and Diet C reduced objective sleep onset latency; Diet B improved objective sleep efficiency. | (All p’s < 0.05) | |||||||||||||||||||||||||||||||||||||||||||||
Bravo et al. (2013) [43] | Spain | Older adults with sleep difficulties | ( n = 35) | Age (55–75 years) | L-TRP (60 mg) enriched cereal for breakfast and dinner | 1 week | Blind assay | Objective Sleep Quality Measures (Actigraphy): | Time in bed | Assumed sleep | Actual sleep time | Sleep onset latency | Sleep efficiency (%) | Number of awakenings | Immobile time | Total activity | Fragmentation index (indicator of quality of rest) | Improvements in objective sleep measures including increase in actual sleep time (p < 0.01); increase in sleep efficiency (p < 0.001); increase in immobile time (p < 0.01); reduction in sleep latency (p < 0.01); wake bouts | ( p < 0.05); total activity (p < 0.01); fragmentation index (p < 0.001). | |||||||||||||||||||||||||||||||||||||||||||||||
5-HTP | Bruni et al. (2004) [44] | Italy | Children with sleep terrors | ( n = 45) | Age (3.2–10.6 years) | 2 mg/kg | (Daily) | 20 days | Randomized, controlled | Frequency of sleep terrors | After 1-month: | Sleep terrors reduced > 50% from | baseline in 93.5% of children treated with 5-HTP ( p < 0.00001). | After 6 months: | 51.6% were sleep-terror free | ( p < 0.001). | ||||||||||||||||||||||||||||||||||||||||||||||||||
Melatonin | Scheer et al. (2012) [45] | USA | Hypertensive adults on beta blockers | ( n = 16) | Age (45–64 years) | 2.5 mg | (nightly, 1 h before bedtime) | 3 weeks | Randomized, | Double-blind | Placebo-controlled | Parallel-group design | Objective Sleep Quality Measures | (Polysomnography): | Sleep stages | Total sleep time | Time in bed | Sleep efficiency (%) | Objective Sleep Quality Measures (Actigraphy): | Sleep onset latency | Total sleep time | Sleep efficiency (%) | Increased total sleep time by 32 min | ( p = 0.046); increased sleep efficiency by 7.6% (p = 0.046). Decreased sleep onset latency to stage 2 NREM sleep by 14 min (p = 0.001) and increased the duration of stage 2 NREM sleep by 42 min (p = 0.037). | ||||||||||||||||||||||||||||||||||||||||||
Grima et al. (2018) [46] | Australia | Adults with sleep disturbance post onset of traumatic brain injury | ( n = 33) | Age (37 ± 11 years) | 2 mg | (nightly 2 h before bedtime) | 4 weeks | Randomized, | Double-blind | Placebo-controlled | Two-period | Two-treatment | Crossover study | Objective Sleep Quality Measures (Actigraphy) | Sleep onset latency | Total sleep time | Sleep duration | Sleep efficiency (%) | Sleep Diary: | Sleep onset/offset | Sleep duration | Subjective Sleep Quality Measures: | PSQI | ESS | FSS | Improved subjective sleep quality | ( p < 0.0001) and objective sleep efficiency (p < 0.04). | |||||||||||||||||||||||||||||||||||||||
Xu et al. (2020) [47] | China | Adults with primary insomnia (n = 97) | Age (45–60 years) | 3 mg | (nightly 1 h before bedtime) | 4 weeks | Randomized, | Double-blind | Placebo-controlled | Parallel study | Objective Sleep Quality Measures | (Polysomnography): | Sleep stages | Total sleep time | Sleep onset latency | Wake after sleep onset | Sleep efficiency (%) | Subjective Sleep Quality Measures: | PSQI | ESS | ISI | Decreased objective sleep measures including early morning wake (p = 0.001) and decreased percentage of Stage 2 NREM sleep (p = 0.031). | ||||||||||||||||||||||||||||||||||||||||||||
L-Cysteine | Sadasivam et al. (2011) [48] | India | Adults with obstructive sleep apnea | ( n = 20) | Age (53.1 ± 2.3 years) | 600 mg (Mucinac, | Cipla), three times per day | 30 days | Randomized, | Placebo-controlled | Objective Sleep Quality Measures | (Polysomnography): | Sleep stages | Total sleep time | Sleep onset latency | Wake after sleep onset | Sleep efficiency (%) | Sleep apnea | Snoring | Subjective Sleep Quality Measures: | ESS | Improvements in objective slow wave sleep as sleep percent time (p < 0.001) and sleep efficiency. | ( p < 0.05). | Reduction in subjective Epworth Sleepiness Score ( p < 0.001). | ||||||||||||||||||||||||||||||||||||||||||
