Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Hua Li and Version 4 by Rita Xu.
Progesterone is a steroid hormone that is synthesized in the ovaries, placenta, and adrenal glands. The regulation of progesterone on plant growth is concentration-dependent. For example, low progesterone concentrations (0.01–1 μM) stimulate hypocotyl elongation, while high concentrations (100 μM) inhibit hypocotyl growth in Arabidopsis.
- progesterone
- metabolism
- growth and development
1. Introduction
Although progesterone is often considered an important female reproductive hormone, progesterone is also involved in endometrial cancer [1], breast cancer [2], central nervous system development [3], brain injury, and various neurological diseases [4][5][6]. The physiological functions and mechanisms of progesterone in mammals have therefore been studied extensively. However, adequate information regarding the physiological functioning of progesterone in plants is still lacking, which may be due to the question of whether progesterone is indeed ubiquitous in plants or not. For a long time, progesterone was thought to exist only in animals; however, the presence of progesterone was first detected in apple seeds via layer and gas-chromatography in 1968, with the progesterone levels being around 500 ng·g−1 [7]. Since then, researchers have gradually explored and recognized progesterone in plants. Progesterone was detected at 0.08 μg·g−1 in the pollen of Pinus nigra by radioimmunoassay (RIA) [8]. Progesterone levels were detected in dry mature wood, needles, and bark of Pinus taeda at 15.5, 3.85, and 1.19 μg·g−1, respectively [9]. Simons and Grinwich [10] found that progesterone was present in 80% of the plants they examined (128 species from over 50 families). Progesterone has also been detected in seven dicots (including Arabidopsis) and two monocots (including Oryza sativa) by Iino et al. [11]. Moreover, both studies confirmed that progesterone levels differed not only between species, but also between different tissues and organs, where the progesterone content ranged between 3–1600 ng·g−1. Progesterone was also subsequently found in Digitalis purpurea, Nicotiana tabacum, Inula helenium, and Juglans regia, and the progesterone concentrations in these species were comparable to previous studies [12][13]. It is therefore currently accepted that progesterone is ubiquitous in plants at low levels. Progesterone has continued to be recognized as research output has increased, which has demonstrated its important physiological functions in plants.
2. Metabolism of Progesterone in Plants
The processes of progesterone synthesis (Figure 1) are studied by administering 3H or 14C-labeled precursors. By treating Digitalis lanata with 14C-labeled sitosterol, it was found that sitosterol could be converted into progesterone [14]. Research on leaf homogenates of Cheiranthus cheiri showed that progesterone can be derived from cholesterol [15]. Additionally, stigmasterol and campesterol can be catalyzed to produce progesterone by side-chain-cleaving enzymes (SCCE) at a low rate [16][17]. Therefore, sitosterol, cholesterol, stigmasterol, and campesterol are often considered to be the main precursors of progesterone synthesis in plants [18]. In mammals, side-chain-cleavage of these precursors is accomplished by SCCE (P450scc and Cyp11A1) [19][20]. However, the nature of SCCE in plants remains unclear; this is covered in detail in Lindemann’s review [21]. In addition to precursor substances, a progesterone synthesis pathway intermediate also occurs, namely, pregnenolone (pregn-5-ene-3b-ol-20-one). Pregnenolone can be converted to isoprogesterone (pregn-5-ene-3,20-dione) by Δ5-3β-hydroxysteroid dehydrogenase (3βHSD), whereafter isoprogesterone is isomerized to progesterone (pregn-4-ene-3,20-dione) under the action ofΔ5-Δ4-ketosteroid isomerase (3KSI) [21][22]. Unlike SCCE, 3βHSD and 3KSI activities have been confirmed in plants, with 3βHSD specifically having been explored extensively [21].
