1. Brief Medical History of Silver Nanoparticles
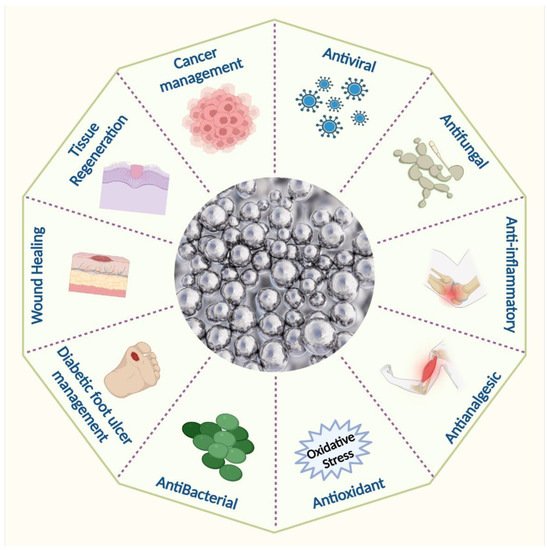
Silver nanoparticles have been extensively used in various medicinal applications, as shown in (
Figure 3). Silver nanoparticles are already widely used in wound dressings and burn treatment in biomedicine and also in the food and textile industries, in paints, household items, catheters, implants, and cosmetics, as well as in combination with a variety of materials to prevent infection [
20,
21,
22,
23,
24,
25]. During World War I, they treated soldiers’ wounds with silver leaves to stop infections and help them heal [
26,
27]. Silver as an ion was used in earlier civilizations, particularly in Egypt. This silver ion was used mainly in wound dressings to treat wounds that were hard to heal. In 1998, Ziehl-Abegg was the first firm to introduce AgNPs into wound dressings, resulting in the AgNPs antimicrobial dressing ActicoatTM. Silver products improve efficacy compared to standard wound dressing [
28,
29]. Acticoat and Actisorb, two silver-based wound dressings, are commercially available [
30]. Dermatology increasingly employs metal nanoparticles to expedite wound healing and to treat and prevent bacterial infections [
31].
Figure 3. Biomedical applications of silver nanoparticles.
Indeed, AgNPs’ excellent antimicrobial properties have already been tested against 650 bacterial strains [
32,
33,
34]. Because of their extensive antimicrobial properties and ability to reduce the chance of infection from antibiotic-resistant strains, silver nanoparticles also found their application in wound management products [
35,
36,
37,
38,
39]. AgNPs are utilized in wound care to prevent secondary infections since they are effective against a spectrum of microbes that can slow down the healing process [
40]. Their size and charge allow them to enter microorganisms [
41,
42,
43]. The AgNPs destroy many multi-resistant strains. It also helps rid pathogenic microbes that can slow or stop the typical stages of wound healing [
44,
45].
The biosynthesis of AgNP utilizing aqueous
Bryonia laciniosa leaf extract resulted in rapid wound epithelialization and scarless wound repair without significant inflammation due to effective cytokine modulation. It was used as a wound healing agent due to its outstanding anti-inflammatory and antibacterial properties [
46]. Biosynthesized AgNPs are made from
Delonix elata leaf aqueous extract for wound healing in human patients who have had anorectal surgery [
47]. Due to their sustained ability to release silver ions, which exhibit concentration-dependent toxicity in HaCaT cells, these nanoparticles have a high potential for usage in dermatology and wound healing [
48]. Because AgNPs are biocompatible and can avoid this involuntary inflammatory action, they are not nullified by the immune system; thus, they could be used as anti-inflammatory agents [
49]. Because of their anti-inflammatory properties, topical application of AgNPs at the wound site minimizes the release of inflammatory cytokines, lymphocytes, and mast cell infiltration, promoting wound healing with minimal scarring. A. Hebeish et al. also evaluated the anti-inflammatory effects of Ag by comparing them to indomethacin. This commercially available anti-inflammatory drug revealed a dose-dependent reduction in inflammation inside the rat bow edema model [
50].
A study showed that AgNPs eradicate 44 strains of six fungi [
51]. Gajbhiye et al. discovered that biogenic AgNPs were effective against
Fusarium semitectum,
Pleospora herbarum,
Trichoderma spp,
Phoma glomerata, and
Candida albicans. They also reported a synergic effect of AgNPs in conjunction with fluconazole [
52,
53]. When AgNPs were added, they changed the growth rates of all tested fungi except Mortierella spp, meaning that Chaetomium and Stachybotrys could not grow on gypsum products.