Rao et al. (2019) [49] | Japan | Healthy adult males | ( n = 22) | Age (27.5 ± 0.9 years) | 4 × 50 mg | (nightly, 1 h before bedtime) | 6 days | Randomized, | Double-blind | Placebo-controlled | Crossover trial | Objective Sleep Quality Measures (Actigraphy): | Time in bed | Wake after sleep onset | Sleep onset latency | Sleep length | Sleep efficiency (%) | Subjective Sleep Quality Measures: | Obstructive Sleep Apnea | Inventory questionnaire | Improvements in objective sleep measures including an increase in objective sleep efficiency (p < 0.047) and reduction in intermittent | wakening ( p < 0.044). | Improvements in subjective sleep measures including feeling of recovery from exhaustion or fatigue scores ( p < 0.042) and improvement in refreshed upon awakening scores | ( p < 0.014). | ||||||||||||||||||||||||||||||||||||||||||
L-Theanine | Lyon et al. (2011) [50] | Canada | Boys with ADHD | ( n = 98) | Age (8–12 years) | 2 × 100 mg | (twice per day, | morning and evening) | 6 weeks | Randomized, | Double-blind | Placebo-controlled | Parallel trial | Objective Sleep Quality Measures (Actigraphy): | Wake after sleep onset | Sleep onset latency | Sleep length | Nocturnal activity | Sleep efficiency (%) | Subjective Sleep Quality Measures: | Pediatric Sleep Questionnaire | Improved objective measures including sleep efficiency (p < 0.05), and reduced nocturnal activity (p < 0.05). | ||||||||||||||||||||||||||||||||||||||||||||
Sarris et al. (2019) [51] | Australia | Adults with GAD | ( n = 46) | Age (40.7 ± 15 years in TG; 32.2 ± 9.29 years in PG) | 225 mg (twice daily); increased to 450 mg (twice daily) if anxiety score did not reduce by ≥35% after 4 weeks | 8 weeks | Randomized, | Double-blind | Placebo-controlled | Multi-center pilot study | Subjective Sleep Quality Measures: | ISI | Improved subjective sleep | satisfaction | ( p < 0.015); improvements in ISI scores for “difficulty in falling asleep” | ( p < 0.049); “Problems waking up too early” ( p < 0.017); and “interference with daily functioning” (p = 0.030) in control. | ||||||||||||||||||||||||||||||||||||||||||||||||||
Hidese et al. (2019) [52] | Japan | Healthy Adults | ( n = 30) | Age (48.3 ± 11.9 years) | 200 mg tablet daily before sleep | 4 weeks | Randomized, | Double-blind | Placebo-controlled | Crossover trial | Subjective Sleep Quality Measures: | PSQI | Improved subjective sleep quality (p < 0.013), reduced sleep onset latency, sleep disturbance and use of sleep medication (All p’s < 0.05). | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Vitamin B12 | Mayer et al. (1996) [53] | Healthy Adults | ( n = 20) | Age (CB12 = 36.6 ± 5.2 years. | MB12 = 36.2 ± 5.2 years) | 3 mg | (cyano-(CB12) or methylcobalamin (MB12)) | 14 days | Randomized | Single-blind | Between subject’s design | Objective Sleep Quality Measures (Actigraphy): | Wake after sleep onset | Sleep onset latency | Sleep length | Nocturnal activity | Sleep efficiency (%) | Subjective Sleep Quality Measures: | Morning and Evening VAS | Reduction in objective sleep time | ( p = 0.036) in MB12 group improvements in sleep quality and daytime alertness (All p’s < 0.05). | |||||||||||||||||||||||||||||||||||||||||||||
Luboshitzky et al. (2002) [54] | Israel | Healthy Adult Males | ( n = 12) | Age (22–26 years) | 100 mg | (5.00 PM) | Once | Randomized | Placebo-controlled | Parallel trial | Objective Sleep Quality Measures (EEG): | Sleep stages (%) | Total recording time | Sleep latency | Actual sleep time | Sleep efficiency (%) | REM latency | No effect. | ||||||||||||||||||||||||||||||||||||||||||||||||