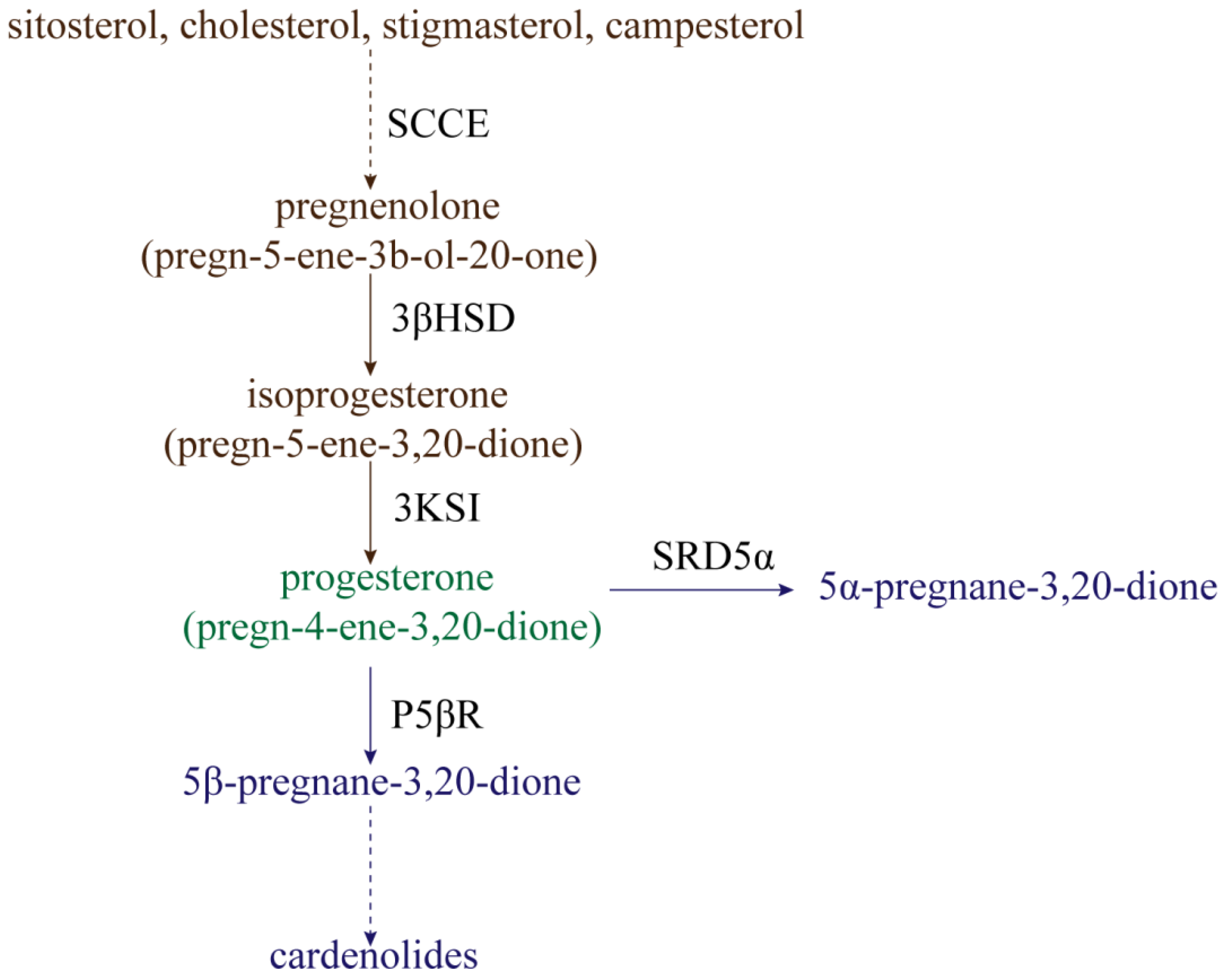
Figure 1. The synthesis and reduction processes of progesterone in plants. SCCE: side-chain-cleaving enzymes; 3βHSD: Δ5-3β-hydroxysteroid dehydrogenase; 3KSI: Δ5-Δ4-ketosteroid isomerase; P5ΒR: progesterone 5β-reductase; SRD5α: progesterone 5α-reductase.
Progesterone can be reduced for use in secondary metabolism in two ways (Figure 1). One metabolic pathway is the reduction of progesterone to 5β-pregnane-3,20-dione by progesterone 5β-reductase (P5βR), whereafter 5β-pregnane-3,20-dione is further converted into cardenolides (such as digitoxigenin, digoxigenin, and gitoxigenin) under the catalyzing activities of various enzymes [23][24][25][26]. Cardenolides, without exception, possess a 5β-configuration instead of a 5α-configuration, and P5βR is therefore a key enzyme in the metabolism of progesterone to cardenolides. Klein et al. [27] identified two genes encoding progesterone 5β-reductase in Digitalis lanata, named DlP5βR1 and DlP5βR2. After down-expression of these two genes by RNA interference (RNAi) technology, shoot cardenolide content decreased significantly. The gene encoding progesterone 5β-reductase in Arabidopsis was identified as VEP1 (At4g24220) [28]; overexpression of the Arabidopsis VEP1 in Digitalis purpurea could thus significantly increase cardenolide production. Compared with non-transgenic plants, digitoxin and digoxin content could increase up to 3.8-fold and 2.2-fold in transgenic plants cultivated in vitro, respectively [29]. Research has continually confirmed that P5βR is not only present in cardenolide-producing plants, but is also highly conserved in cardenolide-free angiosperms [30]. Therefore, P5βR might not only participate in cardenolide synthesis, but might actually have other physiological functions. For example, P5βR2 in Digitalis purpurea was significantly induced by wounding, heat shock, cold shock, and salt stress [31]. Therefore, P5βR might potentially mediate abiotic stress. However, few functional studies exist regarding the P5βR abiotic stress response, and much research is still needed.