J.L. Speshock et al. investigated AgNPs’ potential in prokaryotic and eukaryotic organisms, and AgNPs at about 25 nm or even less were found to have exceptional potential for viral infection suppression [
54]. AgNPs inhibited virus attachment, cell penetration, and the cell’s ability to propagate the virus [
55]. As an HIV-1 antiviral nanohybrid and in the deactivation of SARS-Cov-2 spike proteins, TPU-Ag worked better than PVA-Ag. TPU-Ag and PVA-Ag nanofibrous membranes displayed increased antibacterial activity by increasing Ag content from 2 to 4 wt. Additionally, the developed membranes showed good mechanical and physical properties and antiviral and antibacterial activities [
56].
Silver synthesized from M. Domestica extracts significantly affected breast cancer MCF-7 cells. In contrast, silver synthesized from
O. Vulgare aqueous extracts had a dose-dependent effect on the A549 cell line [
57,
58].
Moringa olifera stem bark extract was used to produce AgNPs. K. Vasanth et al. investigated the anticancer properties of these biosynthesized AgNPs. The flow cytometry analysis indicated that ROS generation caused apoptosis in HeLa cells [
59]. According to the findings, AgNPs effectively prevent the development of HepG2 cells by inducing apoptosis [
60]. Venkatesan et al. found that the human breast cancer cells MDA-MB-231 were killed by chitosan-alginate-biosynthesized AgNPs that were highly permeable (IC
50 = 4.6 mg) [
61]. A recent study found that packed quinazolinone polypyrrole with chitosan silver chloride nanocomposite was active against Ehrlich ascites carcinoma cells [
62]. I.M. El-Sherbiny et al. found that Chitosan-silver hybrid nanoparticles cause HepG2 cells to die by turning down the BCL2 gene and the P53 gene [
63]. A significant decrease in cyclobutene-pyrimidine-dimer creation demonstrated their chemo-preventive efficacy in HaCaT cells after UVB-induced DNA damage, which has a good potential for avoiding skin cancer [
64]. The UVB-protective effectiveness of AgNPs in human keratinocytes is proportional to their size [
65]. As a result, pre-treating HaCaT cells with smaller AgNPs (10–40 nm) helped shield skin cells from UVB-induced DNA damage and UV-induced apoptosis. Using 60 and 100 nm AgNPs, no prevention was obtained. AgNPs are increasingly used in healthcare and consumer products, so many commercial products now include these nanoparticles for topical administration to human skin.
Over one million people die from malaria yearly, caused by protozoal vector-borne diseases, the most prevalent and dangerous infections in wealthy nations [
66]. Z. Jiang et al. are creating novel antimalarial strategies to control the malaria vector. AgNPs were tested against
Plasmodium falciparum malarial parasites and other antimalarial medications [
67]. The bio-reduction of AgNPs was 5%. The malaria vector Anopheles stephensi and chloroquine-sensitive and resistant
P. falciparum strains were all treated with
Cassia occidentalis leaf broth [
68].
2. Wound Healing Properties of Silver Nanoparticles
Compounds of silver, such as silver nitrate and silver sulfadiazine, are often used to treat infections in chronic wounds and burns [
69]. AgNPs help fibroblasts change into myofibroblasts, which makes wounds tighter and speeds up the healing of diabetic wounds. AgNPs accelerate wound healing by enhancing keratinocyte proliferation and migration [
70,
71]. AgNPs may engage with sulphur-containing proteins in bacterial membrane cells and, ideally, attack the respiratory chain, resulting in apoptosis [
72]. When it comes into contact with the injured region, they cause neutrophil apoptosis by lowering mitochondrial function, which reduces cytokine production. As a result, the inflammatory response is modulated or reduced, resulting in faster healing [
73,
74]. However, because of their small size, AgNPs can easily penetrate biofilms and cell membranes, causing DNA damage, inhibiting cell proliferation, and inhibiting cellular ATP production [
75]. The silver nanoparticles change the amount of m-RNA in the wound environment. Aside from antibacterial activities, silver surgical textiles exhibit an increase in healing properties; as an outcome, silver exploitation has an optimistic effect on cell migration and proliferation quality [
76,
77,
78]. Cytokine modulation is mediated by silver nanoparticles’ anti-inflammatory activity [
79]. As stated in the preceding section, Cytokines can stimulate fibroblasts and chondrocytes to generate ROS [
80]. Thus, silver nanoparticle modulation of cytokine production can reduce ROS levels to avoid severe cellular damage and lag wound healing [
81]. Silver has many antibacterial effects, making it less likely that bacteria will become resistant and more effective against microorganisms resistant to multiple drugs. When the amount of AgNPs in the dressing increases, the wound area becomes smaller and more collagen is deposited, which is linked to macrophage and fibroblast migration [
82,
83]. Sustained release mechanisms can decrease silver ion toxicity and stimulate local antibacterial activity [
84,
85].
