1. The Pathways between Gut Microbiota and the Nervous System
As shown in
Figure 1, the bidirectional connection between the gut and the brain is based on metabolic, endocrine, neural, and immunological pathways. It includes the vagal nerve, the
hypothalamic–pituitary–adrenal axis (HPA
) axis, the production of bacterial metabolites, immune mediators, and entero–endocrine signaling
[1][2][50,99].
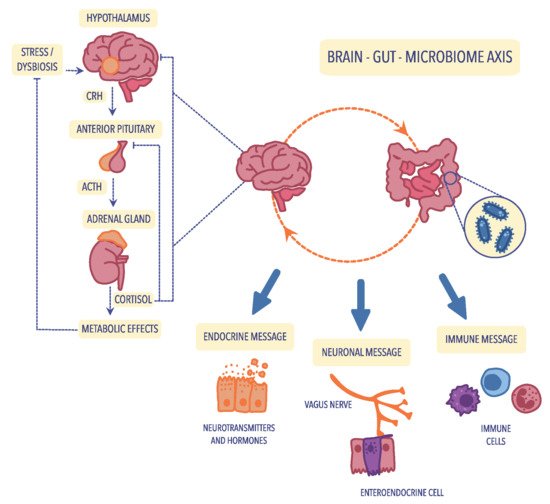
Figure 1. The bidirectional communication of the microbiota–gut–brain axis is mediated by the immune, neuroendocrine, and neuronal pathways. The activation of the hypothalamic–pituitary–adrenal (HPA) axis is associated with the occurrence of stress factors or dysbiosis, which increase the release of corticotropin-releasing hormone (CRH) from the hypothalamus, which subsequently stimulates the transport of adrenocorticotropic hormone (ACTH) from the anterior pituitary. Under the influence of ACTH, the adrenal gland begins to produce and secrete the stress hormone (cortisol), which is responsible for the modulation of intestinal immune and barrier functions. Figure was created using the Vectornator application.
1.1. The Hypothalamic–Pituitary–Adrenal (HPA) Axis
The cerebral–intestinal axis is controlled at several levels, and the primary regulator is the nervous and endocrine (with the primary role of the HPA axis) and the immune pathway. Immune regulation of the HPA axis occurs mainly through the modification of cytokine secretion. In contrast, nervous regulation occurs primarily via the transmission of impulses in the autonomic nervous system, including the vagus nerve, afferent and centrifugal fibers, and the enteric nervous system (ENS). ENS, known as the “gut brain”, was first described in 1998 by Michael Gershon of Columbia University Medical Center
[3][100]. The enteric nervous system is not only responsible for the direct regulation of muscles, mucosa, and vessels in the digestive tract but also for its activity. It comprises a large number of nerve fibers that form an impressive network of connections, and it is worth pointing out that over 30 different neurotransmitters are involved in the functioning of this system. There are about 40 neurons for each intestinal villi
[4][101]. Contrary to the peripheral nervous system, the neuronal elements of the enteric nervous system are not surrounded by collagen and Schwann cells; instead, they are enveloped by glia that resemble
central nervous system (CNS
) astrocytes. The ENS is formed by the Meissner plexus, located in the submucosa of the intestine, and the Auerbach plexus, located between the layer of circular and longitudinal muscles
[5][102]. Due to this location, the ENS, through numerous transmitters and cytokines, remains in close contact with intestinal-associated lymphoid tissue (GALT) and the systemic humoral defense system of mucosa-associated lymphoid tissue (MALT). The neurotransmitters of the intestinal nervous system act, among others, on receptors in Peyer’s patches and lymphocytes. Most of GALT consists of lymphocytes of the entire immune system (70%), constituting the first line of defense and playing a particularly important role in the immune response to external antigens
[6][7][103,104]. Likewise, microorganisms that inhabit the intestines, certain species of bacteria and fungi, transmit signals to both GALT and ENS through the synthesis and secretion of many different neurotransmitters
[8][105]. Hormonal regulation of cerebral–intestinal communication occurs primarily through the HPA axis, also known as the stress axis, which primarily regulates the course of the stress response. Hypothalamic hormones-corticoliberin, together with vasopressin, start a hormonal cascade along the HPA axis, stimulating the anterior pituitary gland to produce and secrete the corticotropic hormone ACTH, which, along with the bloodstream, goes to the adrenal cortex and stimulates it to secrete glucocorticoids, mainly cortisol
[9][69].
