1. Liquid-Phase Reduction
Zhang et al. (2009) first verified the possibility of developing the reduction of GO via L-aa [
51]. The method developed was performed in an aqueous solution at room temperature under vigorous stirring. The study showed the monitoring of the reduction progress by optical absorption spectroscopy. The UV–vis spectrum of GO is typically characterized by the typical π–π* transition peak at 233 nm from the C=C bond, and the
n–π* transition peak at around 300 nm from the C=O bond [
34]. The red-shift of the π–π* transition of GO originates related to the extension of its π-conjugated structure in the r-GO and the decreasing intensity of peaks centered at 300 nm, which is caused by the decrease in the C=O bond, allowing analysis of the reduction. The reduction that occurred was confirmed by FT-IR, Raman, and AFM results. The obtained r-GO, which showed a strongly restacked sheet arrangement with a wrinkled texture, provided a low specific surface area of 11.8 m
2/g and specific capacity of 128 F/g at a current density of 50 mA/g [
51].
In the same period, Gao et al. (2010) presented a “green” reduction of GO using L-aa, analyzing the possible use of L-tryptophan as a stabilizer, to produce a stable dispersion of r-GO in an aqueous solution [
66]. In brief, an aqueous solution of GO, L-aa, L-tryptophan, and NaOH was treated by ultrasonication at 80 °C for 24 h. After that, the mixture was cooled to room temperature, followed by another 1 h of ultrasonication. Thus, a large amount of stably dispersed aqueous r-GO was easily obtained. The experimental results showed the efficiency of L-tryptophan as a stabilizer to avoid the agglomeration and precipitation of the resulting r-GO sheets. L-tryptophan contains an electron-rich aromatic group that can function as an electron donor and be absorbed onto the r-GO sheet, based on the π–π interaction. In the meantime, the terminal carboxylic acid can supply enough negative charge, and the electrostatic repulsion can make the r-GO dispersions stable [
66].
Successively, Fernández-Merino et al. (2010) [
54] verified the use of L-aa to obtain stable suspensions of highly reduced r-GO in some common organic solvents, such as N,N-dimethylformamide (DMF) and N-methyl-2-pyrrolydone (NMP). In addition, the comparison of the deoxygenation efficiency of GO by different reductants (i.e., hydrazine, sodium borohydride, and pyrogallol) showed that only L-aa was found to yield highly reduced suspensions in a way that was comparable to those provided by hydrazine [
54].
Sui et al. (2011) analyzed the effect of the amount of L-aa to reduced GO hydrogel and demonstrated that the mass ratio of L-aa to GO, temperature, and pH value of the reaction mixtures play a significant role in the formation of irreversible r-GO agglomerates in the form of hydrogels [
71]. The hydrogels consist of a 3D cross-linked network of r-GO sheets self-assembling into a well-defined and interconnected 3D porous network through π–π interaction during gelation. Furthermore, this study demonstrated that r-GO in the form of a hydrogel with an excess of L-aa as a bioactive component can easily be used to release it in a diffusion-controlled manner. After the complete release of L-aa, the hydrogel exhibits excellent mechanical and electrical properties, and for this reason, it can be advantageously used in the fields of tissue engineering, drug delivery, soft machines, regenerative medicine, biosensors, etc. [
71].
A complete study about the pH effect on the morphology of the as-prepared hydrogel r-GO was presented by Ha et al. (2019) [
72]. This work shows that the reduction of GO in the liquid phase was completed in 1 h. The morphological characterization performed by scanning electron microscopy (SEM) demonstrated the formation of the 3D cross-linked spherical structure of r-GO by the hydrogel process with a diameter from 4 to 2 μm and with a specific surface area of 150 m
2/g, when the pH of the solution was 2. The experimental measurement showed that more spherical and compact structures were obtained at pH 10 with an improved specific surface area of 216 m
2/g.
The effect of pH on the degree of dispersion, packed with r-GO, can be explained by zeta potential analysis. The colloid of GO dispersion has a zeta potential −43 mV when the pH is 10 [
73]. It is well kwon that Zeta potential values greater than −30 mV are generally considered to exhibit sufficient mutual repulsion to ensure the stability of dispersion [
74]. When the droplets are generated from the GO colloid fabricated at pH 10, a more densely compacted structure of r-GO can be formed by the capillary-force-driven self-assembly of well dispersed GO sheets in the droplet during the solvent evaporation. Similar experimental results for the formation of the r-GO hydrogels were confirmed by Kondratowicz et al. (2017) [
75]. This study explained that the by-products of reduction, such as dehydroascorbic acid and water molecules, may form additional hydrogen bonds with residual carboxylic groups on r-GO planes and contribute to the final structure of hydrogels [
75].
