Annona muricata is a member of the family Annonaceae and is familiar for its medicinal properties. A. muricata has been identified to have promising compounds that could potentially be utilized for the treatment of cancer. The most prevalent phytochemical components identified and isolated from this plant are alkaloids, phenols, and acetogenins.
- Annona muricata
- ethnomedicinal
- bioactive metabolites
- pharmacological activities
- anticancer activity
1. Introduction
2. Botanical Description and Distribution
The Annonaceae family includes about 130 genera and 2300 species, including A. muricata L., also known as soursop, graviola, guanabana, pawpaw, and sirsak [30,31][30][31]. A. muricata is native to the warmest tropical areas of South and North America, but now it has spread across the world’s tropical and subtropical countries, including India, Malaysia, Nigeria, Australia, and Africa [32]. Evergreen, terrestrial tree A. muricata grows to a height of 5 to 8 m with a broad, glossy, dark green, open, and round canopy. Individual yellow flowers on woody stalks are larger on this tree. The edible fruits of the tree are large, oval, or heart-shaped, green in color with more than 4 kg weight, with a diameter of 15 to 20 cm. The white juicy fibrous segments that make up the fruit pulp form an elongated receptacle. Fruit may have 5–200 seeds [33]. The skin is reticulated and has short spines, making it look leathery. It has a creamy, granular inner surface that easily separates from the soft pithy base [28].3. Bioactive Metabolites Responsible for Various Pharmacological Activities in A. muricata
Phytochemicals are constitutive metabolites produced by the primary or secondary metabolism of various parts of plants and have important plant functions (Figure 1). Plant growth and metabolism are also influenced by primary and secondary metabolites [34].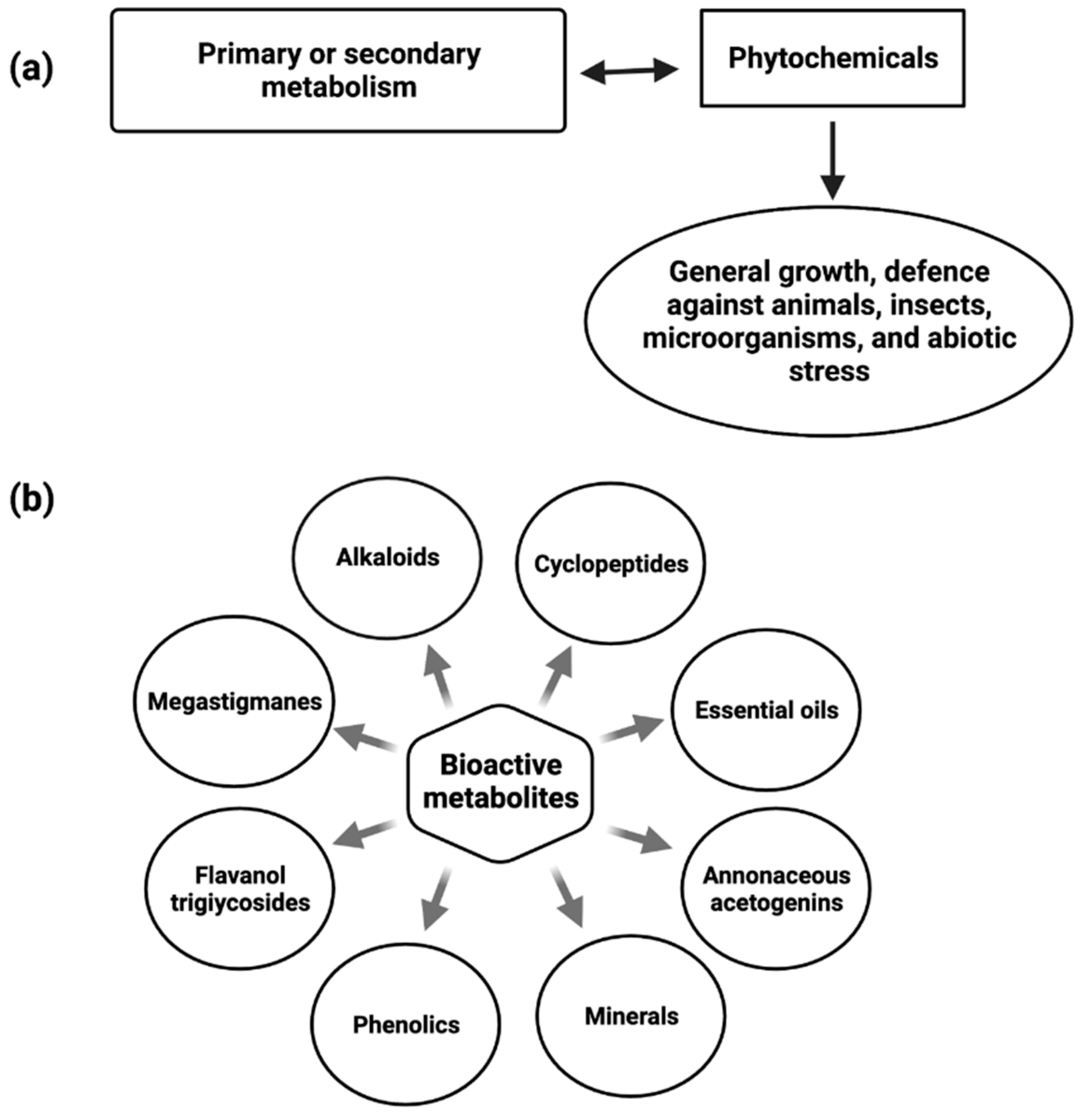
4. Ethnomedicinal Uses
Due to their therapeutic potential, the Annonaceae family has been widely investigated in recent years. The medicinal uses of the Annonaceae family have been recorded for a long time, and this species has gained attention in recent years due to its bioactivity and traditional uses [50][38]. Medicinal herbs are widely recognized as the foundation for human life and health care. Chronic degenerative diseases have attained widespread levels and are now regarded as serious health problems; as a result, treatment of these diseases is given clinical priority [68][39]. A. muricata has been proposed as an insecticide [69][40] and a parasiticide [70][41] in ethnobotanical studies. Fever [70][41], sedative [71][42], respiratory disease [70][41], malaria [72[43][44],73], gastrointestinal disorders, liver, heart, and kidney disorders [74,75,76][45][46][47] have all been treated with fruit juice and leaf/branch infusions. In recent years, this has been widely used for hypoglycemic [42][48], hypotensive [70][41], and cancer treatments [77,78][49][50] (Table 1).|
Ethnomedicinal Uses |
Plant Parts Used |
Graviola Extract/Chemical Compound |
References |
|---|---|---|---|
|
Insecticide |
Seed, leaves, barks, stems, roots and flowers |
Acetogenins |
|
|
Parasiticide |
Leaf |
Ethanolic extract and its fractions, methanol extracts, and acetogenins, ethyl acetate extract |
|
|
Hypotensive |
Leaf, fruit |
Aqueous extract, the alkaloids, isoquinoline, coreximine, and anomurine |
|
|
Fever |
Leaf |
Flavonoids |
|
|
Respiratory illness |
Leaf |
Essential Oil |
|
|
Sedative |
Leaf |
Hydroalcoholic extract |
|
|
Malaria |
Seed, leaf |
Ethanolic extract |
|
|
Gastrointestinal disorders |
Leaf |
Ethyl acetate extract |
|
|
Liver, heart, and renal disorders |
Fruit, Leaf |
Ethyl acetate and ethanol extracts |
|
|
Hypoglycemic |
Leaf, branch |
Ethanolic extract |
|
|
Cancer |
Leaf, fruit, stem, bark and branch |
Annonaceous acetogenins, alkaloids, flavonoids, sterols, and others |
Discussed in detail in the following sections |
5. Role of Annona muricata against Various Types of Cancer
5.1. Pancreatic Cancer