The cerebral–gut axis (which strongly connects the brain, intestines, and intestinal microbiota) is a two-way communication pathway, where brain–gut communication occurs through the autonomic nervous system (AUN), mainly the vagus nerve. Research has revealed that 90% of the impulses within the cerebral–intestinal axis are transmitted centripetally, i.e., from the intestines to the brain, and only 10% centrally. According to many observations, it has been proven that after vagotomy, the intestines continue to function correctly
[4][101].
1.2. Neuroendocrine Pathways
Cortisol plays a key role in the endocrine mechanisms that regulate the gut–brain axis because it affects the cells of the immune system by modulating the secretion of cytokines that act on the HPA axis, but also significantly affects the functioning and differentiation of the intestinal microbiota
[10][106]. On the other hand, special attention should be paid to the fact that intestinal bacteria produce numerous substances such as γ-aminobutyric acid (GABA) (
Lactobacillus spp.,
Bifidobacterium spp.), acetylcholine (
Lactobacillus spp.), serotonin (
Escherichia spp.,
Candida spp.,
Enterococcus spp.), dopamine (
Bacillus spp.), or noradrenaline (
Bacillus spp.,
Saccharomyces spp.). These substances are involved not only in communication within the intestinal microflora but also in systemic and peripheral effects that affect brain functioning
[11][107].
The intestinal microbiota also influences the level and metabolism of glutamic acid (glutamate), one of the main stimulants of the CNS. Glutamic acid is synthesized in glutamine-derived glial cells with the participation of glutaminase. The most important receptor of the glutamatergic system is the
N-methyl-
d-aspartate receptor (NMDA)
[12][108]. Under physiological conditions, glutamic acid is the basis of learning and memory. Therefore, it has been widely applied to treating memory disorders and nervous exhaustion. However, an abundance of this acid causes an overactivation of glutamatergic receptors and consequently damages neurons. Both glutamine and glutamic acid are converted into GABA, the main inhibitory neurotransmitter, in other metabolic pathways. Several studies reported that the gut microbiota affects not only the level of GABA but also its metabolism
[13][109].
1.3. Tryptophan Metabolism
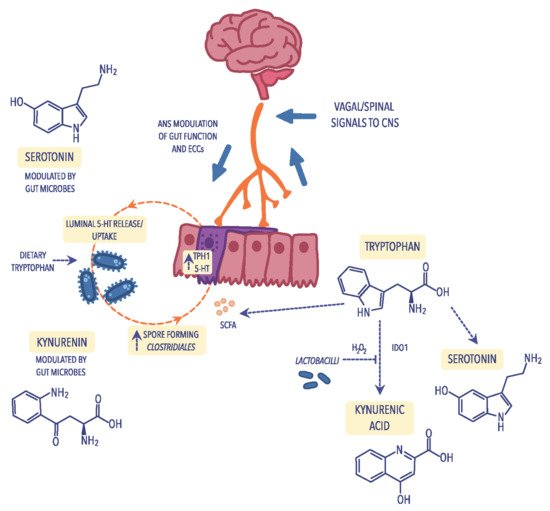
A particular example of how the gut microbiota can influence the brain–gut axis is the synthesis of serotonin, commonly known as the “happiness hormone”. Serotonin deficiency in the CNS is one of the factors that cause depression, sadness, apathy, and anxiety and, according to modern concepts, is the leading cause of depressed mood. Therefore, drugs belonging to the class of selective serotonin reuptake inhibitors (SSRIs) have widespread use in treating mood disorders
[14][110]. Serotonin is produced due to the transformation of tryptophan, one of the essential amino acids, of which about 2% of tryptophan ingested with food is converted to serotonin. In the body, serotonin is produced in the digestive tract, nervous system, and immune system (
Figure 2)
[15][111]. In the
gastrointestinal (GI
) tract, even 95% of serotonin is produced by the mucosa’s enterochromatophilic cells (ECCs), microorganisms that are part of the intestinal microbiota, and neurons of nerve plexuses in the submucosa and muscular layers of the intestine
[16][112]. Only 3% of serotonin is produced in thrombocytes and 2% in the pineal gland. The primary function of serotonin in the nervous system is neurotransmission, where serotonergic neurons play an important role in regulating pain, sleep, mood changes, and memory processes. In addition, serotonin is also an essential transmitter in the ENS
[17][113]. Serotonin receptors in the GI tract are found not only in the neurons of the submucosa and muscle plexuses but also in enterocytes and smooth muscle cells. Through receptors, serotonin acts on the activity of the GI tract (both inhibiting and stimulating its function)
[16][112]. Studies have revealed that altered serotonin metabolism is involved in the pathogenesis of certain GI diseases. In other words, in inflammatory bowel diseases, GI infections, and also in appendicitis, an increase in serum serotonin concentration has been observed
[18][114]. Studies also demonstrated that the intestinal bacteria
Bifidobacterium infantis affect the level and metabolism of tryptophan, thus increasing its level in the body
[19][29].