The other way in which progesterone is reduced is by conversion into 5α-pregnane-3,20-dione under the action of progesterone 5α-reductase (SRD5α) [32]. The Arabidopsis protein DET2 shares about 40% sequence identity with SRD5α in mammals, and has been determined to have the activity of steroid 5α-reductase. The reason for this is that, when DET2 is expressed in human embryonic kidney 293 cells, DET2 can catalyze the 5α-reduction of several steroids, including progesterone, testosterone, and androstenedione [33]. The 5α-reductase (LeDET2) identified in tomato (Solanum lycopersicum) has 76% homology with the DET2 protein in Arabidopsis thaliana. Similar to AtDET2, it is active on the substrates progesterone, testosterone, and rostenedione, but has a very low reducing activity on campestenone [34]. Two separate 5α-reductase activities were also detected in the calli and leaves of Solanum malacoxylon, which can perform 5α-reduction with progesterone and campestenone as substrates, but not testosterone and androstenedione [35]. DET2 might therefore catalyze different substrates in different plants, but progesterone reduction by DET2 remains ubiquitous in plants.
3. Research on Progesterone Receptors in Plants
Mammals have multiple progesterone receptors (PR), such as intracellular receptors, membrane-associated progesterone receptor component 1 (PGRMC1), and membrane progesterone receptors (mPR), which can mediate various physiological processes by activating downstream genes or proteins [4]. In Arabidopsis, a putative membrane steroid binding protein 1 (MSBP1) was identified, which can bind progesterone and other molecules (such as 5-dihydrotestosterone, 24-epi-brassinolide, and stigmasterol) with different in vitro affinities, and among them, MSBP1 has the highest affinity with progesterone [36]. Iino et al. [11] soon discovered two homologous genes of AtMSBP1 in Arabidopsis, AtMSBP2 and AtSBP (steroid binding protein), and cloned OsMSBP1, OsMSBP2, and OsSBP in rice. Furthermore, progesterone-binding membrane proteins were found to be widely distributed in plants by aligning and analyzing EST date in a variety of plants [11]. Tissue expression analysis showed that MSBP1, MSBP2, and SBP were expressed in various tissues [11], whereas AtMSBP1 was weakly expressed in roots and difficult to detect in mature flowers and siliques, and that the expression level of MSBP1 could be significantly suppressed by darkness [36].