Pancreatic cancer, the deadliest malignancy in the world and the 4th largest source of malignancy fatalities, seems to have a 5-year survival rate of only 8% [110][83]. Due to a lack of early clinical symptoms, there is a high mortality rate in late diagnosed patients. Late diagnosis, resistance to available chemotherapy treatments, and cancer’s high aggressive behavior have encouraged new early detection markers, as well as research and evolution of chemo-preventive and chemotherapy agents. Even though several plant chemicals have been studied for pancreatic cancer treatment, none have yet been scientifically proven [111][84]. A. muricata capsules containing leaf and stem powder have anti-proliferative and antitumor effects in pancreatic cancer cell lines (IC50 values were 200 µg/mL in FG/COLO357 and 73 µg/mL in CD18/HPAF), and in subcutaneous xenografts, these activities included inducing cell cycle arrest with apoptosis. The migratory capacity of pancreatic cells has been similarly diminished upon treatment with the extract at a concentration of 100 µg/mL by a transwell assay [112][85]. Similar anti-proliferative effects have been identified for the hexane fraction of A. muricata leaves against pancreatic cancer cell line, Capan-1 (IC25 values were 7.8–8 μg/mL), which is rich in flavonoids [113][86]. A. muricata also inhibited the motility and invasion of PC cells by downregulating the mucin MUC4 (Figure 2) [114][87]. Despite MUC4′s enormous pathological importance in various cancers, A. muricata’s high therapeutic suitability for various tumors, especially PC, is indicated by MUC4 down-regulation. A. muricata, in addition to downregulating MUC4, has been shown to cause cell death by modifying glucose metabolism and inducing metabolic catastrophe [112,115][85][88].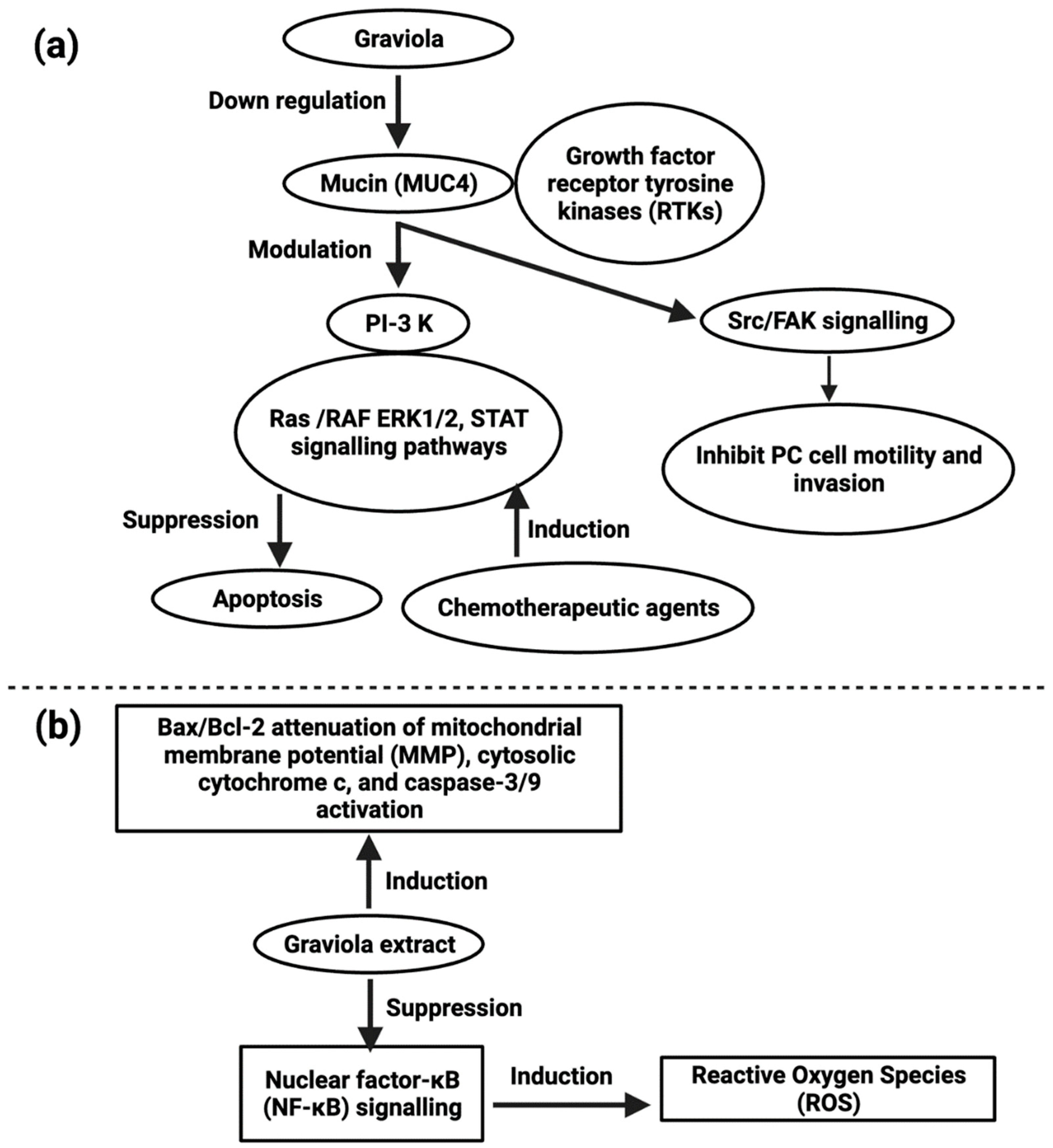
5.2. Lung Carcinoma
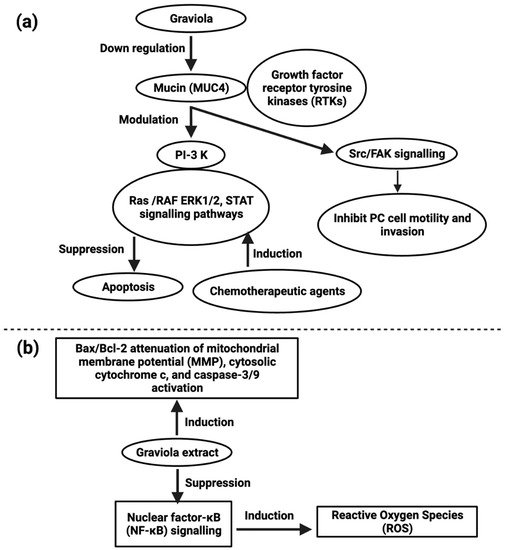
Lung cancer is the most common cancer-related death worldwide [110][83]. Because of chemotherapeutic tolerance, many patients with lung cancer succumb to the disease. In vitro studies of A549 (human lung adenocarcinoma) cell lines revealed that A. muricata leaf extract possesses cytotoxic activity and the IC50 values for hexane, ethyl acetate, and methanol extracts were 21.05 ± 0.42 µg/mL, 5.09 ± 0.4 µg/mL and ≥ 100 µg/mL respectively, caused cell cycle arrest at the G0/G1 phase and apoptosis (Figure 2) [121][91]. cis-Annonacin-A-one and trans-Annonacin-A-one, cis- gigantetrocinone and trans-gigantetrocinone, cis-isoAnnonacin and trans-isoAnnonacin and squamolone isolated acetogenins possess cytotoxic activity against A549 cell lines, and the ED50 values were 3.39 × 10−2, 9.74 × 10−3, 4.42 × 10−5, ≥ 10, 1.48 × 10−3, respectively [122][92]. A. muricata leaf ethyl acetate extract (AMEAE) possesses a cytotoxic effect on the A549 cell line, and the IC50 value was 5.09 ± 0.41 μg/mL after 72 h of treatment, inducing apoptosis. This was proved by multiple high-content screening cytotoxicity studies. The results showed that A549 cells treated with the A. muricata extract inhibited growth potential, and their apoptosis pathway was upregulated [121][91]. Graviola extract inhibits nuclear factor-κB (NF-κB) signaling, increases ROS production, and enhances the Bax/Bcl-2 ratio–mediated inhibition of mitochondrial membrane potential, activation of cytosolic cytochrome c, and caspase-3/9 as reported in A549 cell line [121][91].5.3. Prostate Carcinoma
Prostate carcinoma is the highest source of cancer-related fatalities in Western developed countries [110][83], with over 1,64,690 records and 29,430 deaths in 2018. Though rapid innovations in early identification and novel therapy techniques can significantly boost these patients’ lives, a significant proportion of them acquire aggressive and refractory tumors with a bad prognosis. Several bioactive compounds have been tried as adjuvants to existing therapy for treating and preventing hormone-refractory pancreatic cancer, but no clinical success has been found [123][93]. MTT and colony formation assays revealed that A. muricata fruit pulp extract has potent antiproliferative activity in prostate cancer (PCa) cell lines 22Rv1, LNCaP, and PC-3 at a concentration of 1–5 μg/mL [124][94]. They also discovered that fruit extract had antiproliferative effects, which were mediated by lowering HIF-1 expression and inhibiting NOX activity (Figure 3) [125][95]. Muricin J, muricin K, and muricin L induced antiproliferative and apoptotic effects on PC-3 cells at 20 µg/mL [126][96]. Muricin M, muricin N, and muricenin obtained from the A. muricata fruit bioactive ethanolic extract were shown to have antitumor effects at a concentration of 20 µg/mL [124][94].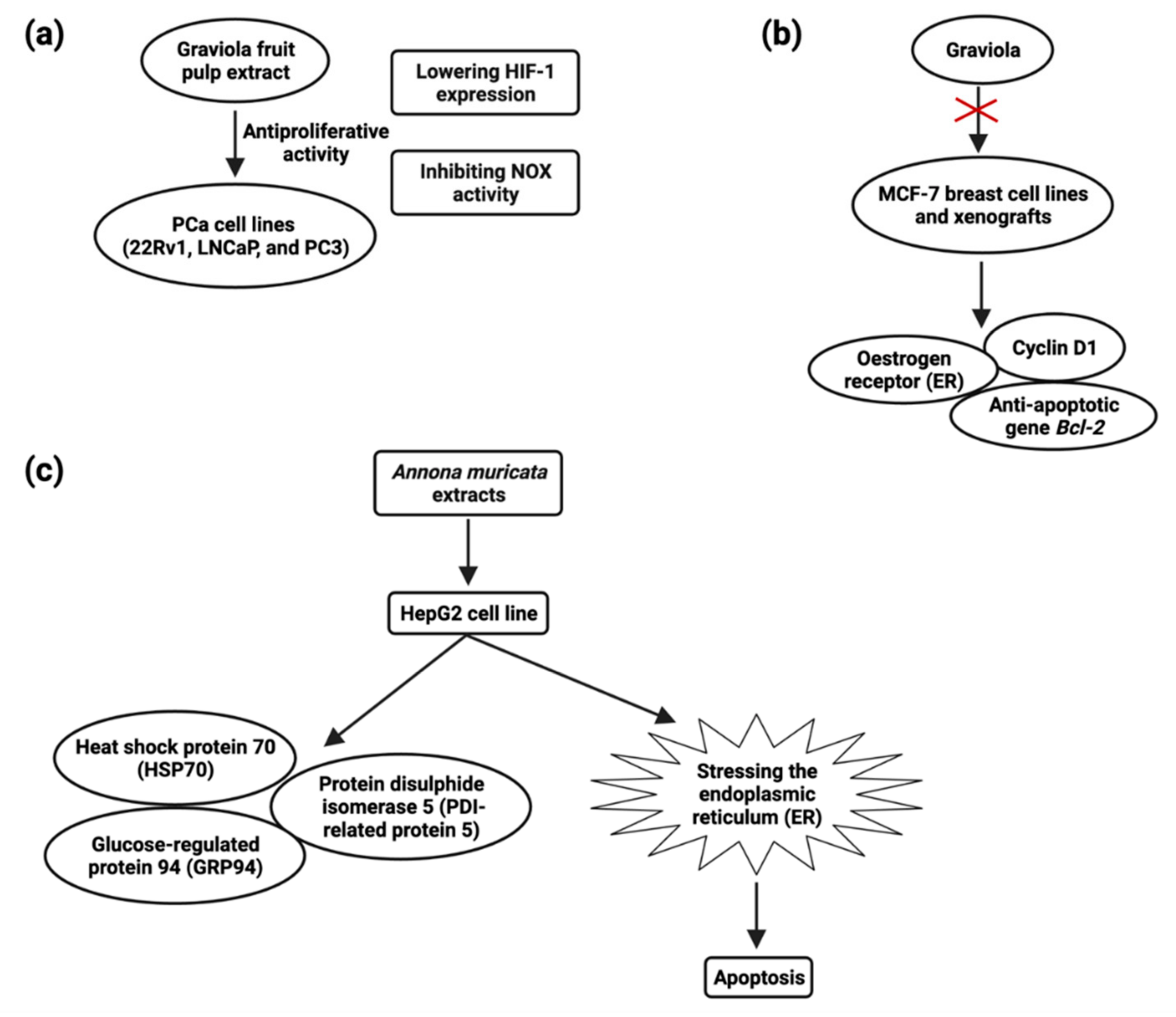