Figure 2. Metabolism of tryptophan through different pathways: the kynurenine pathway that leads to the production of kynurenine and its derivatives in reliance on indoleamine 2,3-dioxygenase (IDO); the serotonin pathway, including the enterochromaffin cells (ECCs) in which tryptophan is transformed into serotonin (5-HT) and its derivatives and is dependent on short-chain fatty acids (SCFAs) which are biosynthesized by spore-forming Clostridiales. ECCs can be activated by the autonomic nervous system (ANS) to secrete 5-HT into the gut lumen, where it can communicate with the intestinal microbiota. Figure was created using the Vectornator application.
1.4. Immunological Mechanisms
In recent years, the amount of evidence that points to an inflammatory basis of a psychiatric disorder has increased. There are indications that anti-inflammatory cytokines influence both neurohormonal and neurochemical functions in this process
[20][21][115,116]. The primary factor leading to the systemic inflammatory response is increased intestinal permeability, also known as a leaky gut syndrome (LGS)
[22][117]. Mechanisms involved in LGS include, first of all, disturbances in the intestinal microbiota within the GI tract, damage to enterocytes, weakening of tight connections between enterocytes, but also stress, which plays a particularly important role in the pathophysiology of depression
[23][118]. As a result of the development of this syndrome, the translocation of Gram-negative bacteria containing lipopolysaccharide (LPS) causes overactivation of the immune system
[24][96]. This stimulation causes an increase in the concentration of pro-inflammatory cytokines, the excess of which is destructive to host cells, including cells of the CNS. Since immune cells produce several cytokines, chemokines, and inflammatory mediators, leading to general inflammation of the organism, one of the hypotheses of the development of affective disorders is inflammation of the organism
[25][119].
According to numerous observations, intestinal dysbiosis is the cause of the development of many intestinal diseases such as intestinal candidiasis, Crohn’s disease, ulcerative colitis, pseudomembranous enteritis, and inflammatory bowel disease, but also food allergies and intolerances, or colorectal cancer
[26][27][120,121]. It should be noted that disturbances in the amount and composition of the intestinal microbiota promote the development of pathological conditions within the intestines and systemic diseases, allergies, obesity, metabolic syndrome, and autoimmune diseases. Therefore, the question can be asked whether the intestinal microbiota influences cognitive disorders and whether intestinal dysbiosis will affect the dysregulation of these processes? The answer is neither simple nor unambiguous. However, some premises, observations, and facts allow the formation of theories and hypotheses. The truth is that there is close communication between the brain and the intestines. It is known that the brain directly regulates the functioning of the intestines. However, special attention should be paid to the reverse direction of this communication. In particular, the processes in the intestines and the intestinal microbiota can affect the CNS’s functioning.
Pro-inflammatory cytokines also affect the concentration of serotonin by activating the kynurenine pathway in which tryptophan, the precursor of serotonin, is metabolized
[28][122]. Increasing the concentration of pro-inflammatory cytokines activates indoleamine 2,3-dioxygenase (IDO), which results in a decrease in the concentration of tryptophan and serotonin (5-HT), simultaneously intensifying the symptoms of affective disorders and a rise in the level of tryptophan catabolites
[19][29]. Under the influence of IDO, tryptophan is converted to kynurenine, quinolinic acid, and 3-hydroxy-kynurenine, which reduces serotonin production while increasing the concentration of toxic metabolites in the CNS
[29][30][123,124]. Moreover, the weakened intestinal barrier allows the penetration of nutrients (food antigens). In addition, in contact with IgG antibodies, formed immune complexes are moved with the blood circulation, among others, for the choroid plexus of the CNS. Chronic accumulation of such complexes causes the initiation of inflammation and the progression of chronic ailments.