Vitamin B6 | Ebben et al. (2002) [55] | USA | Healthy Adults | ( n = 12) | Age (18–28 years) | 100 mg | 250 mg | Placebo | (All nightly before bed) | 5 days per treatment | Placebo-controlled | Double-blind | Crossover trial | Subjective Sleep Quality Measures: | Sleep questionnaire | Dream Salience Scale | Increase in dream salient scores in | 250 mg B6 treatment compared to placebo ( p = 0.05). | ||||||||||||||||||||||||||||||||||||||||||||||||
Aspy et al. (2018) [56] | Australia | Healthy Adults | ( n = 100) | Age (mean = 27.5) | 120 mg | (pyridoxine hydrochloride) | Vitamin B Complex | (120 mg pyridoxine hydrochloride + other B vitamins) | Placebo | (All nightly before bed) | 5 days | Randomized | Double-blind | Placebo-controlled trial | Subjective Sleep Quality Measures: | Sleep log | Increased the amount of dream content recalled (p = 0.032) and decrease in sleep quality (p = 0.014) in B complex group. | |||||||||||||||||||||||||||||||||||||||||||||||||
Vitamin D | Ghaderi et al. (2017) [57] | Iran | Adults undergoing Methadone Treatment. | ( n = 68) | Age (25–70 years) | 50,000 IU | (once per fortnight) | 12 weeks | Randomized | Double-blind | Placebo-controlled trial | Subjective Sleep Quality Measures: | PSQI | Improvement in subjective sleep score | ( p = 0.02). | |||||||||||||||||||||||||||||||||||||||||||||||||||
Mason et al. (2016) [58] | USA | Overweight menopausal females with low VitD | ( n = 218) | Age (50–75 years) | 2000 IU vitamin D3 | (daily) | 12 months | Randomized | Double-blind | Placebo-controlled trial | Subjective Sleep Quality Measures: | PSQI | Increase in PSQI score (p = 0.01) and increase in need to take sleep medication (p < 0.01). | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Vitamin C | Dadashpour et al. (2018) [59] | Iran | Adults on hemodialysis with sleep disorder | ( n = 90) | Age (18–70 years) | 500 mg /5 cc intravenously–3 times per week | 8 weeks | Randomized | Double-blind | Trial | Subjective Sleep Quality Measures: | PSQI | VAS | Reductions in subjective sleep quality, sleep latency, daytime dysfunction | (All p’s = 0.001). | |||||||||||||||||||||||||||||||||||||||||||||||||||
Yeom et al. (2007) [60] | Korea | Adults with Stage IV cancer | ( n = 39) | Age (53.5 ± 10.5 years) | 10 g vitamin C intravenously twice with 3-day interval, then | 4 g oral supplement daily | 1 week | Prospective study | Subjective Sleep Quality Measures: | European Organization for | Research and Treatment of Cancer Core Quality-of-Life questionnaire (EORTC QLQ-C30)-Korean Version | Lower subjective scores for sleep disturbance and fatigue (p < 0.005). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Murck et al. (2000) [61] | Germany | Older adults without sleep disturbances (n = 12) | Age (60–80 years) | 10 mmol for 3 days, then | 20 mmol for 3 days, then | 30 mmol daily for 14 days | Randomized | Placebo-controlled | Crossover design | Objective Sleep Quality Measures (EEG): | Sleep stages (%) | Total recording time | Sleep latency | Actual sleep time | Sleep efficiency (%) | REM latency | Increase in slow wave sleep (p < 0.05), delta and sigma waves (p < 0.05 for both). | |||||||||||||||||||||||||||||||||||||||||||||||||
Magnesium | Abbasi et al. (2012) [62] | Iran | Older adults | ( n = 43) | Age (65 ± 4.6 years) | 414 mg magnesium oxide (250 mg Mg) | Twice per day | 8 weeks | Double-blind | Placebo-controlled trial | Subjective Sleep Quality Measures: | ISI | Sleep Log | Increase in subjective sleep time | ( p = 0.002) and subjective sleep efficiency (p = 0.03); decrease in subjective sleep onset latency (p = 0.04), and insomnia severity index (p = 0.006). | |||||||||||||||||||||||||||||||||||||||||||||||||||
