Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Naveed Ahmed Khan and Version 2 by Rita Xu.
Gut microbial composition codevelops with the host from birth and is influenced by several factors, including drug use, radiation, psychological stress, dietary changes and physical stress. Importantly, gut microbial dysbiosis has been clearly associated with several diseases, including cancer, rheumatoid arthritis and
Clostridium difficile
-associated diarrhoea, and is known to affect human health and performance.
- gut microbiota
- microbiota modulation
- microbiota development
1. Introduction
The link between human health and the gut microbiome is profound and has been speculated upon for thousands of years. In 400 B.C., it was suggested by Hippocrates that “bad digestion is the root of all evil” and “death sits in the bowels” [1]. It is now well-known that humans are inhabited by microorganisms, including bacteria, viruses and archaea, that live in harmony with them, known as microflora, microbiota or normal flora [2][3][2,3]. It is estimated that the human microbiome is comprised of a hundred trillion bacteria cells, which amounts to ten times more than there are human cells in the human body [2][4][2,4]. With a surface area of 200 m2 to 300 m2 [5], the gut alone accounts for over 70% of the human microbiota [3]. During the average human lifespan, along with approximately 60 tonnes of food, a plethora of microorganisms from the environment pass through the human gastrointestinal (GI) tract, and this results in coevolution forming intricate and mutually beneficial relationships, leading to the formation of the “gut microbiota” [6]. The gut microbial composition develops concomitantly with the host from birth and is influenced by several factors, including genetic predisposition and nutrition [7]. Even within the gut microbiota, striking differences are observed at different locations such as the duodenum, ileum and colon [8]. In addition, alterations in the gut microbiota may be influenced by environmental factors, toxins, pathogens, diet and drugs [9]. While the gut microbiota is present in the digestive tract, its effect is not limited to that location and is even known to modulate brain development in mammals and later behaviour in adults [10]. Many aspects of human health are also influenced by the gut microbiota, as they may provide energy and nutrients to the host by aiding the digestion of nondigestible dietary components and can also contribute to inflammation, infection, gastrointestinal diseases, diabetes mellitus and obesity [11]. Interestingly, the composition of the human gut microbiota shifts with age, leading to influenced changes in the host’s health [12]. In this regard, the disturbance of the microbiome has been suggested as a new hallmark of ageing in the recent meeting held in Copenhagen on March 2022, in addition to the original nine hallmarks of ageing proposed by López-Otín and colleagues in 2013 [13].
2. Gut Microbiota Development and Composition
Formerly, it was thought that initial contact with microbial species takes place during birth, as was proposed by Tissier in 1900. The placenta was thought to prevent microorganisms from entering the bloodstream of the fetus, thus maintaining a sterile environment [14][15][14,15]. Nonetheless, recently it has emerged that diverse microbial communities may exist in human semen and in the womb [14][16][14,16]. Furthermore, a variety of other reports state that microbial species may inhabit diverse niches, comprising the placenta, umbilical cord blood and amniotic fluid, suggesting that select microbes may colonize in utero [14][17][18][19][14,17,18,19]. A recent study was conducted in mice whereby pregnant mice were colonized with genetically labelled Enterococcus faecium, that was isolated from human breast milk; the labelled strain was successfully cultured from the amniotic fluid of mice 2 days before full term was achieved [17]. However, even though the findings suggest transfer of some microbial species prior to birth, the results remain controversial because of the probability of contamination while assessing the specimens, and so prospective studies are necessitated [20]. Nonetheless, if this is the case, such microbial studies could be extrapolated and studied in order to determine their unique properties.
The human gut microbiota typically undergoes an age-affiliated microbiota shift from 3 days after birth to 2, 3, 4 or 5 years later when the gut microbiota reaches an adult-like configuration [21][22][23][24][21,22,23,24]. Effects of the microbiota on the host can be both beneficial and harmful, and while it is estimated that 40% of the microbiota is yet to be identified or cultured, links between the gut microbiota composition and human health have been established, even in infants [25]. For example, in infants, reduced numbers of bifidobacterial species have been linked to atopic sensitization and future development of obesity, while increased numbers of Clostridium difficile have been linked to colicky infants [26][27][28][26,27,28].
Initially, the gut microbiota is influenced by prenatal factors, including maternal diet, antibiotic use during pregnancy, maternal stress, smoking status and obesity [29][30][29,30]. The microbiota is then affected by the type of birth, mainly vaginally and through Caesarean section, whereby vaginally delivered children, as compared to those delivered through Caesarean section, displayed lower rates of asthma, atopic symptoms and diabetes, while interestingly, specific colonization of the microbiota in children delivered through Caesarean section was delayed by up to one 1 month and the diversity and number of colonies in their microbiota were lower [31][32][33][34][31,32,33,34]. Newborns are typically exposed to vaginal microbes that are typically dominated by Lactobacilli and Prevotella spp. [35]. The microbiome of babies born via Caeserean section is normally dominated by Staphylococcus, Propionibacterium spp. and Corynebacterium [35][36][35,36]. Whether modulation of these microbiomes at an earlier stage can affect ageing and the precise manner in which this may occur needs to be further investigated. For example, inoculation with maternal vaginal microbiota immediately following C-section delivery is denoted as “vaginal seeding” [37]. Nonetheless, there are limitations pertaining to this method as there is a lack of comprehension of the precise mechanisms in early microbiota initialization and maturation. A major limitation is the ability to study neonates in sufficient number. Moreover, the link between impaired maternal microbiota transfer at birth and acute or chronic illnesses in infants born by C-section needs to be determined, and future work is needed.