1.5. Bacterial Cell Wall Sugars
Some researchers emphasize that in healthy people, the dose of bacterial LPS may be associated with a more frequent occurrence of depression and anxiety and also contributes to an increase in norepinephrine and pro-inflammatory cytokines and cortisol in blood plasma and saliva, respectively
[31][32][125,126]. Interestingly, some studies have shown some effects of LPS on emotional memory, suggesting that this ability depends on the dose
[33][127]. Evidence in people with psychiatric disorders indicates decreased secretion of gastric acid that contributes to the development of small intestinal bacterial overgrowth (SIBO). SIBO is characterized by several symptoms that directly indicate changes in the composition and quality of the microbiota in individual sections of the intestine. The presence of bacteria in the small intestine, which live in the large intestine under physiological conditions, causes their physiological and metabolic processes to lead to numerous symptoms in the digestive system
[34][35][14,128]. In addition, when decomposing proteins, bacteria such as
Clostridium, Proteus, and
Enterobacteriaceae produce ammonia, a potent neurotoxin. The high concentration of this substance in the blood leads to a deficiency of alpha-ketoglutaric acid, responsible for the removal of ammonia from the CNS and consequently causes a toxic effect in the enlargement of neurological symptoms
[36][129]. It should be noted that some species of
Clostridium can reduce the secretion of dopamine beta-hydroxylase, the enzyme responsible for converting dopamine to norepinephrine, resulting in a deficiency of norepinephrine and an excess of dopamine
[37][130]. The imbalance of the mentioned compounds leads not only to the development of compulsive behaviors and obsessive states but also to their disturbed proportion, characteristic of SCZ or ASD
[38][131].
1.6. Bacterial Metabolites
The products or metabolites of the gut, such as short-chain fatty acids, are carboxylic acids with aliphatic tails of 1 to 6 carbon atoms produced during anaerobic bacterial fermentation, mainly of dietary fiber
[39][132]. More than 95% of SCFAs are butyric acid (butyrate), acetic acid (acetate), and propionic acid (propionate), although lactic acid was also found in smaller amounts
[40][133]. Among SCFAs-producing bacteria there are
Clostridium spp.,
Eubacterium spp.,
Fusobacterium spp.,
Butyrivibrio spp.,
Megasphaera elsdenii,
Mitsuokella multiacida,
Rosburia intestinalis,
Faecalibacterium prausnitzii, and
Eubacterium halli [41][26]. The primary source of energy for colonocytes is butyrate, which not only nourishes colonocytes but is also an essential factor that stimulates the growth and differentiation of these cells
[42][19]. In addition, SCFAs play various roles in the human body. For example, they stimulate saprophytic microorganisms’ growth while inhibiting pathogenic bacteria such as
E. coli,
Campylobacter sp. Or
Salmonella sp., which compete with saprophytic bacteria for the site of colonization
[43][134]. They also accelerate healing and support intestinal epithelium, stimulate biosynthesis of mucus in the intestinal epithelium, and maintain the correct pH in the intestines, thus contributing to the protection of the GI tract against disturbances in the intestinal microbiota
[44][45][135,136]. Furthermore, SCFAs support the maintenance of the proper intestinal barrier, e.g., by reducing the absorption of inulin, which stimulates the development of normal gut microbiota
[46][137]. SCFAs also have an anti-inflammatory effect by inhibiting the activity of inflammatory mediators in the intestinal epithelium, reducing IL-8 secretion while blocking the anti-inflammatory cytokine cascade
[47][138].