Hornyak et al. (2004) [63] | Germany | Alcohol dependent adults in subacute withdrawal with sleep disturbance | ( n = 11) | 30 mmol Magnesium | L-aspartate hydrochloride (10 mmol morning and 20 mmol evening) daily | 4 weeks | Open Pilot Study | Objective Sleep Quality Measures (Polysomnography): | Sleep stages | Total sleep time | Sleep onset latency | Wake after sleep onset | Sleep efficiency (%) | Periodic leg movements in sleep (PLMS) | Subjective Sleep Quality Measures: | PSQI | Decrease in objective sleep latency | ( p = 0.03), improvement in subjective sleep quality (p = 0.05). | ||||||||||||||||||||||||||||||||||||||||||||||||
Zinc | Saito et al. (2017) [64] | Japan | Healthy Adults | ( n = 94) | Age (20–84 years) | Group A: Placebo | Group B: 15 mg | Group C: 15 mg + Astx | Group D: Placebo + 16 mg + Astx | 12 weeks | Randomized | Double-blind | Placebo-controlled | Parallel group trial | Objective Sleep Quality Measures (Actigraphy): | Wake after sleep onset | Sleep onset latency | Sleep length | Frequency | Nocturnal activity | Sleep efficiency (%) | Subjective Sleep Quality Measures: | PSQI | Improvements in objective sleep efficiency in group B (p = 0.025); objective sleep onset latency in Group B and D (p < 0.032) and (p = 0.004), respectively. | ||||||||||||||||||||||||||||||||||||||||||
Gholipour et al. (2018) [65] | Iran | ICU nurses | ( n = 54) | Age (31.2 ± 5.42 years) | 1 × 220 mg | (every 72 h) | 1 month | Multi-center | Randomized | Two parallel group | Placebo-controlled trial | Subjective Sleep Quality Measures: | PSQI | Improvements in subjective total sleep quality (p < 0.002); sleep onset latency | ( p < 0.003), sleep duration (p < 0.02) and total sleep quality score (p < 0.008). |
Note: L-TRP-L-Tryptophan; A-LAC–alpha-lactalbumin; EEG–Electroencephalography; 5-HTP–5-hydroxytryptophan; SWS–Slow Wave Sleep; REM–Rapid Eye Movement; NREM–Non-Rapid Eye Movement; ADHD–Attention Deficit Hyperactivity Disorder; GAD–Generalized Anxiety Dissorder; TG–Treatment Group; PG–Placebo Group; PSQI–Pittsburgh Sleep Quality Index; ICU–Intensive Care Unit; ESS–Epworth Sleepiness Scale; FSS–Fatigue Severity Scale; ISI–Insomnia Severity Index; VAS–Visual Analogue Scale; Astx-Astaxanthin.
3. DNutraceutiscussiocals as Potential Targets for the Development of a Functional Beverage for Improving Sleep Quality
References
- Özen, A.E.; Bibiloni, M.D.M.; Pons, A.; Tur, J.A. Fluid intake from beverages across age groups: A systematic review. J. Hum. Nutr. Diet. 2014, 28, 417–442.
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875.
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467.
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206.
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310.
- Roberfroid, M.B. Concepts and strategy of functional food science: The European perspective. Am. J. Clin. Nutr. 2000, 71, 1660S–1664S.
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334.
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and its Benefits on Health and Mental Health: A Literature Review. Clin. Pr. Epidemiology Ment. Heal. 2020, 16, 156–164.
- Pan, S.-Y.; Litscher, G.; Gao, S.-H.; Zhou, S.-F.; Yu, Z.-L.; Chen, H.-Q.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ko, K.-M. Historical Perspective of Traditional Indigenous Medical Practices: The Current Renaissance and Conservation of Herbal Resources. Evid. Based Complement. Altern. Med. 2014, 2014, 1–20.
- Zahiruddin, S.; Basist, P.; Parveen, A.; Parveen, R.; Khan, W.; Gaurav; Ahmad, S. Ashwagandha in brain disorders: A review of recent developments. J. Ethnopharmacol. 2020, 257, 112876.