3. Mechanistic Understanding of Silver Nanoparticles (AgNPs)
The usual quantity of silver in human plasma is less than 2 µg/mL, and this concentration comes from diet and particulate matter inhalation. Oral exposure to silver can also come via dietary supplements, contaminated water, or from eating fish and other aquatic species [
86]. Ionic silver can be ingested orally, inhaled, or absorbed through wounds to enter the body. AgNPs are believed to be transported inside the body by two processes: pinocytosis and endocytosis. The development of a revolutionary medication delivery method was prompted by the discovery that nanoscale particles penetrate far deeper than bulk particles. Although the precise mode of action of AgNPs is not yet known, numerous ideas for their antibacterial qualities have been put forth. Its antibacterial effect is thought to solely be caused by the ongoing release of silver in its ionic state [
87,
88,
89]. Silver ions cling to the cytoplasmic membrane and cell wall because of the sulphur protein affinity and electrostatic attraction. This increases the permeability of the membrane and causes the bacterial cell to rupture and degenerate. Reactive oxygen species are produced, and the respiratory enzymes are essentially deactivated when the silver ion enters the bacterial cell. Reactive oxygen species, a critical element in the mechanism of action for silver, contribute significantly to the disruption of the cell membrane and DNA damage (by interacting with sulphur and phosphorus in the DNA molecule), which hinder replication and reproduction and ultimately lead to microbe death. By denaturing ribosomes, silver ions also prevent the synthesis of ATP and hinder the formation of proteins. After anchoring and observing the cell’s surface, silver nanoparticles build up in the cellular wall pits of microorganisms, causing the denaturation and degeneration of the cell membrane. Due to their micro size, they can easily enter cells, rupturing cell organelles and even causing cell lysis. They interfere with the phosphorylation of protein substrates, which can cause cell death and proliferation, which has an impact on the bacterial transduction process as well. Due to their cellular walls being shorter than those of Gram-positive bacteria, Gram-negative bacterial strains are more susceptible to the effects of AgNPs [
86,
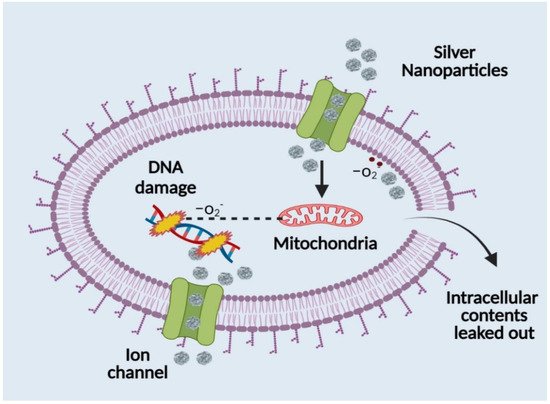
89]. Silver nanoparticles have a significant downside in that bacterial biofilms make them less effective and penetrating. Due to their intricate structure, biofilms typically change the transport chain to shield the membrane from both silver ions and nanoparticles. The nanoparticle size, which is around 50 nm, severely obstructs the path of penetration that is currently being used. Additionally, it has been observed that silver nanoparticle adsorption and deposition on bacterial biofilms reduces the nanoparticles’ ability to diffuse into bacterial cells (
Figure 4). Silver’s interaction with a molecule containing a thiol group in bacterial, fungal, and fungus cells provides the basis for silver nanoparticles’ antibacterial effect (
Figure 4). It has been observed that bacterial and fungal cells undergo structural changes after coming into touch with silver nanoparticles, albeit the precise process is yet unclear. Silver nanoparticles have higher antibacterial and antifungal characteristics than normal silver particles because of their extensive surface area, which enables better interaction with bacterial and fungal pathogens. Additionally, the gel made of silver nanoparticles penetrates bacteria and fungi in addition to attaching to cell membranes. Silver penetrates cells and connects to the cell membrane and wall, which prevents the cell from respiring [
90,
91]. When silver is present, Escherichia coli is prevented from absorbing phosphate and from releasing mannitol, succinate, proline, and glutamine. As a result, silver nanoparticles can be used as effective growth inhibitors in a wide range of microbes and are helpful in many antibacterial control systems [
92,
93].