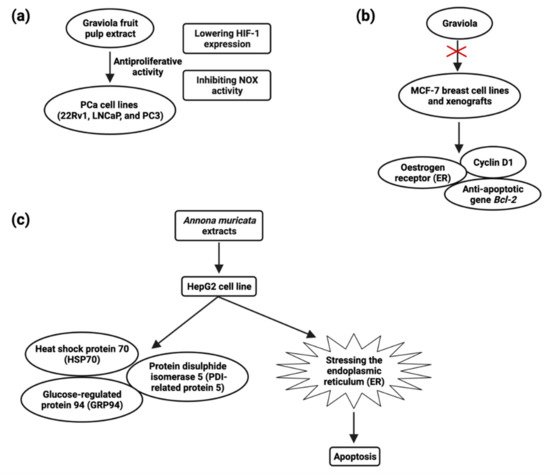
5.4. Breast Cancer
The most prevalent malignancy among women worldwide is breast cancer [110][83]. Early-stage breast cancer may be treated, but advanced breast cancer has no treatment options. New chemopreventive and chemotherapeutic drugs are desperately needed in order to impede the formation of tumors and reduce associated morbidity. Although certain natural chemicals have been examined in vitro and shown to be safer and less hazardous than synthetic compounds [134][102], these natural compounds’ low clinical efficacy has hampered their translational use. Recent studies have demonstrated A. muricata’s strong antiproliferative and antitumor ability. Exposure of cancer cells to A. muricata leaf extract and ethyl acetate fraction resulted in morphological alterations indicative of apoptosis, a process characterized by the rupture and loss of the membrane and nucleus of cells. Reduced Bcl-2 and PARP-1 and increased caspase-9 and caspase-3 expression are responsible for the cytotoxic activity observed in MCF7 cells [62][103]. Treatment of Annonacin (0.5–1 µM) induced MCF breast cancer cell line death at 48 h and treatment of Annonacin (0.1 µM) in xenografts tumor decreases the expression of ER, cyclin D1, and Bcl2 (Figure 3) [135][104]. A. muricata fruit extract inhibited MDA-MB-468 cells (IC50 = 4.8 µg/mL) and significantly downregulated EGFR mRNA expression, cell cycle arrest, and apoptosis but not in MCF-10A cell lines. In the xenograft mouse model, 5-week dietary treatment with fruit extract (200 mg/kg diet) decreased expression of EGFR, p-EGFR, and p-ERK in MDA-MB-468 tumors by 56–32.5% [136][105]. MDR is the predominant source by which tumor cells gain therapeutic resistance, leading to therapy failure and tumor progression. By depleting ATP content, ACG bullatacin (1 µg/mL) is cytotoxic to the MCF-7/ADR cell line of multidrug resistant breast cancer [137][106]. ACGs from A. muricata offer a particular benefit against MDR breast tumors, whereas ACGs extracted from Annona Squamosa seeds alter mitogen-activated protein kinase (MAPK) signaling and triggered apoptosis in MCF-7/ADR cells. Annosquacin B (AB), dramatically reduced cell viability on MCF-7/ADR (IC50value-14.69 µM), induced apoptosis followed by elevated levels of caspase-3, caspase-9, Bax/Bcl-2, p-p38 MAPK and lowered p-JNK [138][107] .5.5. Colon Carcinoma
CRC (colorectal carcinoma) is the third most common cause of cancer-related fatalities [110][83]. The biggest problems for CRC patients are therapeutic resistance and toxicity against current medications, which are connected to a poor prognosis [145][108]. A. muricata and other natural products have proven to be effective in preventing and treating CRC. The molecular mechanisms of various A. muricata extracts against CRC have recently been elucidated. In the COLO-205 (human colon adenocarcinoma) cell line, the A. muricata leaf extract (1422 ng/mL) also showed anticancer properties by increasing the proapoptotic protein caspase-3 [146][109] (Figure 4). The ethyl acetate leaf extract of A. muricata has a substantial cytotoxic effect on HCT-116 (human colorectal carcinoma) and HT-29 (human colorectal adenocarcinoma) cell lines (Figure 4), and IC50 values were 11.43 ± 1.87 µg/mL and 8.98 ± 1.24 µg/mL, respectively, followed by cell cycle arrest, apoptosis, mitochondrial membrane depolarization, cytochrome c leakage and activation of the initiator and executioner caspases, up-regulation of Bax and down-regulation of Bcl-2 proteins, halted the migration and invasion of HT-29 and HCT-116 cells [109][82].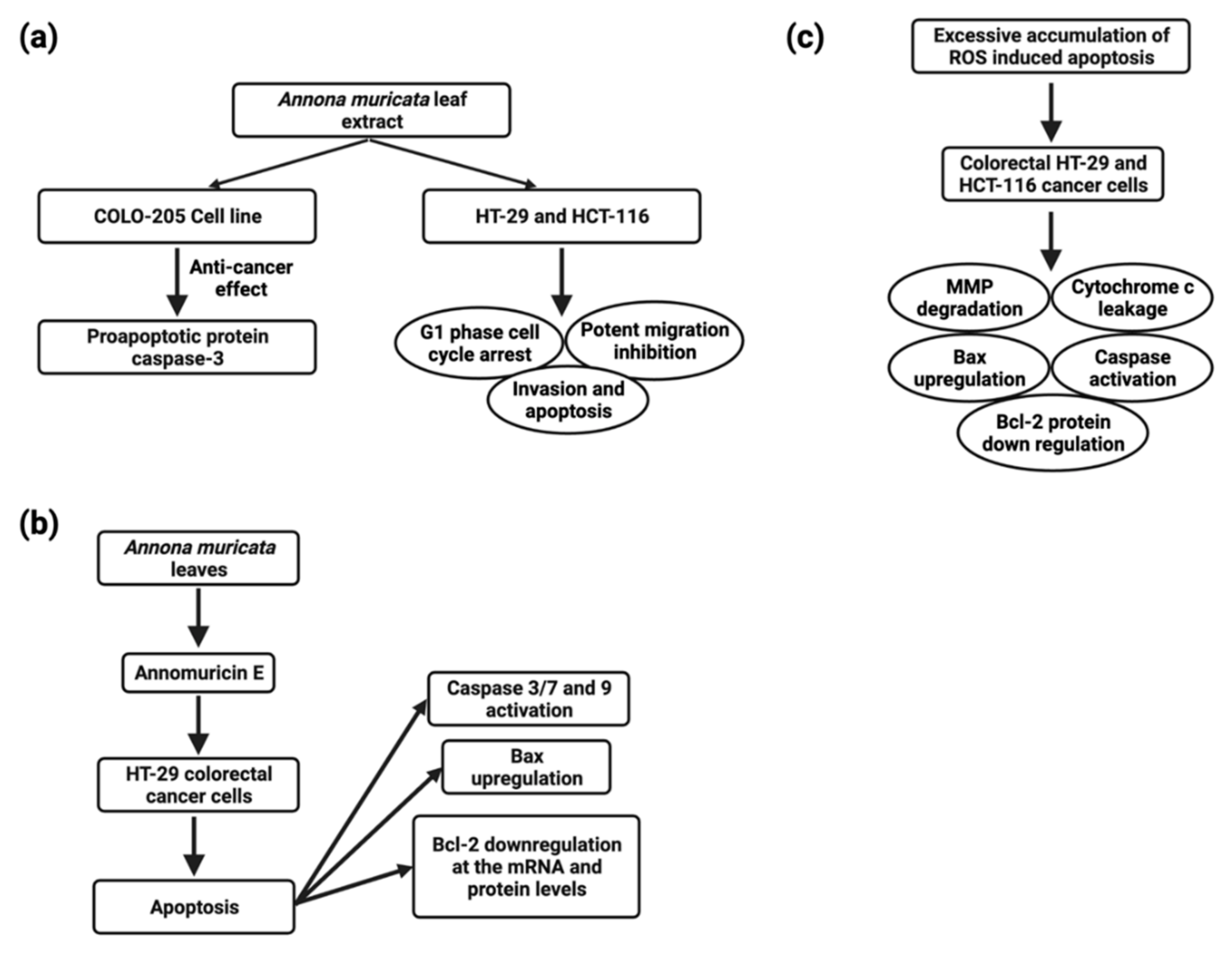
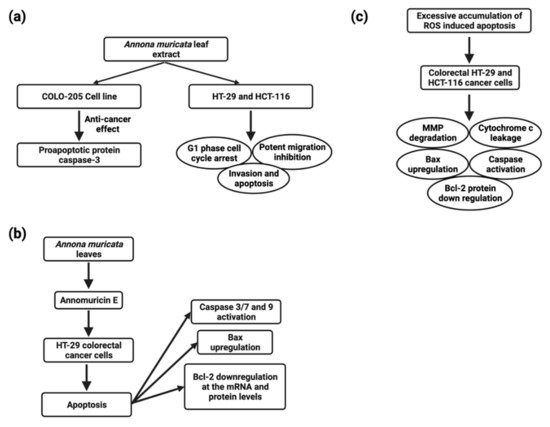