Further studies revealed that MSBP1 can regulate hypocotyl growth and stimulate the anti-gravitropism and gravitropism of hypocotyl and roots, respectively (Figure 2). MSBP1 overexpressing transgenic and antisense transgenic plants showed shorter and longer hypocotyl, respectively, compared to WT. The regulation of hypocotyl growth by MSBP1 was related to its regulation of cell-elongation-related genes. MSBP1 overexpression decreased the expression of Exp1, thus inhibiting cell elongation and hypocotyl growth [36]. Further studies revealed that MSBP1 inhibits hypocotyl growth by negatively regulating brassinosteroid (BR) signaling. MSBP1 could interact with BAK1 (BRI1 associated receptor kinase 1) to promote BAK1 endocytosis, thereby reducing BR response [37]. Additionally, the upstream regulators of MSBP1 have been found through electrophoretic mobility shift assay (EMSA) and chromatin IP (ChIP) assay. HY5 (Long Hypocotyl 5) and HYH (HY5 Homolog) can bind to the GAGA-box in the promoter to activate the transcription of MSBP1, thereby inhibiting the elongation of the hypocotyl [38]. Moreover, MSBP1 was found to stimulate root gravitropism and antigravitropism of the hypocotyl by regulating auxin redistribution [39].
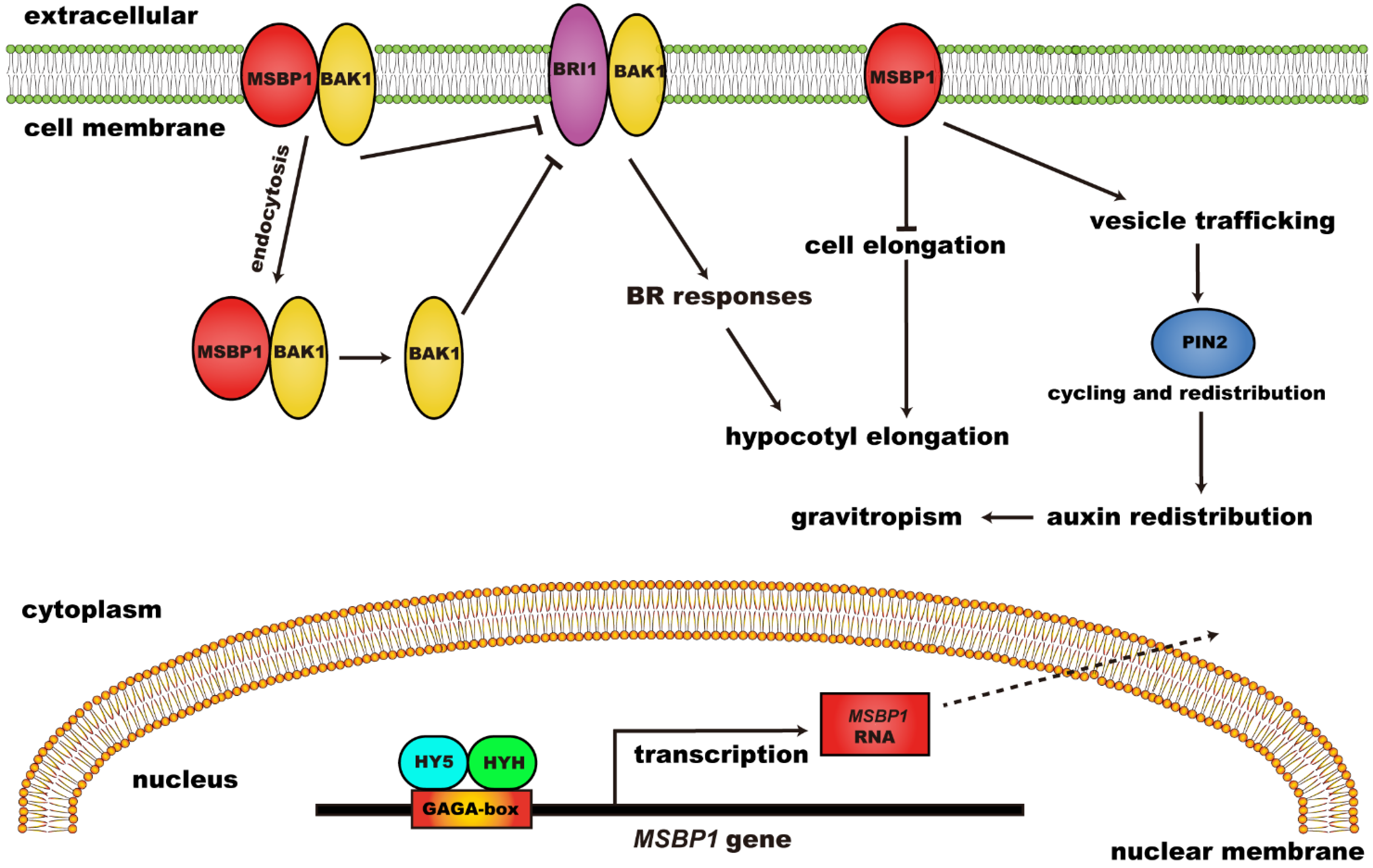
Figure 2. The regulatory function and mechanism of MSBP1 on hypocotyl growth and gravitropism. MSBP1: membrane steroid binding protein 1; BRI1: brassinosteroid-insensitive 1; BAK1: BRI1 associated receptor kinase 1; BR: brassinosteroid; HY5: long hypocotyl 5; HYH: HY5 homolog.
Progesterone also has specific binding sites in the membrane and cytoplasm of wheat (Triticum aestivum) cells. In non-vernalized and vernalized wheat cell membrane extracts, the specific binding of progesterone was 31.0 and 18.1 fmol/mg protein, respectively. However, the specific bindings of progesterone were 4.9 and 21.3 fmol/mg protein in the non-vernalized and vernalized wheat cell cytosolic fractions, respectively [40]. Janeczko et al. [41] confirmed that specific progesterone binding sites exist in the wheat cell membrane and cytoplasm, and the number of binding sites varies with differing drought-resistant varieties and water conditions. This suggests that steroid binding proteins are present in wheat, and that the membrane and cytoplasm content of steroid binding proteins varies significantly with vernalization and drought treatment. Unfortunately, the genes encoding for steroid binding proteins in wheat have not yet been identified.
4. The Regulation of Progesterone on Plant Growth and Development
In addition to the above-mentioned, progesterone may be involved in hypocotyl growth and root gravitation through its receptor MSBP1 [36][38][39], and the application of exogenous progesterone significantly affects plant shoot and root growth, seed germination, and reproductive development (Table 1, Figure 3).