Figure 4. Antibacterial mechanism of silver nanoparticles.
4. Silver Nanoparticles and Their Synthesis
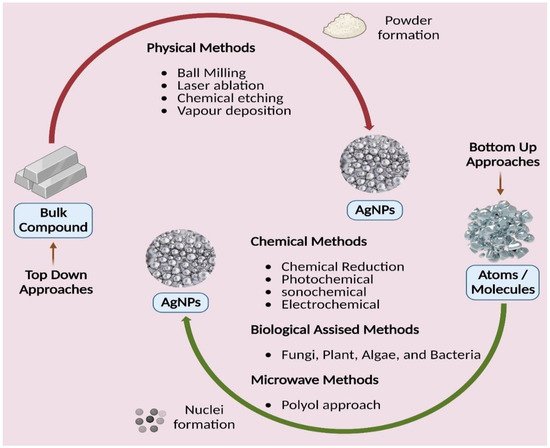
Figure 5. Different approaches of silver nanoparticles synthesis.
New alternative techniques that are cost-efficient, energy-efficient, and environmentally benign are swiftly becoming available to counteract these negative environmental consequences. There has been a thorough literature assessment on the development of green or biological synthesis [
98,
99,
100,
101]. In green synthesis, an ecologically friendly substance such as microbial (fungal and bacterial) enzymes and phytochemicals from plant extracts are used instead of the capping/stabilizing and reducing agents used in chemical reduction procedures (leaves, roots, barks, flowers, fruits, peels, and seeds). These biological processes generate biocompatible nanoparticles suitable for pharmaceutical and biomedical applications [
102,
103]. While utilizing microbes as reducing agents in nanoparticle manufacturing is quite tricky and involves several methods, it is more advantageous than chemical and physical techniques. Because they are widely accessible, simple to extract, and do not need laborious processes, plant extracts are considered a solution to the issues mentioned above in the manufacturing of AgNP. For the most part, because they are so readily available, plant extracts are employed to make nanoparticles. Manufacturing AgNPs with regular shapes and sizes is the main difficulty with plant-based synthesis, and the reduction processes of biological approaches are not well understood [
104,
105,
106,
107,
108].
5. Electrospinning
An electrospinning machine consists of four main parts: a high-power source, a syringe pump, a syringe needle carrying solutions, and a fiber deposition collector, as shown in (
Figure 6). A positive electrode is attached to the needle, and a negative electrode is connected to the collector to create the applied electric field. As a result, the repulsion charge accumulates near the hemispherical needle tip when a voltage is applied. A Taylor cone is made when the repulsive charge surpasses the surface tension. The negative electrode, which serves as the collector in this procedure, is where the polymer solution is directed to create fibers. The polymer solution evaporates, leaving behind dry fibers ranging in size from nanometers to micrometers deposited on the collector [
109,
110].
6. Advantages of Silver and Fibre Platforms
The ideal wound dressing should fulfill a number of criteria, including those listed below: (i) representing a physical barrier that is permeable to oxygen while also maintaining or providing a moist environment; (ii) being sterile, non-toxic, and protective against microorganism infections; (iii) providing an appropriate tissue temperature to favour epidermal migration and promote angiogenesis; and (iv) being non-adherent to prevent traumatic removal after healing. An ideal wound dressing should have all the qualities listed above, but it is challenging for one kind of dressing to meet every one of these needs. Creating a moist wound environment reduces dehydration and cell death. It facilitates angiogenesis and epidermal migration. It preserves moisture at the site of the wound. Excess exudate must be removed for the wound to heal, but it can also cause healthy tissue to macerate, creating a persistent wound. It enables gaseous exchange. Oxygenation regulates exudate levels and promotes fibroblast and epithelial growth. It prevents infection by prolonging the inflammatory phase and preventing epidermal migration and collagen formation; microbial infections slow the healing of wounds. Low adherence and painless removal of adherent dressings can be uncomfortable and can worsen existing granulation tissue damage. The cost-effective and optimal dressing should promote wound healing while remaining reasonably priced [
128,
129,
130]. The main categories of wound-dressing materials are fibers, gels, membranes, films, sponges, and hydrocolloids, as shown in (
Table 2). Nanofiber mats are a superior option for drug delivery compared to all other biomaterials because of their numerous advantages and inherent properties, as shown in (
Table 2). The incorporation of nanoparticles into nanofiber scaffolds constitutes a novel approach to “nanoparticle dressing” that has acquired significant popularity for wound healing (
Table 2). Due to their remarkable antibacterial capabilities, silver nanoparticles are attractive materials for wound healing. Numerous wound-dressing materials have been created in this area (
Table 3), based either on synthetic or natural polymers.