5.6. Head and Neck Cancers
The majority of head and neck malignancies arise from the mucosal epithelium of the oral cavity, pharynx, and larynx, and are generally referred to as head and neck squamous cell carcinoma (HNSCC) [151][114] and are the sixth highest prevalent cancer worldwide. Several anticancer drugs, such as etoposide, cisplatin, topotecan, doxorubicin, and fluorouracil (Figure 5), have been linked to adverse side effects that severely limit their utility. Through current advancements in the understanding and treatment of the disease, patients with HNSCC have a poor prognosis due to resistance to available chemoradiotherapy. As a result, there is a critical need to investigate less toxic anticancer compounds.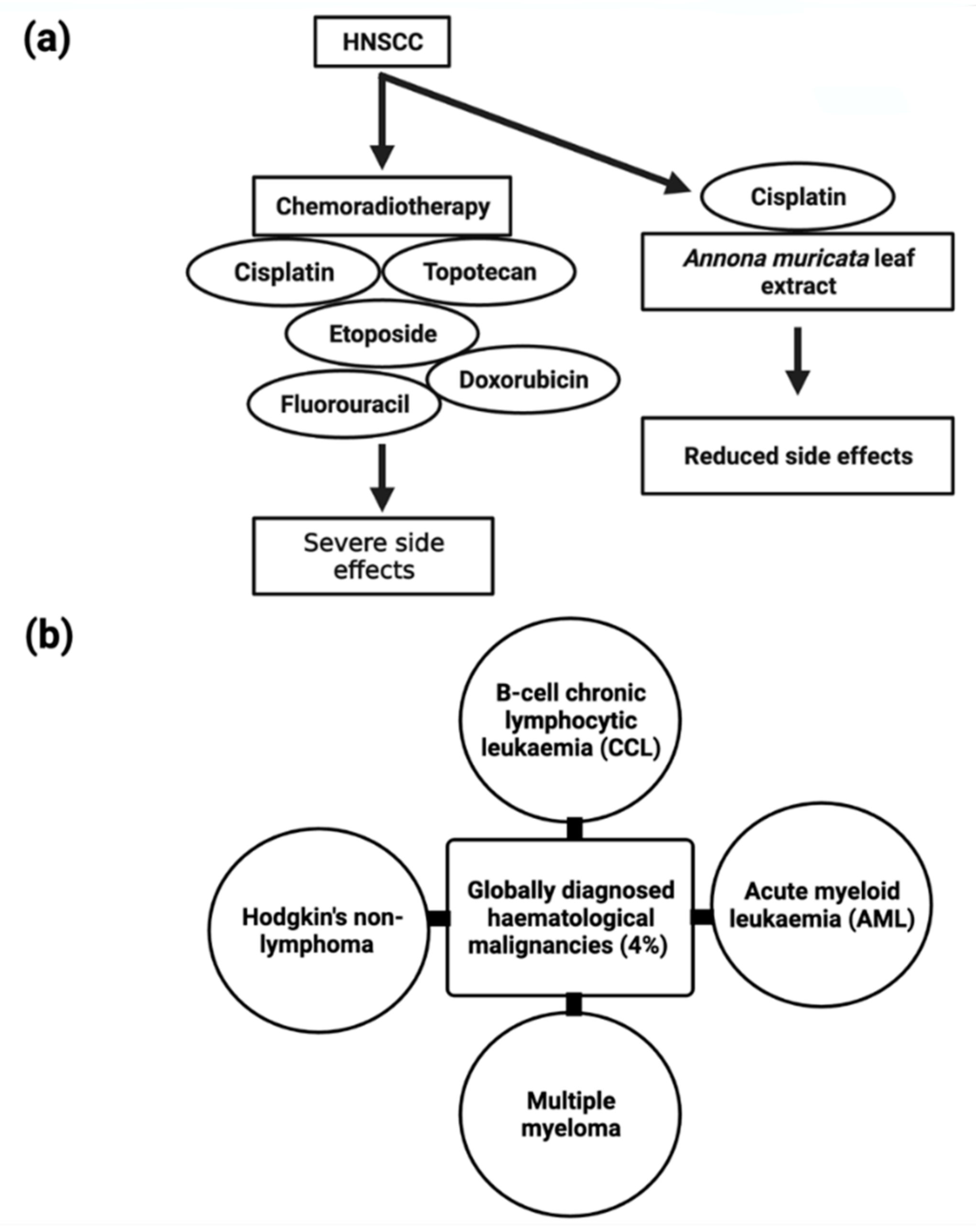
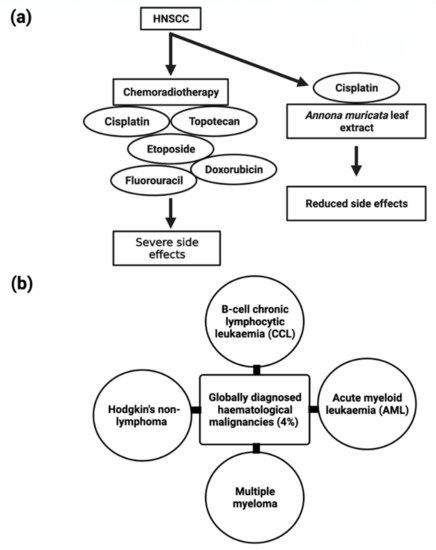
5.7. Hematological Malignancies
B-cell chronic lymphocytic leukemia, acute myeloid leukemia, multiple myeloma, and non-lymphoma Hodgkin’s make up about 4% of cancers diagnosed globally (Figure 5) [110][83]. Despite the disease’s biological characteristics and complexity, it poses particular clinical challenges. In clinical trials, phytocompounds and derivatives have been evaluated, but the majority of compounds have been unsuccessful due to inadequate effectiveness, resulting in resistance; therefore, it is imperative to develop novel natural chemical compounds with improved therapeutic capabilities. Though random screening is a high-priced and tedious process, focused research into commonly used medicinal plants can speed up the development of new anticancer drugs [155][118]. A. muricata twig, root, and leaf extract possess antiproliferative activity, and the IC50 values were found to be 49 ± 3.2 µg/mL, 9 ± 0.8 µg/mL, 14 ± 2.4 µg/mL, respectively, and apoptotic effects have been linked to cell cycle arrest and MMP loss in mechanical experiments [156][119]. Furthermore, ethanol and methanol A. muricata leaf extract-induced apoptosis in K562 (human myelogenous leukemia), CCRF-CEM (human T-leukemia), and CEM/ADR5000 (multidrug-resistant leukemia) cells have been reported [101][74]. In this study, it was found that A. muricata can be used to identify phenotypes of multidrug-resistant malignancy and is thus an outstanding method for the evolution of new therapeutic medicines for hematological malignancies. Methanolic extracts from seeds, leaves, and pericarp have shown antiproliferative activity, and the IC50 values were found to be fruit pericarp 4.58 ± 0.25 µg/mL, leaves 0.57 ± 0.02 µg/mL, and seeds 0.36 ± 0.03 µg/mL against CCRF–CEM cells and induced apoptosis, whereas pericarp and leaf extracts induce apoptosis in leukemia cells CEM/ADR5000 [37]. An ethanolic leaf extract at a 50 µg/mL concentration significantly increased caspase3 activity to induce apoptosis in K562 leukemia cancer cells, as reported by the TUNEL assay [157][120] .5.8. Liver Cancer
The plant extracts have been shown to have a cytotoxic impact on hepatic cancer cells, hinting that they could be employed as a hepatic cancer treatment. The HepG2 cell line was shown to be inhibited in growth and viability after being incubated with an ethanol extract of A. muricata; after 24 and 48 h of treatment and LD50 values were 180 and 80 µg/mL, respectively, and apoptosis through inducing ROS pathway [159][121]. A. muricata leaf extracts possess antiproliferative activity, and the IC50 values were 150 μg/mL for both HepG2 and HCT116 cells, inducing apoptosis at a concentration of 120 μg/mL against HepG2 cells. HSP70, GRP94, and PDI-related protein 5 (Figure 3) were all found to be upregulated after HepG2 treatment [160][122] .5.9. Cervical Cancer
In HeLa cervical cancer cells, different solvent leaf extracts were able to induce antiproliferative activity. As shown by the (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazole) (MTT) reduction method, A. muricata methanol leaf extract was able to inhibit the proliferation of the HEp-2 (laryngeal cancer) cell line [132][100]. However, later HEp-2 was confirmed to be a HeLa cell line that had been cross-contaminated [162][123].References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789.