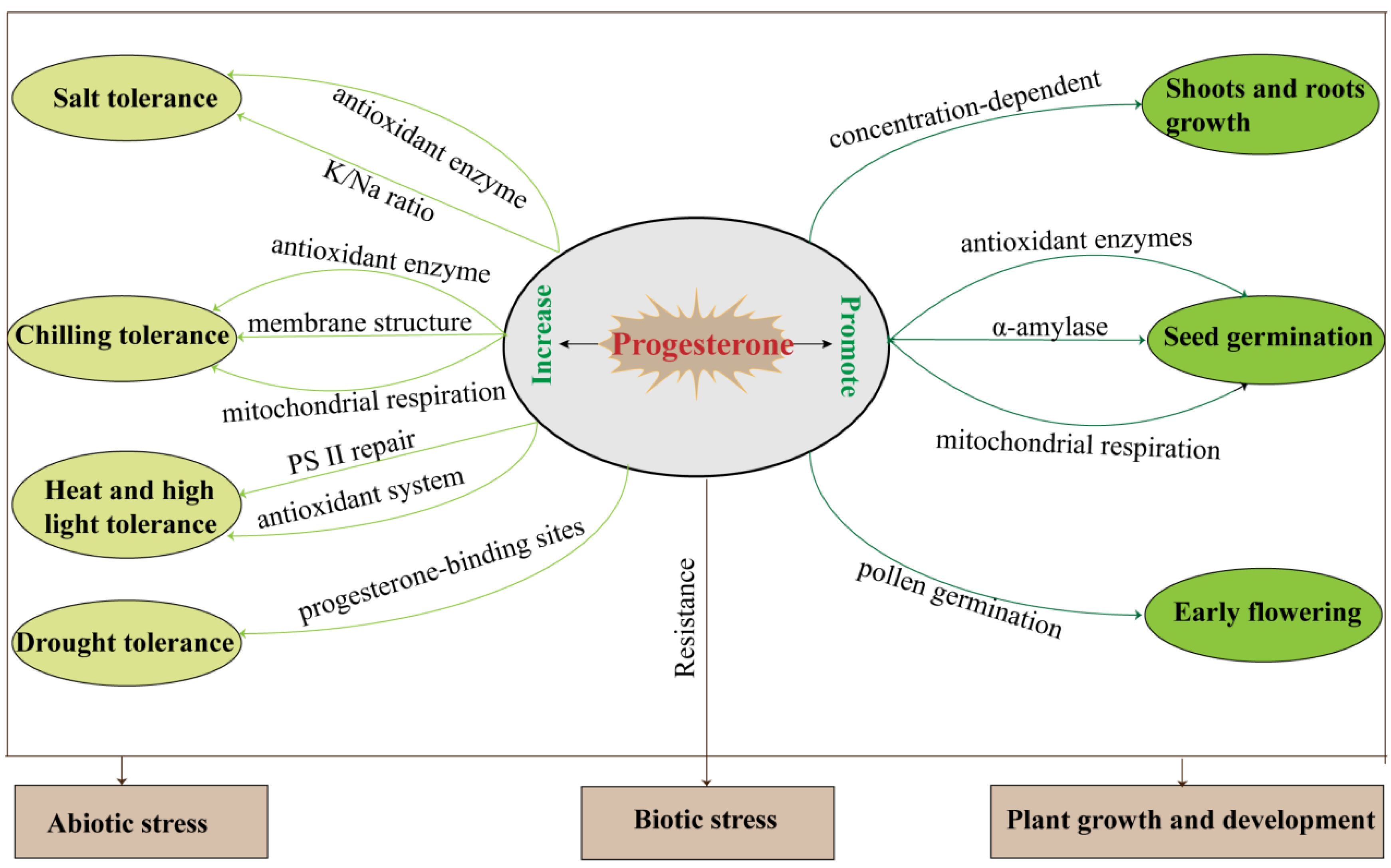
Figure 3. The summary of plant progesterone regulation during growth, development, and biotic/abiotic stress responses.
Table 1. The regulation of progesterone on plant growth and development.
| Plant Growth and Development | Progesterone Action | Plant Species | Reference |
|---|---|---|---|
| Shoot and root growth | Progesterone regulated plant growth in a concentration dependent manner. | Arabidopsis thaliana | [11] |
| Helianthus annuus | [42] | ||
| Cicer arietinum | [43] | ||
| Tissue culture | Progesterone improved shoot and callus formation. | Onobrychis sativa | [44] |
| Progesterone regulated responded embryogenic callus and regenerable callus induction. | Triticum aestivum | [45] | |
| Seed germination | Progesterone accelerated seed germination. | Cicer arietinum | [46] |
| Phaseolus vulgaris | [47] | ||
| Zea mays | [48][49] | ||
| Reproductive development | Progesterone increased gradually with pollen germination. | Actinidia deliciosa | [50] |
| Progesterone stimulated pollen germination and tube elongation. | Nicotiana tabacum | [51][52] | |
| Progesterone induced plant flowering and promoted reproductive growth. | Triticum aestivum | [53][54] | |
| Arabidopsis thaliana | [55] |
4.1. Shoot and Root Growth
The regulation of progesterone on plant growth is concentration-dependent. For example, low progesterone concentrations (0.01–1 μM) stimulate hypocotyl elongation, while high concentrations (100 μM) inhibit hypocotyl growth in Arabidopsis [11]. A high progesterone concentration (0.25 μg/plant) promotes shoot growth and inhibits root growth in sunflower (Helianthus annuus), while a low progesterone concentration (0.1 μg/plant) induces the opposite [42]. Numerous progesterone concentrations (10−4–10−15 M) can promote chickpea (Cicer arietinum) shoot and root growth, with 10−4 M having the best effect [43]. Progesterone also affects tissue culture. Progesterone concentrations of 10−4 and 10−5 mM improve shoot formation from cotyledons explants and callus formation of sainfoin (Onobrychis sativa), respectively [44]. The responded embryogenic callus (REC) and regenerable callus (RE) induction from mature wheat embryos varied under different progesterone concentrations. The maximum REC rate (75%) was obtained with 10−8 mM progesterone treatment, but the REC rate decreased significantly under the 10−6 and 10−4 mM progesterone; 10−6 mM progesterone could increase RE, while RE was inhibited at the concentrations of 10−8 and 10−4 mM [45]. These results suggest that shoots and roots are generally growth-promoted at low concentrations of progesterone and growth-inhibited at high concentrations, but that optimal concentrations vary between species and at different growth stages. This feature is very similar to the regulation of plant growth by auxin and BR. Studies have confirmed that there is a certain relationship between progesterone and BR signaling, but whether there is a link between progesterone and auxin signaling has not been explored, which is worthy of attention and discussion.