The absence of microorganisms that produce SCFAs is believed to cause multiple brain disorders. In studies in axenic mice, mice lacking bacterial microbiota exhibit severe disorders of microglial cell development
[48][139]. In turn, in mice with intestinal dysbiosis, significant changes in the functioning of these cells were observed. After colonizing mice with the microbiota of a very diverse composition, a substantial improvement in the function of microglial cells was observed
[49][50][140,141]. Interestingly, the administration of SCFAs to mice also improved the functioning of microglial cells. Furthermore, many changes in the neurotransmitter system and its receptors in various parts of the brain were observed in GF mice
[51][142]. Additionally, a significant increase in serotonin levels was observed in the hippocampus and a reduced degree of receptor expression. It is noteworthy that GF mice showed in the cerebral cortex and amygdala a decreased expression of a brain-derived neurotrophic factor (BDNF)
[52][8]. This neurotrophin has been shown to affect not only the differentiation and synaptic plasticity of neurons but also changes in different brain areas. BDNF is believed to be a factor that plays an important role in the development of mental diseases, especially the development of SCZ and MDD; however, its exact contribution to the development of these diseases is not fully known
[13][53][109,143]. Żełobowska et al.
[54][144] pointed to the reduced concentration of this active peptide as a common pathogenetic factor in cognitive impairment, depression, dementia, and type 2 diabetes. Based on samples from Alzheimer’s disease (AD) donors, decreased BDNF expression has been shown in the hippocampus, indicating its role in AD
[55][145]. The lower level of BDNF protein in the blood serum compared to healthy people has been shown in patients with SCZ by Libman-Sokołowska et al.
[56][146].
2. The Intestinal Microbiota in Neurological and Psychiatric Disorders
2.1. Depression (Major Depressive Disorder; MDD)
Depression is described as a common mental disorder state, characterized by a continuous feeling of sadness and apathy lasting at least two weeks, as a result of interactions covering social, psychological, and biological factors, for example, significant changes in life, family matters, chronic health problems, or addiction
[57][58][147,148]. It is also a common cause of disability and a cause of suicide death. According to WHO, approximately 280 million people worldwide suffer from MDD and every year, and more than 700,000 people die from committing suicide
[59][149]. The data described many factors that link this mental disorder with the components of the intestinal microbiota, which was confirmed by Naseribafrouei et al.
[60][150]. They have shown that the level of the Alistipes genus associated with inflammation and
Oscillibacter, which has valeric acid involved in is associated with depression, was elevated in patients with MDD.
Zhang et al.
[61][151] showed, using a mouse model, that microbiota dysbiosis was associated with greater intestinal permeability and systemic inflammation. As a result of endogenous melatonin reduction (EMR), the composition of the mice microbiota changed and consisted of a decrease in the relative abundance of
Bacteroidetes, an alteration of the
Firmicutes/Bacteroidetes ratio, and growth of the relative abundance of
Lactobacillus. The study also revealed improved gut permeability (leaky gut) and systemic inflammation in EMR mice.
The determination of SCFAs content could be helpful in the analysis of the microbiota composition by patients with MDD. The study concerning the SCAFs profile conducted by Skonieczna-Żydecka et al.
[62][152] on a group of 116 women aged 52.0 (±4.7) years, in which 40.52% of them recognized depression, revealed a lower level of propionic acid and a higher content of isocaproic acid compared to healthy subjects. However, due to the small group size, it cannot be conclusively stated that SCAFs contribute to the depressive phenotype. Studies on animal models revealed the connection between intestinal microbiota composition and its personalities and behavior, such as anxiety or depression. Gan et al.
[63][153] showed changes in the behavior of shy personalities of Mongolian gerbils (Meriones unguiculates) after transplantation of the gut microbiota of bold individuals. Shy gerbils often exhibited bold behavior after “bold fecal microbiota” transplantation, suggesting the association between the gut microbiota and the host’s personality.
2.2. Schizophrenia (SCZ)
Schizophrenia is a multifactorial disorder involving emotional, occupational, and cognitive impairments
[64][154]. Due to cardiovascular, metabolic, and infectious diseases, adults with SCZ are at risk of early death. For individuals in the United States with SCZ, the average potential life lost is estimated to be 28.5 years
[65][155]. According to Owen et al.
[66][156], SCZ demonstrated three different dimensions determined as positive symptoms (hallucinations and loss of contact with reality), negative symptoms (decrease in motivation and withdrawal), and cognitive weakness (limited efficiency compared to controls).