Successively, the influence of pH and surfactants used for reduction by L-aa on the adsorption mechanism of organic contaminants, such as phenanthrene (a representative nonpolar, nonionic, and aromatic contaminant) and 1-naphthol (a representative polar, aromatic contaminant) was studied by Wang et al. in 2019 [
76]. The study shows that the pH of the solution had a negligible effect on phenanthrene adsorption for both GO and r-GO, and it inhibited 1-naphthol adsorption at high pH because of the electronic repulsion. This was mainly attributed to the hydrophobic interaction, π–π interaction, and H-bonding between graphene sheets and organic contaminants. The same study shows that the use of the surfactants had different effects on the adsorption of polar and nonpolar aromatics onto graphene materials. For sodium dodecyl benzene sulfonate (SDBS), the exfoliation effect could enhance the adsorption affinity; thus, it could counteract the inhibition effect caused by competition. Cetyltrimethylammonium bromide (CTAB) could form hemimicelles on reduced graphene oxide, which may provide a favorable media for organic contaminant partitioning. In addition, this study shows that the r-GO could be regenerated and reused with high recyclability over five cycles. These findings could provide a promising material for wastewater treatment and the understanding of the fate and transport of organic contaminants in aquatic environments.
De Silva et al. (2018) [
77] monitored the reduction of GO by L-aa in an aqueous solution in 10-min intervals up to 1 h in order to study how structural and morphological changes would take place. The reduced products obtained at different time periods were characterized in detail by UV–visible spectroscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), attenuated total reflectance Fourier transform infrared (ATR-FT-IR) spectroscopy, Raman spectroscopy, thermogravimetric analysis (TGA), atomic force microscopy (AFM), and scanning electron microscopy (SEM). The UV–visible spectra displayed a complete removal of the GO peak by 50 min, while other characterization techniques revealed the presence of residual oxygen functionalities. In particular, the XPS results showed that the decline in the oxygen atomic percentage was mainly due to the removal of the hydroxyl and epoxy groups located at the basal planes of the GO sheets and, to a small extent, due to edge carbonyl groups. AFM characterization indicated that at the intermediate stages of reduction, both GO and r-GO coexist in the material, as confirmed by XRD results.
The study of the impact of ultrasounds on the rate of GO reduction in L-aa aqueous solutions was carried out by Abulizi et al., 2014 [
78]. They found that the r-GO formation under ultrasound treatment was accelerated in comparison with the conventional mechanical mixing treatment. To understand the effects of ultrasound, the authors compared the experimental results on the trend rates of r-GO formation, as a function of temperature, under ultrasound and mixing treatment, showing that this rate was increased by ultrasound treatment. The authors proposed that physical effects such as shear forces, microjets, and shock waves during acoustic cavitation enhanced the mass transfer and reaction of L-aa with GO to form r-GO, as well as the change in the surface morphology of GO. Furthermore, the rates of r-GO formation were suggested to be affected by local high temperatures of cavitation bubbles [
78].
Similarly, the increase in kinetics and in the GO reduction degree under UV irradiation were analyzed by Go et al., 2018 [
79]. They demonstrated that when the reactant solution was placed under UV irradiation (254 nm), L-aa improved its chemical activity caused by its UV-sensitive oxidation property [
80]. In particular, the reduction was performed under various conditions, (i) without any reducing agent, (ii) using L-aa, (iii) using L-aa under UV irradiation (254 nm), and monitored by using UV–visible spectroscopy up to 24 h to explore the effect of UV irradiation on the reduction of GO by L-aa. The evolution of UV–Visible spectra for these different conditions, confirmed that the UV irradiation (254 nm) improves the activity of L-aa.
In fact, the UV–visible spectrum of GO showed the typical π–π* transition peak at 233 nm from the C=C bond, and the
n–π* transition peak at around 300 nm from the C=O bond [
33]. The red-shift of the GO π–π* transition can be used as an indicator of its reduction [
51]. Consequently, analyzing the trend of this shift for all three different reduction conditions, it is possible to deduce the advantageous effect of UV radiation on a reduction of GO [
79,
80].