The admixture of compounds and antigens in breast milk, together with its range of bifidobacteria and lactobacilli strains, are the next factors that may affect the development of the microbiota [25]. A recent study revealed that having older siblings positively impacted bacterial diversity and richness in 18-month infants, inducing effects that are more pronounced than early-life infections [38]. Notably, the administration of antibiotics in infants also affects the development of their microbiome transiently or persistently [39]. Autochthonous bacteria—indigenous bacteria of the gut flora—inhibit the growth of allochthonous species—bacteria from water, food or other organs—through the production of specific substances, which is partly responsible for keeping the gut flora constant [40].
3. Gut Microbiota Modulation
While it is known that following initial colonization, the gut flora of humans remains mostly constant throughout their adult life [41], evidence of intestinal microbiota changes in adult humans have been reported and linked to specific phenomenon, including drug use, radiation, psychological stress, dietary changes, altered gastrointestinal tract peristalsis and physical stress (Figure 1) [1][42][1,42].
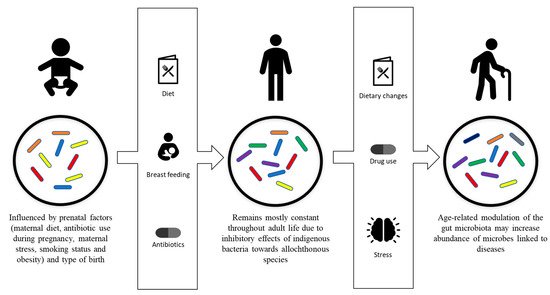
Figure 1. Development and modulation of the gut microbiota. Gut microbiota is initially influenced by prenatal factors, including maternal diet, antibiotic use during pregnancy, maternal stress, smoking status, obesity and type of birth. Breastfeeding, diet and antibiotic usage are the factors that affect the development of the gut microbiota into its stable adult composition. The adult composition of the gut microbiota may be modulated by drug usage, dietary changes, psychological stress and physiological stress.
The most commonly used administration route for drugs is the oral route, owing to the fact that it allows for the uptake of drugs without medical intervention, which exposes the gut microflora to drugs [43]. For example, conventional anti-inflammatory drugs such as naproxen, ibuprofen and aspirin, when taken daily over months, affect intestinal microbial composition [44]. In addition, proton-pump inhibitors that are often taken in combination with anti-inflammatory drugs to reduce the formation of stomach ulcers have been reported to alter the gut microbiome [45]. The impact of drugs on intestinal microbes is not limited to anti-inflammatory drugs and has also been linked to other type of medications, such as the use of metformin in the treatment of type 2 diabetes, which has been shown to change the gut microbiota composition [46].
However, the most significant and common source of changes in normal gut microbiota has been linked to antibiotic use [47][48][47,48]. Pharmacokinetics, spectrum of activity, length of administration and dosage are some of the factors of antibiotic usage that influence the impact of the antimicrobial agent on the gut flora [1]. Antimicrobial agents may severely impact the gut microbiota, leading to an overgrowth of microorganisms, such as fungi, that are already present [47][49][47,49]; a decrease in the production of short-chain fatty acids, responsible for electrolyte and water absorption, leading to electrolyte imbalances [50][51][50,51]; and/or a decrease in colonization resistance by autochthonous bacteria, resulting in increased susceptibility to intestinal pathogens [52][53][52,53]. While most orally administered antimicrobial agents will trigger changes in the gut microbiota, the effects might be transient or long-lasting [43]. For example, some antibiotics, such as amoxicillin, do not have long-term impacts on the gut microbiome [54], while others, such as ciprofloxacin, leave a long-lasting signature [55]. Furthermore, in elderly people, it has been observed that repeated exposure to antibiotics may lead to destabilization of the gut flora resulting in antibiotic-resistant pathogenic bacteria outgrowth [56].
Moreover, it has been demonstrated that the integrity of indigenous microflora can be altered for several days due to psychological stress [57]. Changes in the gut microbiota of Soviet cosmonauts were also identified and linked primarily to stress and increased risk of colonization by pathogenic microorganisms [58]. It is now recognized that microgravity environments can affect gut microbial composition [59]. In addition, 20% to 30% changes in levels of some bacterial species were revealed in faeces of humans when experiencing anger or fear, and concentrations of the bacteria returned to normal after the resolving the situations [60]. Furthermore, a recent study that examined changes in the gut microbiome of a world-class ultramarathon runner before and after competing revealed shifts in human gut microbiome composition after acute exercise [61]. In another study, it was demonstrated that gut microbial modulation with probiotics during a stressful ship voyage to Antarctica was able to regulate gut microbiota homeostasis and reduce sea sickness prevalence as well as other physiological complications [62].
Nutrition can also affect the gut microbiota, as variations in food intake may cause both transient changes and cause shifts in specific bacteria leading to typical signatures [63][64][63,64]. It has been shown that the level, both low and high, of uptake of animal proteins and amino acids modulates the levels of certain bacteria and increases the activity of certain bacterial enzymes [65][66][67][65,66,67]. The amount of carbohydrates or sugars in regimes also affects the gut microbiota [1][43][1,43]. Food rich in sulphur also modulates the microbiota by promoting the growth of sulphate-reducing bacteria [68]. Fibres in food also have a predominant role in variation of the gut microbiota, since they are indigestible and cause microbial fermentation [43]. Moreover, it has been shown that a Western diet, comprising of higher fat/higher sugar and higher processed food intake, can lead to gut microbial changes [69][70][69,70].