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 31 August 2022).
- Global Cancer Facts & Figures|American Cancer Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/global.html (accessed on 31 August 2022).
- Minkler, S.; Lucien, F.; Kimber, M.J.; Sahoo, D.K.; Bourgois-Mochel, A.; Musser, M.; Johannes, C.; Frank, I.; Cheville, J.; Allenspach, K.; et al. Emerging Roles of Urine-Derived Components for the Management of Bladder Cancer: One Man’s Trash Is Another Man’s Treasure. Cancers 2021, 13, 422.
- Sahoo, D.K.; Borcherding, D.C.; Chandra, L.; Jergens, A.E.; Atherly, T.; Bourgois-Mochel, A.; Ellinwood, N.M.; Snella, E.; Severin, A.J.; Martin, M.; et al. Differential Transcriptomic Profiles Following Stimulation with Lipopolysaccharide in Intestinal Organoids from Dogs with Inflammatory Bowel Disease and Intestinal Mast Cell Tumor. Cancers 2022, 14, 3525.
- Sahoo, D.K.; Mosichuk, A.P.; Lucien, F.; Frank, I.; Cheville, J.C.; Allenspach, K.; Mochel, J.P. Abstract 3092: Urine-Derived Urinary Carcinoma Organoids: A Novel Tool for Providing New Insights into Human and Canine Bladder Cancer Treatment. Cancer Res. 2022, 82, 3092.
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural Products to Prevent Drug Resistance in Cancer Chemotherapy: A Review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27.
- Gullett, N.P.; Ruhul Amin, A.R.M.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.J.; Kucuk, O. Cancer Prevention with Natural Compounds. Semin. Oncol. 2010, 37, 258–281.
- Borchardt, J.K. Natural Product Chemistry for Drug Discovery. Drug News Perspect. 2002, 15, 187–192.
- WHO Establishes the Global Centre for Traditional Medicine in India. Available online: https://www.who.int/news/item/25-03-2022-who-establishes-the-global-centre-for-traditional-medicine-in-india (accessed on 5 September 2022).
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. 2016, 79, 629–661.
- Siddiqui, A.A.; Farah, I.; Siddiqui, S.; Sahu, K. Role of Natural Products in Drug Discovery Process. Int. J. Drug Dev. Res. 2014, 6, 172–204.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Parekh, J.; Chanda, S. In Vitro Antibacterial Activity of the Crude Methanol Extract of Woodfordia Fruticosa Kurz. Flower (Lythraceae). Braz. J. Microbiol. 2007, 38, 204–207.
- Prasad, P.M.; Palthur, S.; Chitta, S.K. Umar Nutraceuticals: Concept and Regulatory Scenario. Int. J. Pharm. Pharm. Sci. 2010, 2, 14–20.
- Urbi, Z.; Hossain, M.S.; Rahman, K.M.H.; Zayed, T.M. Grape: A Medicinal Fruit Species in the Holy Qur’an and Its Ethnomedicinal Importance. World Appl. Sci. J. 2014, 30, 253–265.
- Dafni, A.; Böck, B. Medicinal Plants of the Bible-Revisited. J. Ethnobiol. Ethnomed. 2019, 15, 57.
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial Activity of Some Ethnomedical Plants Used by Paliyar Tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35.
- Syed Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Abd Rahman, N.M.A.N. Anti-Cancer Effect of Annona muricata Linn Leaves Crude Extract (AMCE) on Breast Cancer Cell Line. BMC Complement. Altern. Med. 2016, 16, 311.
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of Survival, Proliferation, Invasion, Angiogenesis, and Metastasis of Tumor Cells through Modulation of Inflammatory Pathways by Nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434.
- Rady, I.; Bloch, M.B.; Chamcheu, R.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxid. Med. Cell Longev. 2018, 2018, 1826170.
- Chan, W.J.J.; McLachlan, A.J.; Hanrahan, J.R.; Harnett, J.E. The Safety and Tolerability of Annona muricata Leaf Extract: A Systematic Review. J. Pharm. Pharmacol. 2020, 72, 1–16.
- Foster, K.; Younger, N.; Aiken, W.; Brady-West, D.; Delgoda, R. Reliance on Medicinal Plant Therapy among Cancer Patients in Jamaica. Cancer Causes Control 2017, 28, 1349–1356.
- Clement, Y.N.; Mahase, V.; Jagroop, A.; Kissoon, K.; Maharaj, A.; Mathura, P.; Quan, C.M.; Ramadhin, D.; Mohammed, C. Herbal Remedies and Functional Foods Used by Cancer Patients Attending Specialty Oncology Clinics in Trinidad. BMC Complement. Altern. Med. 2016, 16, 399.
- Rojas Rojas, T.; Bourdy, G.; Ruiz, E.; Cerapio, J.P.; Pineau, P.; Gardon, J.; Doimi, F.; Deparis, X.; Deharo, E.; Bertani, S. Herbal Medicine Practices of Patients With Liver Cancer in Peru: A Comprehensive Study Toward Integrative Cancer Management. Integr. Cancer Ther. 2018, 17, 52–64.
- Atawodi, S. Nigerian Foodstuffs with Prostate Cancer Chemopreventive Polyphenols. Infect. Agents Cancer 2011, 6, S9.
- Ong, H.G.; Kim, Y.D. Quantitative Ethnobotanical S.Study of the Medicinal Plants Used by the Ati Negrito Indigenous Group in Guimaras Island, Philippines. J. Ethnopharmacol. 2014, 157, 228–242.
- Badrie, N.; Schauss, A.G. Soursop (Annona muricata L.): Composition, Nutritional Value, Medicinal Uses, and Toxicology. In Bioactive Foods in Promoting Health: Fruits and Vegetables; Academic Press: Oxford, UK, 2010; pp. 621–643.
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican Medicinal Plants Used for Cancer Treatment: Pharmacological, Phytochemical and Ethnobotanical Studies. J. Ethnopharmacol. 2011, 133, 945–972.
- Mishra, S.; Ahmad, S.; Kumar, N.; Sharma, B.K. Annona muricata (the Cancer Killer): A Review. Glob. J. Pharma. Res. 2013, 2, 1613–1618.
- Leboeuf, M.; Cavé, A.; Bhaumik, P.; Mukherjee, B.; Mukherjee, R. The Phytochemistry of the Annonaceae. Phytochemistry 1980, 21, 2783–2813.
- Adewole, S.O.; Caxton-Martins, E.A. Morphological Changes and Hypoglycemic Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Pancreatic B-Cells of Streptozotocin-Treated Diabetic Rats. Afr. J. Biomed. Res. 2006, 9, 173–187.
- de Souza, R.; Benassi, E.; da Silva, R.R.; Afonso, S.; Scarminio, I.S. Enhanced Extraction Yields and Mobile Phase Separations by Solvent Mixtures for the Analysis of Metabolites in Annona muricata L. Leaves. J. Sep. Sci. 2009, 32, 4176–4185.