4.2. Seed Germination
Progesterone can significantly accelerate the rate of chickpea seed germination, which is accompanied by increased α-amylase, superoxide dismutase (SOD), and catalase (CAT) activities, but decreased seed malondialdehyde (MDA) content [46]. It was also found that a progesterone concentration of 10−9 M significantly increased the activities of SOD, POD, and CAT during bean (Phaseolus vulgaris) seed germination [47]. Similar results were obtained in maize (Zea mays), where progesterone (10−4–10−15 M) significantly increased the germination rate of maize seeds and enhanced root and coleoptyle length, while the activities of α-amylase, SOD, CAT, peroxidase (POX), and polyphenol oxidase (PPO) were simultaneously induced [48]. Further studies in maize showed that progesterone accelerated seed germination by activating mitochondrial respiration and related pathways, and genes (such as CS, COX19, Pdh1, and ATP6) related to mitochondrial respiration were upregulated [49]. Thus, the promoting effect of progesterone on seed germination might potentially be related to antioxidant enzyme activation, α-amylase, and mitochondrial respiration.
References
- Gompel, A. Progesterone and endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 95–107.
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and breast cancer. Endocr. Rev. 2020, 41, 320–344.
- González-Orozco, J.C.; Camacho-Arroyo, I. Progesterone actions during central nervous system development. Front. Neurosci. 2019, 13, 503.
- Guennoun, R. Progesterone in the brain: Hormone, neurosteroid and neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271.
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr. Neuropharmacol. 2016, 14, 641–653.
- Wright, D.W.; Yeatts, S.D.; Silbergleit, R. Progesterone in traumatic brain injury. N. Engl. J. Med. 2015, 372, 1766–1767.
- Gawienowski, A.M.; Gibbs, C.C. Identification of cholesterol and progesterone in apple seeds. Steroids 1968, 12, 545–550.
- Šaden-Krehula, M.; Tajić, M.; Kolbah, D. Sex hormones and corticosteroids in pollen of Pinus nigra. Phytochemistry 1979, 18, 345–346.
- Carson, J.D.; Jenkins, R.L.; Wilson, E.M.; Howell, W.M.; Moore, R. Naturally occurring progesterone in loblolly pine (Pinus taeda L.): A major steroid precursor of environmental androgens. Environ. Toxicol. Chem. Int. J. 2008, 27, 1273–1278.
- Simons, R.; Grinwich, D. Immunoreactive detection of four mammalian steroids in plants. Can. J. Bot. 1989, 67, 288–296.
- Iino, M.; Nomura, T.; Tamaki, Y.; Yamada, Y.; Yoneyama, K.; Takeuchi, Y.; Mori, M.; Asami, T.; Nakano, T.; Yokota, T. Progesterone: Its occurrence in plants and involvement in plant growth. Phytochemistry 2007, 68, 1664–1673.
- Simerský, R.; Novák, O.; Morris, D.A.; Pouzar, V.; Strnad, M. Identification and quantification of several mammalian steroid hormones in plants by UPLC-MS/MS. J. Plant Growth Regul. 2009, 28, 125–136.
- Pauli, G.F.; Friesen, J.B.; Gödecke, T.; Farnsworth, N.R.; Glodny, B. Occurrence of progesterone and related animal steroids in two higher plants. J. Nat. Prod. 2010, 73, 338–345.
- Bennett, R.; Heftmann, E.; Winter, B.J. Conversion of sitosterol to progesterone by Digitalis lanata. Die Nat. 1969, 56, 463.
- Stohs, S.; El-Olemy, M. Pregnenolone and progesterone from 20α-hydroxycholesterol by Cheiranthus cheiri leaf homogenates. Phytochemistry 1971, 10, 3053–3056.
- Lindemann, P.; Luckner, M. Biosynthesis of pregnane derivatives in somatic embryos of Digitalis lanata. Phytochemistry 1997, 46, 507–513.
- Sundararaman, P.; Djerassi, C. A convenient synthesis of progesterone from stigmasterol. J. Org. Chem. 1977, 42, 3633–3634.
- Janeczko, A. The presence and activity of progesterone in the plant kingdom. Steroids 2012, 77, 169–173.