2. Gel-Phase Reduction
Recently, a gel-phase technique has achieved a growing interest. This technique is based on the reduction of a thin film of GO deposited on a substrate by the diffusion of L-aa molecules in it. The peculiarity of this approach is that GO is not dispersed in a solvent, but it is swollen by water, and it persists in the form of coating stacked to the substrate, and the chemical interaction with L-aa takes place by permeation of the reductant in these lamellar structures. This approach represents an important technological breakthrough because the development of many functional devices can be made directly by the reduction of a large area of GO coating deposited on selected substrates (i.e., polymers, glass, etc.) [
52,
68,
69,
70,
84]. In addition, considering that this approach allows the GO reduction by L-aa at a low temperature, it is possible to overcome the temperature limitation due to the thermal stability of the substrate. This method preserves the properties of substrates, and it avoids the use of surfactants.
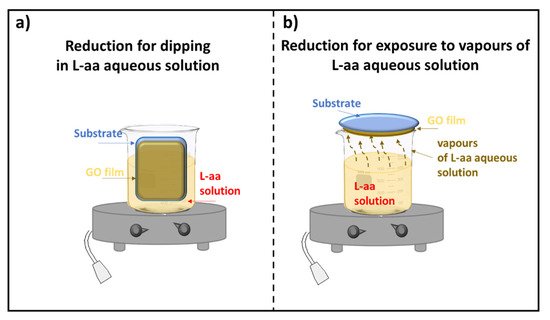
According to the literature, only a few methods have been developed for the reduction of GO thin films. Two approaches for reducing GO film deposited on a substrate were described: the first one is based on the dipping of supported GO in a reducing L-aa solution, and the second method requires the exposure of supported GO to vapors of a reducing L-aa solution. Both procedures, which could need a controlled temperature, are shown in Figure 1.
Figure 1. Scheme of the reduction of a GO film deposited on a substrate (a) by dipping; (b) by exposure to vapors of a L-aa solution.
Liu et al. in 2015 [
52] first reported the use of L-ascorbic acid/water vapor as a reducing agent for GO films. In this paper, GO on cellulose was placed on the top of a glass bottle in a Teflon-lined autoclave containing different concentrations of an aqueous solution of L-aa. Finally, the autoclave was heated at 100 °C for 48 h. The same procedure was used to prepare r-GO–Ag composites that can be used for active substrate surface-enhanced Raman scattering and as antibacterial material.
In 2016, Li et al. [
70] published one article concerning the preparation of porous r-GO membranes reducing GO on copper hydroxide nano-strand freestanding membranes by dipping in a 60 mL L-aa aqueous solution heated at 90 °C for 4 h. The results confirmed that a porous r-GO membrane, fabricated from a graphene oxide sheet via etching copper hydroxide nano-strands by L-aa reduction, provides an effective structural configuration for enhancing its gauge factor.
Tas et al. in 2019 [
69] proposed the reduction of graphene oxide thin films deposited on glass by dipping in an opportune solution of the L-aa at low-temperature. To compare the effectiveness of the reduction process, hydrazine hydrate was also used as a chemical reducing agent following the same method. The results have shown that this reduction process, which does not contain heavy toxic chemicals and does not require nitrogen, argon, etc., is more successful.
Chen et al. in 2020 [
84] reported on the preparation of cellulose/r-GO aerogels for the development of chemical vapor sensors. For the GO reduction, the cellulose/GO hydrogels were put in an aqueous solution of L-aa at 95 °C for 2 h. Sensors based on these aerogels exhibited fast response, good recovery, high sensitivity, and excellent reproducibility. The inexpensive, easy, green, and scalable preparation of this new type of vapor sensor could be expected to lead to new sensing and biomedical applications.
In the same year, Longo et al. [
67,
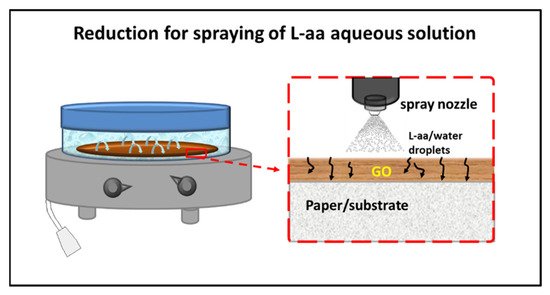
68] published a new method for a green gel-state chemical reduction of GO supported on cellulose substrates. The possibility of having an effective mass transport of the reductant inside the swollen GO deposit was ensured by spraying a reducing solution of L-aa on the GO film, allowing it to reflux for 48 h in a closed microenvironment at 50 °C. A scheme of the apparatus used for reduction is shown in
Figure 2.
Figure 2. Scheme of the reduction of GO film deposited on paper/substrate by spraying of a L-aa aqueous solution [
67].