- Errayes, A.O.; Abdussalam-Mohammed, W.; Darwish, M.O. Review of Phytochemical and Medical Applications of Annona muricata Fruits. J. Chem. Rev. 2020, 2, 70–79.
- Nugraha, A.S.; Haritakun, R.; Lambert, J.M.; Dillon, C.T.; Keller, P.A. Alkaloids from the Root of Indonesian Annona muricata L. Nat. Prod. Res. 2021, 35, 481–489.
- Nguyen, M.T.; Nguyen, V.T.; Minh, L.V.; Trieu, L.H.; Cang, M.H.; Bui, L.B. Determination of the Phytochemical Screening, Total Polyphenols, Flavonoids Content, and Antioxidant Activity of Soursop Leaves (Annona muricata Linn.). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062011.
- Moghadamtousi, S.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.; Kadir, H. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658.
- Gavamukulya, Y.; Wamunyokoli, F.; El-Shemy, H.A. Annona muricata: Is the Natural Therapy to Most Disease Conditions Including Cancer Growing in Our Backyard? A Systematic Review of Its Research History and Future Prospects. Asian Pac. J. Trop. Med. 2017, 10, 835–848.
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complementary Altern. Med. 2013, 10, 5.
- Deewatthanawong, R.; Kongchinda, P.; Deewatthanawong, P.; Pumnuan, J.; Insung, A. GC-MS Analysis and Biopesticide Properties of Different Crude Extracts of Annona Squamosa and Annona muricata. Int. J. Agric. Technol. 2019, 15, 859–868.
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A Comprehensive Review on Its Traditional Medicinal Uses, Phytochemicals, Pharmacological Activities, Mechanisms of Action and Toxicity. Arab. J. Chem. 2018, 11, 662–691.
- Bikomo, E.O.; Magbagbeola, O.A.; Ebuehi, O.A. Antidepressant Activity of Ethanol Leaf Extract of Annona muricata L. In Sprague-Dawley Rats; Scientific & Academic Publishing: Rosemead, CA, USA, 2017; Volume 7, pp. 1–5.
- Parthiban, E.; Arokiyaraj, C.; Ramanibai, R. Annona muricata: An Alternate Mosquito Control Agent with Special Reference to Inhibition of Detoxifying Enzymes in Aedes Aegypti. Ecotoxicol. Environ. Saf. 2020, 189, 11005.
- Ezemuoka, L.C.; Nwankwo, E.N.; Ogbonna, C.U. Toxicity of the Aqueous Leaf and Stem-Bark Extracts of Annona muricata to the 4th Instar Larvae of Aedes Aegypti. J. Entomol. Zool. Stud. 2019, 7, 1047–1052.
- Hemalatha, G.; Sivakumari, K.; Rajesh, S.; Shyamala Devi, K. Phytochemical Profiling, Anticancer and Apoptotic Activity of Graviola (Annona muricata) Fruit Extract against Human Hepatocellular Carcinoma (HepG-2) Cells. Int. J. Zool. Appl. Biosci. 2020, 5, 32–47.
- Ojowu, J.O.; Onwuchukwu, C.N.; Daramola, M.E.; Ebhohon, S.O. Annona Muricata (L.): Investigating the Ameliorative Effect of Leaves Extract on Liver and Kidney Function in Carbon Tetrachloride (CCl4) Induced Rats. J. Biomed. Sci. Res. 2020, 2, 2.
- Priya, M.R.K.; Iyer, P.R. Antiproliferative Effects on Tumor Cells of the Synthesized Gold Nanoparticles against Hep2 Liver Cancer Cell Line. Egypt. Liver J. 2020, 10, 15.
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; da Silva, N.M.; Espindola, F.S. Annona muricata Linn. Leaf as a Source of Antioxidant Compounds with in Vitro Antidiabetic and Inhibitory Potential against α-Amylase, α-Glucosidase, Lipase, Non-Enzymatic Glycation and Lipid Peroxidation. Biomed. Pharmacother. 2018, 100, 83–92.
- Abdul Wahab, S.M.; Jantan, I.; Haque, M.; Arshad, L. Exploring the Leaves of Annona muricata L. as a Source of Potential Anti-Inflammatory and Anticancer Agents. Front. Pharm. 2018, 9, 661.
- Patel, M.S.; Patel, J.K. A Review on a Miracle Fruits of Annona muricata. J. Pharm. Phytochem. 2016, 5, 137.
- Bobadilla, M.; Zavala, F.; Sisniegas, M.; Zavaleta, G.; Mostacero, J.; Taramona, L. Evaluación larvicida de suspensiones acuosas de Annona muricata Linnaeus «guanábana» sobre Aedes aegypti Linnaeus (Diptera, Culicidae). Rev. Peru. Biol. 2005, 12, 145–152.
- Trindade, R.C.P.; Luna, J.d.; de Lima, M.R.F.; da Silva, P.P.; Sant’ana, A.A.E.G. Larvicidal Activity and Seasonal Variation of Annona muricata (Annonaceae) Extract on Plutella Xylostella (Lepidoptera: Plutellidae). Rev. Colomb. Entomol. 2011, 37, 223–227.
- Langenberger, G.; Prigge, V.; Martin, K.; Belonias, B.; Sauerborn, J. Ethnobotanical Knowledge of Philippine Lowland Farmers and Its Application in Agroforestry. Agrofor. Syst. 2009, 76, 173–194.
- Miranda, N.C.; Araujo, E.C.B.; Justino, A.B.; Cariaco, Y.; Mota, C.M.; Costa-Nascimento, L.A.; Espindola, F.S.; Silva, N.M. Anti-Parasitic Activity of Annona muricata L. Leaf Ethanolic Extract and Its Fractions against Toxoplasma Gondii in Vitro and in Vivo. J. Ethnopharmacol. 2021, 273, 114019.
- Bories, C.; Loiseau, P.; Cortes, D.; Myint, S.H.; Hocquemiller, R.; Gayral, P.; Cave, A.; Laurens, A. Antiparasitic Activity of Annona muricata and Annona Cherimolia Seeds. Planta Med. 1991, 57, 434–436.
- Osorio, E.; Arango, G.J.; Jiménez, N.; Alzate, F.; Ruiz, G.; Gutiérrez, D.; Paco, M.A.; Giménez, A.; Robledo, S. Antiprotozoal and Cytotoxic Activities in Vitro of Colombian Annonaceae. J. Ethnopharmacol. 2007, 111, 630–635.
- Nwokocha, C.R.; Owu, D.U.; Gordon, A.; Thaxter, K.; Mccalla, G.; Ozolua, R.I.; Young, L. Possible Mechanisms of Action of the Hypotensive Effect of Annona muricata (Soursop) in Normotensive Sprague–Dawley Rats. Pharm. Biol. 2012, 50, 1436–1441.
- Banne, Y.; Barung, E.N.; Juyta; Dumanauw, J.M. Antipyretic Effect of Soursop Leaves Extract (Annona muricata L.) on Rats. Nat. Prod. Chem. Res. 2017, 5, 5.
- Kossouoh, C.; Moudachirou, M.; Adjakidje, V.; Chalchat, J.C.; Figuérédo, G. Essential Oil Chemical Composition of Annona muricata L. Leaves from Benin. J. Essent. Oil Res. 2007, 19, 307–309.
- Souza, D.O.; Dos Santos Sales, V.; de Souza Rodrigues, C.K.; de Oliveira, L.R.; Santiago Lemos, I.C.; de Araújo Delmondes, G.; Monteiro, Á.B.; do Nascimento, E.P.; Sobreira Dantas Nóbrega de Figuêiredo, F.R.; Martins da Costa, J.G.; et al. Phytochemical Analysis and Central Effects of Annona muricata Linnaeus: Possible Involvement of the Gabaergic and Monoaminergic Systems. Iran. J. Pharm. Res. 2018, 17, 1306–1317.
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and Cytotoxic Activities of Ethnopharmacologically Selected Medicinal Plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427.
- Moghadamtousi, S.Z.; Rouhollahi, E.; Karimian, H.; Fadaeinasab, M.; Abdulla, M.A.; Kadir, H.A. Gastroprotective Activity of Annona muricata Leaves against Ethanol-Induced Gastric Injury in Rats via Hsp70/Bax Involvement. Drug Des. Dev. Ther. 2014, 8, 2099.
- Padma, P.; Chansouria, J.P.; Khosa, R.L. Hepatoprotective Activity of Annona muricata Linn. and Polyalthia Cerasoides Bedd. Anc. Sci. Life 1999, 19, 7–10.
- Adewole, S.O.; Ojewole, J.A.O. Protective Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Serum Lipid Profiles and Oxidative Stress in Hepatocytes of Streptozotocin-Treated Diabetic Rats. Afr. J. Tradit. Complementary Altern. Med. 2009, 6, 30–41.