The results of biochemical and neuroimaging studies also try to explain the etiopathogenesis of SCZ. So far, it has been possible to establish some dependencies in the systems of certain neurotransmitters, which, at least to an extent, may explain the development of clinical symptoms of SCZ. The most significant seems to be the participation of dopamine
[67][157], although, in light of recent discoveries, dopamine plays a rather indirect role in this pathophysiology. At the same time, sources of SCZ should be found in the links between other neurotransmitters
[68][69][158,159]. Kozłowska et al.
[70][160] pointed to the association of immune/inflammatory processes and the etiology of SCZ, in which host peptides/proteins called alarmins activate signaling pathways, which lead to the development of many infection-induced or sterile inflammatory diseases. There is a growing amount of evidence that points to the significant role of the glutamatergic system. This mainly concerns the neuregulin 1 gene, a substance that activates NMDA receptors, located on the 8p12 chromosome, and the G72 and G30 genes located on chromosome 13q33, the first of which acts as an activator of amino acid oxidase (D-serine amino acid oxidase inhibitor—DAOA)
[71][161]. Recognition of these genes supports the neurodevelopmental concept of SCZ and the role of the glutamatergic system in this process
[72][162].
Today, it is known that both the increase in dopaminergic transmission in the mesolimbic part and the inhibition of glutamatergic transmission play an important role in the pathogenesis of positive symptoms of SCZ. Notably, the increasing number of premises proves that kynurenic acid (KYNA) may be a modulator of both these mechanisms
[73][163]. KYNA is a nonselective antagonist of ionotropic receptors for excitatory amino acids: the NMDA receptor and the kainic acid receptor, and it is the AMPA receptor and also an antagonist of the strychnine-independent glycine site in the NMDA receptor complex. Research reveals that the level of KYNA in the CSF of patients with SCZ is elevated. Thanks to KYNA research, more and more is known about its potential role in the physiology and pathology of the CNS. However, the mechanism by which KYNA affects CNS function and, thus, the clinical picture of various diseases has not been directly described. However, significant differences in KYNA concentration in sick and healthy people indicate the participation of this compound in the pathogenesis of neurological and psychiatric diseases
[74][164]. Because KYNA poorly penetrates the blood–brain barrier and is difficult to determine in the blood, scientists also pay attention to other metabolites of the kynurenine pathway. Recent studies have shown a 3-hydroxykynureine predictable concentration value concerning the reduction of psychopathological symptoms during the treatment of the first episode of SCZ. This offers great hope in finding biological factors that can predict the effectiveness of antipsychotic drugs. Unfortunately, despite advanced research, no effective cure for psychotic disorders, including SCZ, has yet been found, just as it has not been possible to clearly indicate the factors involved in the etiopathogenesis of this mysterious and still incurable disease.
2.3. Bipolar Disorder (BD)
Another serious mental illness is bipolar disorder (BD), a chronic and recurrent disease characterized by the return of hypermanic episodes or subsequent depressive episodes, with some symptoms similar to SCZ
[75][76][165,166]. Worldwide, it was estimated that in 2017–2019, 46 million people suffered from BD, with New Zealanders accounting for the most significant percentage
[77][78][167,168]. Although the pathophysiology of BD still requires some elucidation, changes in immune-inflammatory activity, oxidative and nitrosative stress (O&NS), and neuroregulatory tryptophan catabolites (TRYCATs) have been indicated as the etiology and course of BD
[79][169]. With a meta-analysis prepared by Hebbrecht et al.
[80][170], TRYCAT levels measured in cerebrospinal fluid (CSF) or serum/plasma in BD patients were lower than in healthy controls. Furthermore, the impact of the gut microbiota should be considered in the development of BD. Modifying the intestinal microbiota composition of patients with BD indicates the association between GM dysbiosis and disease progression
[81][171]. According to studies considering intestinal microbiome diversity, an increased amount of
Coriobacteriaceae was associated with a raised cholesterol level
[82][172], and an increased level of
Lactobacilli contributes to the development of obesity associated with BD
[83][173]. The low amount of
Faecalibacterium, an autochthonous intestinal bacterium, can also be correlated with diseases
[84][174]. In patients diagnosed with BD, the number of
Clostridiaceae involved in the fermentation of carbohydrates leading to the production of SCFAs was four times lower than in the control group
[85][86][87][175,176,177].
