Amino acids (AAs) are indispensable building blocks of diverse bio-macromolecules as well as functional regulators for various metabolic processes. The fact that cancer cells live with a voracious appetite for specific AAs has been widely recognized. Glioma is one of the most lethal malignancies occurring in the central nervous system. The reprogrammed metabolism of AAs benefits glioma proliferation, signal transduction, epigenetic modification, and stress tolerance. Metabolic alteration of specific AAs also contributes to glioma immune escape and chemoresistance. For clinical consideration, fluctuations in the concentrations of AAs observed in specific body fluids provides opportunities to develop new diagnosis and prognosis markers.
- amino acid
- glioma
- metabolomics
- metabolism
1. Introduction
2. Glioma AA Metabolism Adapted to Proliferation
One of the hallmarks of cancer cell metabolism is their uncontrollable proliferation abilities. This does not mean the malignant cells adopted some metabolic pathways that were unique to tumor cells. Most frequently, cancer cells change certain enzymes’ expression or activities to meet their abnormal catabolic and anabolic requirements [19]. The relevant mechanisms include the mutations of specific enzyme genes, the accumulation of specific activators/inhibitors, the altered post-translational modification activities and the over-activated/inhibited regulation signals. One-carbon unit metabolism is closely linked to many biological processes. The production of ATP, NADPH, lipids and nucleotides greatly relies on one-carbon units, especially the de novo synthesis of purine. Cell proliferation is largely determined by the availability of nucleotides [22]. Jain, M et al. profiled the extracellular consumption and release of 219 metabolites for 60 primary human cancer cell lines including U251 glioblastoma (GBM) cells [23][24][23,24]. They found glycine was consumed massively by rapidly proliferating cells and released by slowly proliferating ones. The uptake of extracellular glycine is mediated by the transporter of GLYT1 [25]. The intracellular biosynthesis of glycine could occur both in the cytosol and the mitochondria. Most cells default to the mitochondria for glycine synthesis [26]. Mitochondrial serine hydroxymethyltransferase 2 (SHMT2) catalyzes the reversible transformation from glycine to serine [27]. SHMT2 was frequently found to be overexpressed in GBM tissues [28][29][28,29]. Isotope tracing analysis indicated mitochondria contribute about two-thirds of the needed glycine to rapidly proliferating glioma cells [24]. The consumed glycine was either converted to one-carbon units to support purine synthesis or incorporated into glutathione (GSH) to clear reactive oxygen species (ROS) [30][31][30,31]. Glycine decarboxylase (GLDC) plays a key role in converting glycine into one-carbon units. The activity of GLDC is regulated by acetylation modification. This post-translational modification of GLDC is inhibited by the mechanistic target of rapamycin complex 1 (mTORC1). Acetylated GLDC is prone to be degraded in the proteasomes and results in impaired pyrimidine synthesis and growth inhibition of gliomas [32]. Many glioma cells exhibit highly expressed mTORC1 and GLDC [32][33][32,33]. γ-Glutamylcyclotransferase (GGCT), one of the key enzymes promoting GSH synthesis, was demonstrated to be highly expressed in glioma cells. Suppression or depletion of GGCT compromised glioma cells but not normal cells’ proliferation [34][35][34,35], implying the different GSH-related vulnerabilities of the normal and the transformed cells. In addition to GGCT, SHMT2 also promoted GSH synthesis [26]. Collectively, glycine confers gliomas a proliferative advantage by providing one-carbon units and reduction substances (Figure 1). The voracious appetite for glycine of gliomas renders glycine a promising imaging tracer to aid glioma aggressiveness evaluation [36]. Additionally, one-carbon metabolism has been identified as one of the potential targets for treating GBM [37].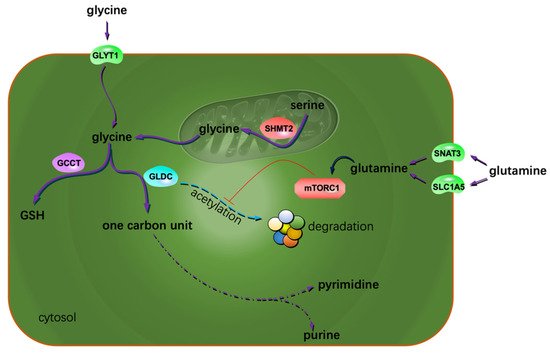
3. Glioma Migration, Invasion, and AA Metabolism
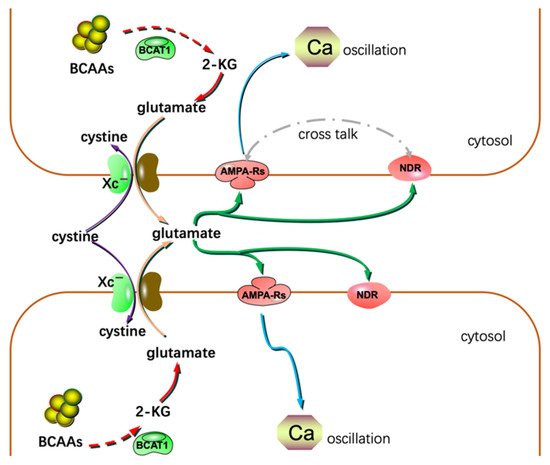
Although the metastasis of glioma is seldom found in patients, the infiltrative growth feature of gliomas is very harmful. Thus, understanding the mechanisms of glioma invasion and migration would benefit patient care. System Xc− is an Na+-independent antiporter. It mediates the exchanging of extracellular cystine and intracellular glutamate [64]. Most gliomas highly express system Xc−. Glutamate excreted by gliomas is cytotoxic to the adjacent normal brain cells. Through this, gliomas create extra space to expand and invade. Lyons S. et al. reported that glutamate could activate Ca2+-permeable α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPA-Rs) in the manner of autocrine and/or paracrine signaling. Activation of AMPA-Rs resulted in intracellular Ca2+ oscillation, one of the essential signals to trigger glioma invasion and migration (Figure 3). Most of the glioma tissues highly express AMPA-Rs. Agonists of AMPA-Rs facilitated glioma invasion and migration [65]. Additionally, glutamate showed a high affinity to N-methyl-D-aspartate receptors. Crosstalk between AMPA-Rs and N-methyl-D-aspartate receptors has been demonstrated to synergistically promote glioma invasion, especially in a glutamate-rich microenvironment. BCAA transaminase 1 (BCAT1) initiates the breakdown of BCAAs. Some catabolic products of BCAAs could be used as the carbon skeleton for glutamate synthesis. Inhibition of BCAT1 resulted in a decreased efflux of glutamate and brought about the impaired invasiveness of glioma cells [66]. In conclusion, except for its cytotoxic effects, glutamate could act as a hormone to stimulate invasion and migration (Figure 3) [67].