- Mishra, B.; John, E. A Systematic Review on Neuro-Psychopharmacological effects of Celastrus paniculatus (Malkangani) Oil. Res. J. Pharm. Technol. 2020, 13, 2452.
- Purnima, B.M.; Kothiyal, P. A review article on phytochemistry and pharmacological profiles of Nardostachys jatamansi DC-medicinal herb. J. Pharmacogn. Phytochem. 2015, 3, 102–106.
- Dwivedi, S.; Chopra, D. Revisiting Terminalia arjuna—An Ancient Cardiovascular Drug. J. Tradit. Complement. Med. 2014, 4, 224–231.
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2011, 49, 173–183.
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23.
- Altemimi, A.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem. Central J. 2015, 9, 1–15.
- Altemimi, A.B.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42.
- Speer, H.; D’Cunha, N.M.; Davies, M.J.; McKune, A.J.; Naumovski, N. The Physiological Effects of Amino Acids Arginine and Citrulline: Is There a Basis for Development of a Beverage to Promote Endurance Performance? A Narrative Review of Orally Administered Supplements. Beverages 2020, 6, 11.
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920.
- Wootton-Beard, P.C.; Ryan, L. Improving public health?: The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011, 44, 3135–3148.
- Businesswire. Global Relaxation Drinks Market (2019 to 2027)—CAGR of 14.06% Expected During the Forecast Period—ResearchAndMarkets.com. 2020. Available online: https://www.businesswire.com/news/home/20200317005330/en/Global-Relaxation-Drinks-Market-2019-to-2027---CAGR-of-14.06-Expected-During-the-Forecast-Period---ResearchAndMarkets.com (accessed on 17 March 2021).
- Assefa, S.Z.; Diaz-Abad, M.; Wickwire, E.M.; Scharf, S.M. The Functions of Sleep. AIMS Neurosci. 2015, 2, 155–171.
- Donlea, J.M. Roles for sleep in memory: Insights from the fly. Curr. Opin. Neurobiol. 2019, 54, 120–126.
- Holst, S.C.; Landolt, H.-P. Sleep-Wake Neurochemistry. Sleep Med. Clin. 2018, 13, 137–146.
- Mechan, A.O.; Fowler, A.; Seifert, N.; Rieger, H.; Wöhrle, T.; Etheve, S.; Wyss, A.; Schüler, G.; Colletto, B.; Kilpert, C.; et al. Monoamine reuptake inhibition and mood-enhancing potential of a specified oregano extract. Br. J. Nutr. 2010, 105, 1150–1163.
- Chaput, J.-P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping hours: What is the ideal number and how does age impact this? Nat. Sci. Sleep 2018, 10, 421–430.
- Lyon, L. Is an epidemic of sleeplessness increasing the incidence of Alzheimer’s disease? Brain 2019, 142, e30.
- Longordo, F.; Kopp, C.; Lüthi, A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur. J. Neurosci. 2009, 29, 1810–1819.
- Guarnieri, B.M.; Sorbi, S. Sleep and Cognitive Decline: A Strong Bidirectional Relationship. It Is Time for Specific Recommendations on Routine Assessment and the Management of Sleep Disorders in Patients with Mild Cognitive Impairment and Dementia. Eur. Neurol. 2015, 74, 43–48.
- Xi, B.; He, D.; Zhang, M.; Xue, J.; Zhou, D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med. Rev. 2014, 18, 293–297.
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E.; Gilbody, S.; Torous, J.; Teasdale, S.B.; Jackson, S.E.; et al. A meta-review of “lifestyle psychiatry”: The role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020, 19, 360–380.
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and Quality of Sleep and Incidence of Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care 2009, 33, 414–420.
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Hear. J. 2011, 32, 1484–1492.
- Ibarra-Coronado, E.G.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Prospéro-García, O.; Méndez-Díaz, M.; Pérez-Tapia, M.; Pavón, L.; Morales-Montor, J. The Bidirectional Relationship between Sleep and Immunity against Infections. J. Immunol. Res. 2015, 2015, 1–14.
- St-Onge, M.-P.; Mikic, A.; Pietrolungo, C.E. Effects of Diet on Sleep Quality. Adv. Nutr. 2016, 7, 938–949.
- Ikonte, C.J.; Mun, J.G.; Reider, C.A.; Grant, R.W.; Mitmesser, S.H. Mun Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005. Nutrients 2019, 11, 2335.