- Aringer, L.; Eneroth, P.; Nordström, L. Side-chain cleavage of 4-cholesten-3-one, 5-cholesten-3α-ol, β-sitosterol, and related steroids in endocrine tissues from rat and man. J. Steroid Biochem. 1979, 11, 1271–1285.
- Hoyte, R.; RB, H. Enzymatic side chain cleavage of c-20 alkyl and aryl analogs of (20-s)-20-hydroxycholesterol: Implications for the biosynthesis of pregnenolone. J. Biol. Chem. 1979, 254, 2278–2286.
- Lindemann, P. Steroidogenesis in plants–Biosynthesis and conversions of progesterone and other pregnane derivatives. Steroids 2015, 103, 145–152.
- Seidel, S.; Kreis, W.; Reinhard, E. D5-3b-Hydroxysteroid dehydrogenase/D5-D4-ketosteroid isomerase (3b-HSD), a possible enzyme of cardiac glycoside biosynthesis, in cell cultures and plants of Digitalis lanata EHRH. Plant Cell Rep. 1990, 8, 621–624.
- Gärtner, D.E.; Keilholz, W.; Seitz, H.U. Purification, characterization and partial peptide microsequencing of progesterone 5β-reductase from shoot cultures of Digitalis purpurea. Eur. J. Biochem. 1994, 225, 1125–1132.
- Herl, V.; Fischer, G.; Müller-Uri, F.; Kreis, W. Molecular cloning and heterologous expression of progesterone 5β-reductase from Digitalis lanata Ehrh. Phytochemistry 2006, 67, 225–231.
- Gärtner, D.E.; Wendroth, S.; Seitz, H.U. A stereospecific enzyme of the putative biosynthetic pathway of cardenolides: Characterization of a progesterone 5β-reductase from leaves of Digitalis purpurea L. FEBS Lett. 1990, 271, 239–242.
- Caspi, E.; Lewis, D. Progesterone: Its possible role in the biosynthesis of cardenolides in Digitalis lanata. Science 1967, 156, 519–520.
- Klein, J.; Horn, E.; Ernst, M.; Leykauf, T.; Leupold, T.; Dorfner, M.; Wolf, L.; Ignatova, A.; Kreis, W.; Munkert, J. RNAi-mediated gene knockdown of progesterone 5β-reductases in Digitalis lanata reduces 5β-cardenolide content. Plant Cell Rep. 2021, 40, 1631–1646.
- Herl, V.; Fischer, G.; Reva, V.; Stiebritz, M.; Muller, Y.; Müller-Uri, F.; Kreis, W. The VEP1 gene (At4g24220) encodes a short-chain dehydrogenase/reductase with 3-oxo-Δ4, 5-steroid 5β-reductase activity in Arabidopsis thaliana L. Biochimie 2009, 91, 517–525.
- Kairuz, E.; Pérez-Alonso, N.; Capote-Pérez, A.; Pérez-Pérez, A.; Espinosa-Antón, A.A.; Angenon, G.; Jiménez, E.; Chong-Pérez, B. Enhancement of cardenolide production in transgenic Digitalis purpurea L. by expressing a progesterone-5β-reductase from Arabidopsis thaliana L. Ind. Crops Prod. 2020, 146, 112166.
- Bauer, P.; Munkert, J.; Brydziun, M.; Burda, E.; Muller-Uri, F.; Groger, H.; Muller, Y.; Kreis, W. Highly conserved progesterone 5b-reductase genes (P5bR) from 5b-cardenolide-free and 5b-cardenolide-producing angiosperms. Phytochemistry 2010, 71, 1495–1505.
- Pérez-Bermúdez, P.; Moya Garcia, A.A.; Tuñón, I.; Gavidia, I. Digitalis purpurea P5βR2, encoding steroid 5β-reductase, is a novel defense-related gene involved in cardenolide biosynthesis. New Phytol. 2010, 185, 687–700.
- Wendroth, S.; Seitz, H.U. Characterization and localization of progesterone 5 α-reductase from cell cultures of foxglove (Digitalis lanata EHRH). Biochem. J. 1990, 266, 41–46.
- Li, J.; Biswas, M.G.; Chao, A.; Russell, D.W.; Chory, J. Conservation of function between mammalian and plant steroid 5α-reductases. Proc. Natl. Acad. Sci. USA 1997, 94, 3554–3559.
- Rosati, F.; Bardazzi, I.; De Blasi, P.; Simi, L.; Scarpi, D.; Guarna, A.; Serio, M.; Racchi, M.L.; Danza, G. 5α-Reductase activity in Lycopersicon esculentum: Cloning and functional characterization of LeDET2 and evidence of the presence of two isoenzymes. J. Steroid Biochem. Mol. Biol. 2005, 96, 287–299.
- Rosati, F.; Danza, G.; Guarna, A.; Cini, N.; Racchi, M.L.; Serio, M. New evidence of similarity between human and plant steroid metabolism: 5α-reductase activity in Solanum malacoxylon. Endocrinology 2003, 144, 220–229.