- Shukry, M.; El-Shehawi, A.M.; El-Kholy, W.M.; Elsisy, R.A.; Hamoda, H.S.; Tohamy, H.G.; Abumandour, M.M.; Farrag, F.A. Ameliorative Effect of Graviola (Annona muricata) on Mono Sodium Glutamate-Induced Hepatic Injury in Rats: Antioxidant, Apoptotic, Anti-Inflammatory, Lipogenesis Markers, and Histopathological Studies. Animals 2020, 10, 1996.
- Ola-Davies, O.E.; Oyagbemi, A.A.; Omobowale, T.O.; Akande, I.; Ashafa, A. Ameliorative Effects of Annona muricata Linn. (Annonaceae) against Potassium Dichromate-Induced Hypertension in Vivo: Involvement of Kim-1/P38 MAPK/Nrf2 Signaling. J. Basic Clin. Physiol. Pharm. 2019, 30, 20180172.
- Son, Y.; Lee, H.; Son, S.Y.; Lee, C.H.; Kim, S.Y.; Lim, Y. Ameliorative Effect of Annona muricata (Graviola) Extract on Hyperglycemia Induced Hepatic Damage in Type 2 Diabetic Mice. Antioxidants 2021, 10, 1546.
- Florence, N.T.; Benoit, M.Z.; Jonas, K.; Alexandra, T.; Désiré, D.D.P.; Pierre, K.; Théophile, D. Antidiabetic and Antioxidant Effects of Annona muricata (Annonaceae), Aqueous Extract on Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2014, 151, 784–790.
- Choi, M.; Kang, Y.R.; Zu, H.D.; Lim, I.S.; Jung, S.K.; Chang, Y.H. Effects of Time on Phenolics and in Vitro Bioactivity in Autoclave Extraction of Graviola (Annona muricata) Leaf. Biotechnol. Bioprocess. Eng. 2020, 25, 9–15.
- Olugbuyiro, J.A.O.; Omotosho, O.E.; Taiwo, O.S.; Ononiwu, F.O.; Banwo, A.S.; Akintokun, O.A.; Obaseki, O.S.; Ogunleye, O.M. Antimicrobial activities and phytochemical properties of Annona muricata leaf. Covenant J. Phys. Life Sci. 2018, 5, 2.
- Orak, H.H.; Bahrisefit, I.S.; Sabudak, T. Antioxidant Activity of Extracts of Soursop (Annona muricata L.) Leaves, Fruit Pulps, Peels and Seeds. Pol. J. Food Nutr. Sci. 2019, 69, 4.
- Mansour, H.H.; Elkady, A.A.; Elrefaei, A.H.; Hafez, H.F. Radioprotective, Antioxidant and Antitumor Efficacy of Annona muricata L. Leaf Extract. Indian J. Biochem. Biophys. 2018, 55, 205–214.
- Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Gálvez-Ruiz, J.C.; Ruí-Cruz, S.; Chaidez-Quiroz, C.; Morán-Palacio, E.F. Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extra. Plants 2020, 9, 1650.
- Kuete, V.; Dzotam, J.K.; Voukeng, I.K.; Fankam, A.G.; Efferth, T. Cytotoxicity of Methanol Extracts of Annona muricata, Passiflora Edulis and Nine Other Cameroonian Medicinal Plants towards Multi-Factorial Drug-Resistant Cancer Cell Lines. Springerplus 2016, 5, 1666.
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; Madivoli, E.S.; El-Shemy, H.A.; Wamunyokoli, F.; Magoma, G. Green Synthesis and Characterization of Highly Stable Silver Nanoparticles from Ethanolic Extracts of Fruits of Annona muricata. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1231–1242.
- Adaramoye, O.A.; Oladipo, T.D.; Akanni, O.O.; Abiola, O.J. Hexane Fraction of Annona muricata (Sour Sop) Seed Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Biomed. Pharmacother. 2019, 111, 403–413.
- Agu, K.C.; Okolie, P.N. Proximate Composition, Phytochemical Analysis, and in Vitro Antioxidant Potentials of Extracts of Annona muricata (Soursop). Food Sci. Nutr. 2017, 5, 1029–1036.
- Rodrigues, A.M.; Silva, A.A.S.; Pinto, C.C.C.; Santos, D.L.D.; Freitas, J.C.C.D.; Martins, V.E.P.; Morais, S.M.D. Larvicidal and Enzymatic Inhibition Effects of Annona muricata Seed Extract and Main Constituent Annonacin against Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae). Pharmaceuticals 2019, 12, 112.
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, Characterization and Antimicrobial Activity of Gold Nanoparticles from Leaf Extracts of Annona muricata. J. Nanostruct. Chem. 2019, 9, 111–117.
- Oridupa, O.A.; Falade, F.B.; Oyagbemi, A.A.; Abegunde, B.A.; Ekwem, P.C.; Badmus, A.; Omobowale, T.O. Annona muricata Linn Leaves or Curcuma Longa Linn Rhizomes Ameliorates Oxidative Stress Associated with Hypertension in Uninephrectomized Wistar Rats Daily Loaded with Sodium Chloride. Eur. J. Med. Plants 2019, 26, 1–13.
- Ong, H.C.; Norzalina, J. Malay Herbal Medicine in Gemencheh, Negri Sembilan, Malaysia. Fitoterapia 1999, 70, 10–14.
- Moghadamtousi, S.Z.; Karimian, H.; Rouhollahi, E.; Paydar, M.; Fadaeinasab, M.; Kadir, H.A. Annona muricata Leaves Induce G1 Cell Cycle Arrest and Apoptosis through Mitochondria-Mediated Pathway in Human HCT-116 and HT-29 Colon Cancer Cells. J. Ethnopharmacol. 2014, 156, 277–289.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30.
- Stan, S.D.; Singh, S.V.; Brand, R.E. Chemoprevention Strategies for Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 347–356.
- Torres, M.P.; Rachagani, S.; Purohit, V.; Pandey, P.; Joshi, S.; Moore, E.D.; Johansson, S.L.; Singh, P.K.; Ganti, A.K.; Batra, S.K. Graviola: A Novel Promising Natural-Derived Drug That Inhibits Tumorigenicity and Metastasis of Pancreatic Cancer Cells In Vitro and In Vivo Through Altering Cell Metabolism. Cancer Lett. 2012, 323, 29–40.
- Mohamad Rosdi, M.N.; Nik Mat Daud, N.N.N.; Zulkifli, R.M.; Ya’akob, H. Cytotoxic Effect of Annona muricata Linn Leaves Extract on Capan-1 Cells. J. Appl. Pharm. Sci. 2015, 5, 045–048.
- Macha, M.A.; Krishn, S.R.; Jahan, R.; Banerjee, K.; Batra, S.K.; Jain, M. Emerging Potential of Natural Products for Targeting Mucins for Therapy Against Inflammation and Cancer. Cancer Treat. Rev. 2015, 41, 277.
- Degli Esposti, M.; Ghelli, A.; Ratta, M.; Cortes, D.; Estornell, E. Natural Substances (Acetogenins) from the Family Annonaceae Are Powerful Inhibitors of Mitochondrial NADH Dehydrogenase (Complex I). Biochem. J. 1994, 301 Pt 1, 161–167.
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656.
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sánchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene Ablation-Resistant Pancreatic Cancer Cells Depend on Mitochondrial Function. Nature 2014, 514, 628–632.
- Moghadamtousi, S.Z.; Kadir, H.A.; Paydar, M.; Rouhollahi, E.; Karimian, H. Annona muricata Leaves Induced Apoptosis in A549 Cells through Mitochondrial-Mediated Pathway and Involvement of NF-ΚB. BMC Complement. Altern. Med. 2014, 14, 299.
- Zhao, G.X.; Rieser, M.J.; Hui, Y.H.; Miesbauer, L.R.; Smith, D.L.; McLaughlin, J.L. Biologically Active Acetogenins from Stem Bark of Asimina Triloba. Phytochemistry 1993, 33, 1065–1073.
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive Natural Products for Chemoprevention and Treatment of Castration-Resistant Prostate Cancer. Semin. Cancer Biol. 2016, 40–41, 160–169.
- Sun, S.; Liu, J.; Zhou, N.; Zhu, W.; Dou, Q.P.; Zhou, K. Isolation of Three New Annonaceous Acetogenins from Graviola Fruit (Annona muricata) and Their Antiproliferation on Human Prostate Cancer Cell PC-3. Bioorg. Med. Chem. Lett. 2016, 26, 4282–4385.
- Deep, G.; Kumar, R.; Jain, A.K.; Dhar, D.; Panigrahi, G.K.; Hussain, A.; Agarwal, C.; El-Elimat, T.; Sica, V.P.; Oberlies, N.H.; et al. Graviola Inhibits Hypoxia-Induced NADPH Oxidase Activity in Prostate Cancer Cells Reducing Their Proliferation and Clonogenicity. Sci. Rep. 2016, 6, 23135.
- Sun, S.; Liu, J.; Kadouh, H.; Sun, X.; Zhou, K. Three New Anti-Proliferative Annonaceous Acetogenins with Monotetrahydrofuran Ring from Graviola Fruit (Annona Muricata). Bioorg. Med. Chem. Lett. 2014, 24, 2773–2776.