7. Conclusions Challenges and Perspective
Wound healing with nanofibrous platforms loaded with silver nanoparticles has been studied biologically in vivo and in vitro as well as mechanically in this review. Because of their unique physicochemical and biological characteristics, AgNPs have drawn significant attention from researchers working on applications for wound healing. Ag nanoparticle-loaded electrospun nanofiber scaffolds also showed exceptional antibacterial activity, high porosity, non-toxicity, and biodegradability. Due to their hydrophilic qualities and prolonged release pattern, these nanofiber scaffolds have become more and more well-liked on a global scale. By promoting and hastening the healing process, they contribute significantly to wound dressing. Additionally, silver nanoparticles and other antibacterial substances together showed synergistic antibacterial properties. The effectiveness of silver nanoparticles in wound healing and skin regeneration has been established in numerous papers from various researchers. Without a doubt, additional research will produce scaffolds with unique properties beneficial for treating chronic wounds. However, since it avoids hazardous chemicals, manufacturing silver nanoparticles using green chemistry is an intelligent strategy. Silver nanoparticles can be produced through green chemistry and used for wound dressings to make them non-toxic and compatible with the body. Much research has been conducted on silver nanoparticles to improve the antibacterial capability of medical products such as wound dressings. Nonwoven mats made of electrospun nanofibers offer a great deal of potential for use in wound healing since they structurally resemble native extracellular matrix.
Various biomedical applications, including leading-edge research aimed at healing chronic diabetic wounds, have investigated potential methods of nanotechnology-based medication delivery. In addition to their nanometric size, AgNPs were discovered to have possible use in treating diabetic wounds to lessen the likelihood of limb amputation. Their excellent antibacterial activity, anti-inflammatory response, and non-toxic nature make them an ideal and suitable alternative to other nanomaterials for wound dressing. AgNPs’ advantageous physicochemical characteristics support antibacterial effectiveness, and their surface charge also enables surface functionalization by coordinating particular ligands on the surface to try target-specific delivery. Consequential research has demonstrated the biocompatible AgNPs’ promise for treating diabetic wounds effectively, and a handful of the compounds have already been approved for commercialization. Several might be available soon with improved efficacy as an optimal dressing for successful wound healing in diabetes patients, according to a concurrent clinical study in human subjects.
Additionally, those investigators found a combination of AgNPs and biopolymers to be more effective, and the inclusion of growth factors or phytochemicals may hasten wound healing by correcting any tissue damage. The exponential rise in research papers on green synthesis, when considering the AgNPs synthesis methods, is a drawback since it demonstrates the value and interest of plant materials in the production process. The synthesis rate of AgNPs is increased by using this economical and ecologically beneficial technique. However, morphological traits play a significant role in how effective AgNPs are. For the AgNPs to have the desired features, such as superior wound healing properties, it is necessary to standardize the optimization of the plant extracts and other reactive product characteristics.
Additional research is required to link the physiological attributes of AgNPs with the physiological milieu in which they act. Before widespread usage in wound care products, careful consideration of their toxicity must be made because silver nanoparticles are incredibly active relative to their bulk. Extensive research into short- and long-term toxicity studies should be necessary to ascertain the underlying mechanism, and it should take thorough in vivo investigations into consideration as one of the future possibilities in developing AgNPs for wound healing. Additionally, special care must be given to the ideal AgNP dosage in formulations and suitable pairings to achieve a superior response in the diabetic wound. In recent years, nanotechnology has enabled the fabrication of various forms of AgNPs. However, AgNPs’ efficacy is hindered by their propensity for aggregation; surface passivator reagents are usually required to avoid accumulation. Further, silver oxidation may produce reactive oxygen species and radicals that can harm intracellular micro-organelles (such as mitochondria, ribosomes, and vacuoles) and macromolecules such as DNA, protein, and lipids. AgNPs are biocompatible but can occasionally result in argyria, according to research on how risk-free they are for people with DFU infections. However, most current studies on electrospun nanofibers throughout wound healing have been limited to pharmacodynamic assessments. As a result, the precise mechanism underlying nanofiber-assisted wound healing is unknown. Despite their increasing applications, comprehensive biological information still requires additional research due to several controversial results published on their safety. Researchers used chemical reduction methods to create a stable and colloidal dispersion of AgNPs using borohydride and hydrazine as reducing agents. Whereas these reduce the agent’s hyperactivity, they are toxic to the environment, limiting their applications.