- Yang, X.-H.; Xu, Z.-H.; Xue, H.-W. Arabidopsis membrane steroid binding protein 1 is involved in inhibition of cell elongation. Plant Cell 2005, 17, 116–131.
- Song, L.; Shi, Q.-M.; Yang, X.-H.; Xu, Z.-H.; Xue, H.-W. Membrane steroid-binding protein 1 (MSBP1) negatively regulates brassinosteroid signaling by enhancing the endocytosis of BAK1. Cell Res. 2009, 19, 864–876.
- Shi, Q.-M.; Yang, X.; Song, L.; Xue, H.-W. Arabidopsis MSBP1 is activated by HY5 and HYH and is involved in photomorphogenesis and brassinosteroid sensitivity regulation. Mol. Plant 2011, 4, 1092–1104.
- Yang, X.; Song, L.; Xue, H.-W. Membrane steroid binding protein 1 (MSBP1) stimulates tropism by regulating vesicle trafficking and auxin redistribution. Mol. Plant 2008, 1, 1077–1087.
- Janeczko, A.; Budziszewska, B.; Skoczowski, A.; Dybała, M. Specific binding sites for progesterone and 17beta-estradiol in cells of Triticum aestivum L. Acta Biochim. Pol. 2008, 55, 707–711.
- Janeczko, A.; Oklešťková, J.; Siwek, A.; Dziurka, M.; Pociecha, E.; Kocurek, M.; Novák, O. Endogenous progesterone and its cellular binding sites in wheat exposed to drought stress. J. Steroid Biochem. Mol. Biol. 2013, 138, 384–394.
- Bhattacharya, B.; Gupta, K. Steroid hormone effects on growth and apical dominance of sunflower. Phytochemistry 1981, 20, 989–991.
- Erdal, S.; Dumlupinar, R. Mammalian sex hormones stimulate antioxidant system and enhance growth of chickpea plants. Acta Physiol. Plant. 2011, 33, 1011–1017.
- Dadaşoğlu, E.; Tosun, M. The effect of mammalian sex hormones on in vitro properties of sainfoin (Onobrychis sativa L.). Fresenius Environ. Bull. 2018, 27, 7222–7229.
- Turkoglu, A. Effects of mammalian sex hormones on regeneration capacity, retrotransposon polymorphism and genomic instability in wheat (Triticum aestivum L.). 2022; preprints.
- Erdal, S.; Dumlupinar, R. Progesterone and β-estradiol stimulate seed germination in chickpea by causing important changes in biochemical parameters. Z. Nat. C 2010, 65, 239–244.
- Erdal, S. Effects of mammalian sex hormones on antioxidant enzyme activities, H2O2 content and lipid peroxidation in germinating bean seeds. J. Fac. Agric. 2009, 40, 79–85.
- Erdal, S.; Dumlupinar, R.; Cakmak, T.; Genisel, M. Mammalian sex hormones influence germination velocity and enzyme activities in germinating maize seeds. Fresenius Environ. Bull. 2010, 19, 1458–1465.
- Turk, H. Progesterone Promotes Mitochondrial Respiration at the Biochemical and Molecular Level in Germinating Maize Seeds. Plants 2021, 10, 1326.
- Speranza, A.; Crosti, P.; Malerba, M.; Stocchi, O.; Scoccianti, V. The environmental endocrine disruptor, bisphenol A, affects germination, elicits stress response and alters steroid hormone production in kiwifruit pollen. Plant Biol. 2011, 13, 209–217.
- Ylstra, B.; Touraev, A.; Brinkmann, A.O.; Heberle-Bors, E.; Tunen, A. Steroid hormones stimulate germination and tube growth of in vitro matured tobacco pollen. Plant Physiol. 1995, 107, 639–643.
- Spivak, S.; Berdichevets, I.; Yarmolinsky, D.; Maneshina, T.; Shpakovski, G.; Kartel, N. Construction and characteristics of transgenic tobacco Nicotiana tabacum L. plants expressing CYP11A1 cDNA encoding cytochrome P450SCC. Russ. J. Genet. 2009, 45, 1067–1073.
- Janeczko, A.; Filek, W. Stimulation of generative development in partly vernalized winter wheat by animal sex hormones. Acta Physiol. Plant. 2002, 24, 291–295.
- Janeczko, A.; Oklestkova, J.; Novak, O.; Śniegowska-Świerk, K.; Snaczke, Z.; Pociecha, E. Disturbances in production of progesterone and their implications in plant studies. Steroids 2015, 96, 153–163.
- Janeczko, A.; Filek, W.; Biesaga-Kościelniak, J.; Marcińska, I.; Janeczko, Z. The influence of animal sex hormones on the induction of flowering in Arabidopsis thaliana: Comparison with the effect of 24-epibrassinolide. Plant Cell Tissue Organ Cult. 2003, 72, 147–151.
More