The Reduction of GO Film Deposited on Paper/Substrate Spraying of L-aa Aqueous Solution
This gel-state reduction technique, based on spraying an L-aa aqueous solution, represents a convenient approach for a complete reduction of the GO layers supported on thermally unstable substrates [
67,
68]. The most important experimental results published in previous manuscripts [
67,
68] can be summarized as follows. In addition, in order to improve the previous results, a quantitative analysis of the degree of oxidation of GO before and after the reduction is presented.
According to the thermogravimetric investigation, the process temperature selected (i.e., 50 °C) is necessary to increase the mobility of the water and L-aa molecules in the GO inter-layers [
68]. The SEM investigation confirmed a structural modification of the GO coating after the treatment, mainly consisting of an increase in the coating flatness. In addition, SEM confirmed a strong interfacial adhesion between the GO/r-GO coating and the fibrous substrate. This micro-structural characteristic, due to an excellent adhesion at the GO–paper interface, is relevant for achieving a highly flexible r-GO layer supported on paper, and it is vitally necessary for industrial exploitation [
68].
The XRD results of r-GO/paper show the presence of the main peaks of the r-GO pattern combined with the XRD signals of residual GO. In particular, the results showed that the obtained r-GO coating is composed of platelets with an average thickness of ca. 27 nm and a width of ca. 40 nm, which are aligned parallel to the interfacial plane and show good graphitic quality [
67]. These experimental results confirmed the obtained reduction of GO on paper only qualitatively.
To establish the degree of oxidation of GO before and after the reduction, the Fourier transform infra-red spectroscopy was recorded in attenuated total reflectance (ATR) mode in the 4000–700 cm−1 range by using a spectrophotometer (PerkinElmer Frontier NIR, Milan, Italy).
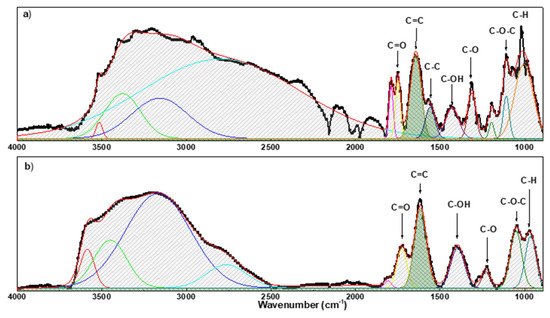
The ATR spectra (see Figure 3) showed that most of the peaks referring to oxygen-containing groups are present in the GO layer. In addition, the broad peak in the spectra in the region 3740–3100 cm−1and the peak at ≈1640 cm−1 can be referring to the water molecules absorbed on the interlayers of GO.
Figure 3. FT-IR spectrum of GO on paper/substrate (a) and r-GO on paper/substrate (b).
To perform a quantitative analysis of the degree of oxidation of GO before and after the reduction by ATR spectra, the following procedure proposed by Guerrero-Contreras et al. [
85,
86] was used:
-
A polynomial baseline was calculated and subtracted from the raw spectra.
-
The resulting spectra were multiplied by −1 in order to have positive bands.
-
The peak deconvolution was obtained by Gaussian fit to achieve the peak area.
The comparison between the spectrum before and after reduction shows a general decrease in intensity of the peaks related to oxygen functional groups. At this point, the degree of oxidation of GO was evaluated by calculating the relative percentage of oxygen-containing functional groups (
RPox) compared to the presence of all functional groups observed in the wavenumber range of 900−1850 cm
−1 (for all peaks in
Figure 3 [
85,
86]).
RPox was calculated using the following formula:
The analysis reveals that the RPox decreases from 68% to 37% after the reduction.
3. Conclusions
The aim of this summury is to highlight the potential for using L-aa as a green reducing agent to improve eco-friendly and large-scale production of r-GO. Liquid-phase and gel phase reduction have been briefly discussed here. As far as the first approach is concerned, experimental results have demonstrated the advantageous use of some factors in improving the reduction process. Higher temperature, the use of sonication, and exposure under UV radiation are factors that (separately or simultaneously) allow an increase in reduction. Furthermore, this review presents an analysis of the role of the pH of the reaction mixture on the formation of irreversible r-GO agglomerates and the use of surfactants to modify the adsorption properties of r-GO. Many studies have demonstrated that this approach can be advantageously used to obtained functional nanomaterials based on r-GO.
More recently, there has been a growing interest in the potential for developing reduction processes in gel-phase.. This approach overcomes some limitations of the reduction of GO in liquid-phase, such as the isolation of r-GO from the solution. In addition, this approach can be used to reduce a large area of GO coating deposited on selected substrates (i.e., polymers, glasses, etc.), and for this reason the approach may provide many technological breakthroughs.
In the future, this green reduction method of GO may provide fascinating results in terms of graphene quality, size and also production.