- Campanini, M.M.Z.; Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Mediterranean Diet and Changes in Sleep Duration and Indicators of Sleep Quality in Older Adults. Sleep 2016, 40.
- Zuraikat, F.M.; Makarem, N.; St-Onge, M.-P.; Xi, H.; Akkapeddi, A.; Aggarwal, B. A Mediterranean Dietary Pattern Predicts Better Sleep Quality in US Women from the American Heart Association Go Red for Women Strategically Focused Research Network. Nutrients 2020, 12, 2830.
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319.
- Markus, C.R.; Jonkman, L.M.; Lammers, J.H.C.M.; Deutz, N.E.P.; Messer, M.H.; Rigtering, N. Evening intake of α-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am. J. Clin. Nutr. 2005, 81, 1026–1033.
- Ong, J.N.; Hackett, D.A.; Chow, C.-M. Sleep quality and duration following evening intake of alpha-lactalbumin: A pilot study. Biol. Rhythm. Res. 2017, 48, 507–517.
- Cubero, J.; Narciso, D.; Terrón, P.; Rial, R.; Esteban, S.; Rivero, M.; Parvez, H.; Rodríguez, A.B.; Barriga, C. Chrononutrition applied to formula milks to consolidate infants’ sleep/wake cycle. Neuro Endocrinol. Lett. 2007, 28, 360–366.
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. AGE 2012, 35, 1277–1285.
- Bruni, O.; Ferri, R.; Miano, S.; Verrillo, E. L-5-Hydroxytryptophan treatment of sleep terrors in children. Eur. J. Nucl. Med. Mol. Imaging 2004, 163, 402–407.
- Scheer, F.A.; Morris, C.J.; Garcia, J.I.; Smales, C.; Kelly, E.E.; Marks, J.; Malhotra, A.; Shea, S.A. Repeated Melatonin Supplementation Improves Sleep in Hypertensive Patients Treated with Beta-Blockers: A Randomized Controlled Trial. Sleep 2012, 35, 1395–1402.
- Grima, N.A.; Rajaratnam, S.M.W.; Mansfield, D.; Sletten, T.L.; Spitz, G.; Ponsford, J.L. Efficacy of melatonin for sleep disturbance following traumatic brain injury: A randomised controlled trial. BMC Med. 2018, 16, 1–10.
- Xu, H.; Zhang, C.; Qian, Y.; Zou, J.; Li, X.; Liu, Y.; Zhu, H.; Meng, L.; Liu, S.; Zhang, W.; et al. Efficacy of melatonin for sleep disturbance in middle-aged primary insomnia: A double-blind, randomised clinical trial. Sleep Med. 2020, 76, 113–119.
- Sadasivam, K.; Patial, K.; Vijayan, V.K.; Ravi, K. Anti-oxidant treatment in obstructive sleep apnoea syndrome. Indian J. chest Dis. Allied Sci. 2011, 53, 153–162.
- Rao, T.P.; Ozeki, M.; Juneja, L.R. In Search of a Safe Natural Sleep Aid. J. Am. Coll. Nutr. 2015, 34, 436–447.
- Lyon, M.R.; Kapoor, M.P.; Juneja, L.R. The effects of L-theanine (Suntheanine®) on objective sleep quality in boys with attention deficit hyperactivity disorder (ADHD): A randomized, double-blind, placebo-controlled clinical trial. Altern. Med. Rev. J. Clin. Ther. 2011, 16, 348.
- Sarris, J.; Byrne, G.J.; Cribb, L.; Oliver, G.; Murphy, J.; Macdonald, P.; Nazareth, S.; Karamacoska, D.; Galea, S.; Short, A.; et al. L-theanine in the adjunctive treatment of generalized anxiety disorder: A double-blind, randomised, placebo-controlled trial. J. Psychiatr. Res. 2019, 110, 31–37.
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients 2019, 11, 2362.
- Mayer, G.; Kröger, M.; Meier-Ewert, K. Effects of Vitamin B12 on Performance and Circadian Rhythm in Normal Subjects. Neuropsychopharmacology 1996, 15, 456–464.
- Luboshitzky, R.; Ophir, U.; Nave, R.; Epstein, R.; Shen-Orr, Z.; Herer, P. The effect of pyridoxine administration on melatonin secretion in normal men. Neuro Endocrinol. Lett. 2002, 23, 213–217.