2.4. Autism Spectrum Disorder (ASD)
One of the most important and dangerous neurodevelopmental diseases related to the composition of the GI microbiota is autism spectrum disorder (ASD). According to the CDC’s Autism and Developmental Disabilities Monitoring Network (ADDM) estimation, approximately 1 in 44 children in the United States has been diagnosed with ASD
[88][89][178,179]. ASD is characterized by unconventional behavior, difficulties in communication and building relationships, and hypo- or hypersensitivity responses to environmental sensory signals
[90][28]. Some studies indicated genetic factors, GI abnormalities, inflammation, or other individual and exterior factors (e.g., pre- and postnatal exposure, stress, GI microbiota, or diet), although none of them is capable of absolutely elucidating this disorder
[91][92][93][31,180,181]. According to Azouz et al.
[94][182], in a group of 40 autistic children aged 3 to 12 years, 82.5% of them showed gastrointestinal symptoms. Furthermore, dysbiosis related to the ratio of Firmicutes and Bacteroides and the phylum number of Firmicutes, Bacteroidetes, Fusobacteria, and Verrucomicrobia was demonstrated in patients diagnosed with ASD
[92][180]. In the same study, the authors revealed that in patients with ASD, the changes also affect the level of SCFAs and volatile organic compounds (VOC), including, among others, indole, which is a metabolite of tryptophan and the precursor of serotonin and melatonin. However, these data should be carefully interpreted due to the possible influence of antibiotic treatment or personalized diet in patients with ASD
[19][29]. To clearly define the role of the gut microbiota in patients, further research and an entire view to connect dependences between GM and ASD are needed.
2.5. Attention-Deficit Hyperactivity Disorder (ADHD)
A common neurodevelopmental disorder is attention deficit hyperactivity disorder (ADHD), which affects 6 million children aged 3–17 years
[95][183]. It manifests in difficulties maintaining attention, and sudden, unexpected behavior
[96][184]. The genes for the dopamine receptors DRD4 and DRD5 and dopamine and serotonin transmitters are considered the main etiological factors of this disease
[97][185]. A growing amount of evidence indicates the connection with the microbiome–gut–brain axis
[98][99][186,187]. In the microbiome study conducted by Aarts et al.
[100][188], 96 participants participated, of whom 19 had been diagnosed with ADHD and 77 were healthy. Results revealed differences in taxa, where in ADHD cases, the phylum Actinobacteria was more abundant (for example,
Bifidobacterium), while the abundance of Firmicutes decreased. Interestingly, the same study revealed that the microbiome of cases of ADHD shows a more remarkable ability to produce cyclohexadienyl dehydratase (CDT), which is involved in synthesizing the dopamine precursor (phenylalanine).
On the contrary, the meta-analysis prepared by Wang et al.
[101][189], which evaluated the intestinal microbiota and ADHD, did not show significant differences at the phylum and family levels beyond the higher level of
Blautia in patients with ADHD compared to healthy control. This microorganism plays a regulatory role in metabolic and inflammatory diseases, as well as biotransformation
[102][190]. Future research that specifies the connection between the microbiome–gut–brain axis and ADHD should cover a larger demographically diverse study group
[103][191]. The risk of developing neuropsychiatric disorders could be reduced through probiotic supplementation in early life. Pärtty et al.
[104][192] showed that early administration of
Lactobacillus rhamnosus GG might decrease the risk of ADHD. It was shown that
L. rhamonsus, through the vagus nerve, regulated emotional behavior and the central GABA receptor expression in a mouse
[105][193]. In addition, the influence of dietary patterns on ADHD patients is important. Since food contains artificial color additives, to decrease the hyperactive behavior of ADHD patients, it is necessary to exclude such products from the diet
[106][194]. Intake of omega-3 PUFAs is also significant, in particular, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), as both are essential for proper membrane fluidity, neurotransmission, and receptor function
[107][195]. In a study based on animal male models of ADHD, nutrition individuals with a diet enriched in omega-3 PUFAs caused decreased impulsivity and improved concentration
[108][196].