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic Interactions among Flavonoids and Acetogenins in Graviola (Annona muricata) Leaves Confer Protection against Prostate Cancer. Carcinogenesis 2015, 36, 656–665.
- Asare, G.A.; Afriyie, D.; Ngala, R.A.; Abutiate, H.; Doku, D.; Mahmood, S.A.; Rahman, H. Antiproliferative Activity of Aqueous Leaf Extract of Annona muricata L. on the Prostate, BPH-1 Cells, and Some Target Genes. Integr. Cancer Ther. 2015, 14, 65–74.
- Moghadamtousi, S.Z.; Rouhollahi, E.; Karimian, H.; Fadaeinasab, M.; Firoozinia, M.; Abdulla, M.A.; Kadir, H.A. The Chemopotential Effect of Annona muricata Leaves against Azoxymethane-Induced Colonic Aberrant Crypt Foci in Rats and the Apoptotic Effect of Acetogenin Annomuricin E in HT-29 Cells: A Bioassay-Guided Approach. PLoS ONE 2015, 10, e0122288.
- de Melo, J.G.; de Sousa Araújo, T.A.; de Almeida Castro, V.T.N.; de Vasconcelos Cabral, D.L.; do Desterro Rodrigues, M.; do Nascimento, S.C.; de Amorim, E.L.C.; de Albuquerque, U.P. Antiproliferative Activity, Antioxidant Capacity and Tannin Content in Plants of Semi-Arid Northeastern Brazil. Molecules 2010, 15, 8534–8542.
- Magadi, V.P.; Ravi, V.; Arpitha, A.; Litha, K.K.; Manjunath, K. Evaluation of Cytotoxicity of Aqueous Extract of Graviola Leaves on Squamous Cell Carcinoma Cell-25 Cell Lines by 3-(4,5-Dimethylthiazol-2-Yl) -2,5-Diphenyltetrazolium Bromide Assay and Determination of Percentage of Cell Inhibition at G2M Phase of Cell c. Contemp. Clin. Dent. 2015, 6, 529–533.
- Ko, E.-Y.; Moon, A. Natural Products for Chemoprevention of Breast Cancer. J. Cancer Prev. 2015, 20, 223.
- Hadisaputri, Y.E.; Habibah, U.; Abdullah, F.F.; Halimah, E.; Mutakin, M.; Megantara, S.; Abdulah, R.; Diantini, A. Antiproliferation Activity and Apoptotic Mechanism of Soursop (Annona muricata L.) Leaves Extract and Fractions on MCF7 Breast Cancer Cells. Breast Cancer Targets Ther. 2021, 13, 447.
- Ko, Y.M.; Wu, T.Y.; Wu, Y.C.; Chang, F.R.; Guh, J.Y.; Chuang, L.Y. Annonacin Induces Cell Cycle-Dependent Growth Arrest and Apoptosis in Estrogen Receptor-α-Related Pathways in MCF-7 Cells. J. Ethnopharmacol. 2011, 137, 1283–1290.
- Dai, Y.; Hogan, S.; Schmelz, E.M.; Ju, Y.H.; Canning, C.; Zhou, K. Selective Growth Inhibition of Human Breast Cancer Cells by Graviola Fruit Extract in Vitro and in Vivo Involving Downregulation of EGFR Expression. Nutr. Cancer 2011, 63, 795–801.
- Oberlies, N.H.; Croy, V.L.; Harrison, M.L.; McLaughlin, J.L. The Annonaceous Acetogenin Bullatacin Is Cytotoxic against Multidrug-Resistant Human Mammary Adenocarcinoma Cells. Cancer Lett. 1997, 115, 73–79.
- Yuan, F.; Bai, G.; Miao, Y.; Chen, Y.; Li, X.; Chen, J. Annosquacin B Induces Mitochondrial Apoptosis in Multidrug Resistant Human Breast Cancer Cell Line MCF-7/ADR through Selectively Modulating MAPKs Pathways. Pharm. Biol. 2016, 54, 3040–3045.
- Qazi, A.K.; Hussain, A.; Aga, M.A.; Ali, S.; Taneja, S.C.; Sharma, P.R.; Saxena, A.K.; Mondhe, D.M.; Hamid, A. Cell Specific Apoptosis by RLX Is Mediated by NFκB in Human Colon Carcinoma HCT-116 Cells. BMC Cell Biol. 2014, 15, 36.
- Abdullah, M.; Syam, A.F.; Meilany, S.; Laksono, B.; Prabu, O.G.; Bekti, H.S.; Indrawati, L.; Makmun, D. The Value of Caspase-3 after the Application of Annona muricata Leaf Extract in COLO-205 Colorectal Cancer Cell Line. Gastroenterol. Res. Pr. 2017, 2017, 4357165.
- Kim, G.S.; Zeng, L.; Alali, F.; Rogers, L.L.; Wu, F.E.; McLaughlin, J.L.; Sastrodihardjo, S. Two New Mono-Tetrahydrofuran Ring Acetogenins, Annomuricin E and Muricapentocin, from the Leaves of Annona muricata. J. Nat. Prod. 1998, 61, 432–436.
- Wu, F.E.; Gu, Z.M.; Zeng, L.; Zhao, G.X.; Zhang, Y.; McLaughlin, J.L.; Sastrodihardjo, S. Two New Cytotoxic Monotetrahydrofuran Annonaceous Acetogenins, Annomuricins A and B, from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 830–836.
- Wu, F.E.; Zeng, L.; Gu, Z.M.; Zhao, G.X.; Zhang, Y.; Schwedler, J.T.; McLaughlin, J.L.; Sastrodihardjo, S. New Bioactive Monotetrahydrofuran Annonaceous Acetogenins, Annomuricin C and Muricatocin C, from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 909–915.
- Xue, J.Y.; Zhou, G.X.; Chen, T.; Gao, S.; Choi, M.Y.; Wong, Y.S. Desacetyluvaricin Induces S Phase Arrest in SW480 Colorectal Cancer Cells through Superoxide Overproduction. J. Cell. Biochem. 2014, 115, 464–475.
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92.
- Qazi, A.K.; Siddiqui, J.A.; Jahan, R.; Chaudhary, S.; Walker, L.A.; Sayed, Z.; Jones, D.T.; Batra, S.K.; Macha, M.A. Emerging Therapeutic Potential of Graviola and Its Constituents in Cancers. Carcinogenesis 2018, 39, 522–533.
- Csuka, O.; RemenÁr, É.; Koronczay, K.; Doleschall, Z.; NÉmeth, G. Predictive Value of P53, Bcl2 and Bax in the Radiotherapy of Head and Neck Cancer. Pathol. Oncol. Res. 1997, 3, 204–210.
- Wahyudiono, A.; Istyawati, E.; Rahaju, P. Annona muricata (Soursop) Leaves Extract Increased Caspase 3 Expression in Patient-Derived WHO III Nasopharyngeal Cancer Cell. In Proceedings of the International Conference on Drug Discovery and Translational Medicine 2018 (ICDDTM ’18) “Seizing Opportunities and Addressing Challenges of Precision Medicine”, Putrajaya, Malaysia, 3 December–5 February 2019.
- Lucas, D.M.; Still, P.C.; Pérez, L.B.; Grever, M.R.; Kinghorn, A.D. Potential of Plant-Derived Natural Products in the Treatment of Leukemia and Lymphoma. Curr. Drug Targets 2010, 11, 812.
- Pieme, A.A.; Kumar, G.G.; Dongmo, S.S.; Moukette, M.M.; Boyoum, F.F.; Ngogang, Y.Y.; Saxena, K.K. Antiproliferative Activity and Induction of Apoptosis by Annona muricata (Annonaceae) Extract on Human Cancer Cells. BMC Complement. Altern. Med. 2014, 14, 516.
- Ezirim, A.U.; Okochi, V.I.; James, A.B.; Adebeshi, O.A.; Ogunnowo, S.; Odeghe, O.B. Odeghe Induction of Apoptosis in Myelogenous Leukemic K562 Cells by Ethanolic Leaf Extract of Annona muricata L. Glob. J. Res. Med. Plants Indig. Med. 2013, 2, 142–151.
- Yang, H.; Liu, N.; Lee, S. Ethanol Extract of Annona muricata L Induces Liver Cancer Cell Apoptosis through ROS Pathway. Biomed. Pharmacol. J. 2016, 9, 919–925.
- Liu, N.; Yang, H.L.; Wang, P.; Lu, Y.C.; Yang, Y.J.; Wang, L.; Lee, S.C. Functional Proteomic Analysis Revels That the Ethanol Extract of Annona muricata L. Induces Liver Cancer Cell Apoptosis through Endoplasmic Reticulum Stress Pathway. J. Ethnopharmacol. 2016, 189, 210–217.
- Capes-Davis, A.; Theodosopoulos, G.; Atkin, I.; Drexler, H.G.; Kohara, A.; MacLeod, R.A.F.; Masters, J.R.; Nakamura, Y.; Reid, Y.A.; Reddel, R.R.; et al. Check Your Cultures! A List of Cross-Contaminated or Misidentified Cell Lines. Int. J. Cancer 2010, 127, 1–8.