- Ebben, M.; Lequerica, A.; Spielman, A. Effects of Pyridoxine on Dreaming: A Preliminary Study. Percept. Mot. Skills 2002, 94, 135–140.
- Aspy, D.J.; Madden, N.A.; Delfabbro, P. Effects of Vitamin B6 (Pyridoxine) and a B Complex Preparation on Dreaming and Sleep. Percept. Mot. Ski. 2018, 125, 451–462.
- Ghaderi, A.; Banafshe, H.R.; Motmaen, M.; Rasouli-Azad, M.; Bahmani, F.; Asemi, Z. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 84–89.
- Mason, C.; Tapsoba, J.D.D.; Duggan, C.; Wang, C.-Y.; Korde, L.; McTiernan, A. Repletion of vitamin D associated with deterioration of sleep quality among postmenopausal women. Prev. Med. 2016, 93, 166–170.
- Dadashpour, S.; Hajmiri, M.S.; Roshani, D. Effect of intravenous vitamin C supplementation on the quality of sleep, itching and restless leg syndrome in patients undergoing hemodialysis; A double-blind randomized clinical trial. J. Nephropharmacol. 2018, 7, 131–136.
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of Terminal Cancer Patients’ Health-related Quality of Life after High Dose Vitamin C Administration. J. Korean Med. Sci. 2007, 22, 7–11.
- Murck, H.; Held, K.; Antonijevic, I.; Künzel, H.; Golly, I.; Steiger, A. Oral Mg2+-supplementation reverses age related sleep-endocrine changes in humans. Eur. Neuropsychopharmacol. 2000, 10, 373.
- Abbasi, B.; Kimiagar, M.; Sadeghniiat, K.; Shirazi, M.M.; Hedayati, M.; Rashidkhani, B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J. Res. Med. Sci. 2012, 17, 1161–1169.
- Hornyak, M.; Haas, P.; Veit, J.; Gann, H.; Riemann, D. Magnesium Treatment of Primary Alcohol-Dependent Patients During Subacute Withdrawal: An Open Pilot Study with Polysomnography. Alcohol. Clin. Exp. Res. 2004, 28, 1702–1709.
- Saito, H.; Cherasse, Y.; Suzuki, R.; Mitarai, M.; Ueda, F.; Urade, Y. Zinc-rich oysters as well as zinc-yeast- and astaxanthin-enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Mol. Nutr. Food Res. 2017, 61, 1600882.
- Baradari, A.G.; Alipour, A.; Mahdavi, A.; Sharifi, H.; Nouraei, S.M.; Zeydi, A.E. The Effect of Zinc Supplementation on Sleep Quality of ICU Nurses: A Double Blinded Randomized Controlled Trial. Work Health Saf. 2018, 66, 191–200.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, ume 13, 757–772.
- Granato, D.; Nunes, D.S.; Barba, F.J. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017, 62, 13–22.
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193.
- DeMuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S., Jr. The Absolute Bioavailability of Oral Melatonin. J. Clin. Pharmacol. 2000, 40, 781–784.
- Scheid, L.; Ellinger, S.; Alteheld, B.; Herholz, H.; Ellinger, J.; Henn, T.; Helfrich, H.-P.; Stehle, P. Kinetics of L-Theanine Uptake and Metabolism in Healthy Participants Are Comparable after Ingestion of L-Theanine via Capsules and Green Tea. J. Nutr. 2012, 142, 2091–2096.
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179.
- Dhakal, S.P.; He, J. Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review. Food Res. Int. 2020, 137, 109326.
- Williams, J.; Kellett, J.; Roach, P.D.; McKune, A.; Mellor, D.; Thomas, J.; Naumovski, N. l-Theanine as a Functional Food Additive: Its Role in Disease Prevention and Health Promotion. Beverages 2016, 2, 13.
- Greene, S.C.; Noonan, P.K.; Sanabria, C.; Peacock, W.F. Effervescent N-Acetylcysteine Tablets versus Oral Solution N-Acetylcysteine in Fasting Healthy Adults: An Open-Label, Randomized, Single-Dose, Crossover, Relative Bioavailability Study. Curr. Ther. Res. 2016, 83, 1–7.
