Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Zhendong Li.
Advanced oxidation process (AOPs) based on sulfate radical (SO4●−) and singlet oxygen (1O2) has attracted a lot of attention because of its characteristics of rapid reaction, efficient treatment, safety and stability, and easy operation. SO4●− and 1O2 mainly comes from the activation reaction of peroxymonosulfate (PMS) or persulfate (PS), which represent the oxidation reactions involving radicals and non-radicals, respectively. The degradation effects of target pollutants will be different due to the type of oxidant, reaction system, activation methods, operating conditions, and other factors.
- sulfate radical
- singlet oxygen
- AOPS
- peroxymonosulfate
1. Characteristics of Persulfate and Peroxymonosulfate
At present, the production of SO4●− mainly comes from the activation of PMS and PS, which are the monosubstituted or symmetrically substituted derivatives of hydrogen peroxide by sulfonic acid group (-SO3), respectively. PMS has been widely used in organic compound synthesis and as a chlorine-free additive for disinfecting swimming pools at a rate of about 1–2 pounds per 10,000 gallons of pool water [11][1]. Under the presence of 25 mg/L PMS and 0.1 mg/L Co2+, the removal rate of E. coli reached 99.99% after 1 h of reaction [12][2]. PMS is white solid powder. It is stable when pH is less than 6 or pH is 12. When pH is 9, it showed the poorest stability where half of HSO5− decomposes to SO52− [4][3]. At present, the widely used potassium bisulfate complex salt (2KHSO5·KHSO4·K2SO4) is composed of three components, potassium peroxymonosulfate, potassium hydrogen sulfate, and potassium sulphate, and its main active substance is potassium peroxymonosulfate (KHSO5). The salt is marketed under the trade names Caroat and Oxone registered by Evonik and DuPont, respectively. Oxone is a white granular powder crystal salt, which is stable, non-toxic, inexpensive, and soluble in water. The peroxide bond (O-O) distance is 1.453 Å, and the bond energy is 140–213.3 kJ/mol. PMS is most stable when the solution pH is less than 6 and equal to 12. When pH is 9, the stability is worst, and the concentration of HSO5− and SO52− in the solution is almost equal. When pH is less than 1, PMS will undergo hydrolysis reaction to produce H2O2 [13][4]. PS, an oxidant with symmetrical structure, was first known as the initiator of polymerization reaction. The O-O distance is 1.497 Å, and the bond energy is 140 kJ/mol [14][5]. PS, often in the form of potassium persulfate or sodium persulfate, has been widely used as bleaching agents, oxidants, emulsion polymerization promoters, and water or soil remediation agents. The related properties of PMS and PS are shown in Table 1. Both PMS and PS are strong oxidants, but their direct reaction rates with most pollutants are very low. Therefore, it is necessary to activate them through appropriate ways to destroy the O-O bond and generate strong oxidizing free radicals, 1O2 and other ROS to degrade organic pollutants quickly and efficiently.
Table 1.
Properties of PMS and PS.
| Properties | PS (Take Potassium Persulfate as an Example) | PMS (Take Potassium Peroxymonosulfate as an Example) | ||||||
|---|---|---|---|---|---|---|---|---|
| CAS Registry Number | 7727-21-1 | 10058-23-8 | ||||||
| Chemical formula | K | 2 | S | 2 | O | 8 | KHSO | 5 |
| Molecular mass | 270.309 | 614.738 | ||||||
| Solubility in water (20 °C) | 520 g/L | >250 g/L | ||||||
| Redox potential | 2.01 V | 1.82 V |
Because PS and PMS are solid powders, they can be transported and stored more easily. Compared with H2O2, the anions of PS and PMS remain stable in water for a much longer time until they are properly activated. In addition, PMS and PS-based AOPs can proceed smoothly in a wide solution pH range from acidic to alkaline (pH = 2–10), while H2O2-based Fenton process requires strict acidic conditions (pH = 2.7–3). Generally, PS and PMS can be activated with the assistance of ultraviolet light, heat, alkali, or metal catalysts, etc. Different types of oxidants and activation methods will produce different ROS. The advanced oxidation processes dominated by SO4●− and 1O2 were discussed in this preseaperrch.
advanced oxidation process (AOPs) based on sulfate radical (SO4●−) and singlet oxygen (1O2) has attracted a lot of attention because of its characteristics of rapid reaction, efficient treatment, safety and stability, and easy operation. SO4●− and 1O2 mainly comes from the activation reaction of peroxymonosulfate (PMS) or persulfate (PS), which represent the oxidation reactions involving radicals and non-radicals, respectively. The degradation effects of target pollutants will be different due to the type of oxidant, reaction system, activation methods, operating conditions, and other factors.
2. Sulfate Radicals-Based Advanced Oxidation
Studies have shown that AOPs based on SO4●− and ●OH is an effective method for the degradation of refractory organic pollutants, such as pharmaceuticals, pesticides, personal care products, steroids, endocrine disruptors, etc. [15][6]. However, AOPs based on ●OH degrades organic pollutants through a non-selective, multi-step approach that typically requires an acidic environment. In addition, the oxidation process is severely limited by the large amount of dissolved organic matter and anions in complex environments, which are the main scavengers of ●OH. SO4●− is inherently more oxidizing than ●OH and lasts longer in aqueous solutions, and in some cases SO4●− can oxidize contaminants that ●OH cannot. In recent years, SO4●−-based AOPs have replaced ●OH-based AOPs to some extent. The essence of the advanced oxidation process based on SO4●− is to activate PMS or PS to form SO4●− to achieve the removal of organic matter. Besides SO4●−, there are also associated or indirect generation of ●OH, superoxide radical (O2●−), or other radicals, but SO4●− plays a leading role in the degradation of pollutants. These free radicals (SO4●−, ●OH, and O2●−) could be detected by using 5,5-dimethyl-1-pyrroline-1- oxide (DMPO) as a spin trapping agent in an electron paramagnetic resonance spectroscopy (EPR) [16][7]. Both ethanol (Et) and tert-butanol (TBA) could quench ●OH rapidly (kEt = 1.2–2.8 × 109 M−1s−1, kTBA = 3.8–7.6 × 108 M−1s−1), and the reaction rate between Et and SO4●− is much faster than that of TBA (kEt = 1.6–7.7 × 107 M−1s−1, kTBA = 4.0–9.1 × 105 M−1s−1). Therefore, Et and TBA can be used as capture agents to identify who contributes more to the degradation of pollutants [17][8]. PMS and PS can be activated to produce SO4●− through energy input, transition metal ions and their oxides, non-metallic materials, etc. Studies on the degradation of pollutants by activating PMS and PS in different ways to generate SO4●− are summarized in Table 2.Table 2.
PMS and PS radical activation with various method for the removal of pollutants.
| Reaction System | Pollutant | Conditions | Reactivity | Dominant ROS | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV(254 nm)/PMS | benzoic acid (BA) | [BA] = 9.90 μM; [PMS] = 100 μM as 1/2 Oxone; pH = 11. |
>90% with 10 min | SO | 4●− | and | ● | OH | [18] | [9] | ||||||||
| UV(254 nm)/PS | Chlorophene | [Chlorophene] = 1 μM, [PS] = 50 μM, pH = 7, UV intensity (254 nm,15 W) = 4.23 mWcm | −2 | . | 100% with 5 min | SO | 4●− | [19] | [10] | |||||||||
| UV-C laser/PS | Iohexol (IOX) | I | UV | = 25 mW/cm | 2 | , [PS] = 1.0 mM, [IOX] = 10 μM, initial pH = 7.0 ± 0.5, temperature 25 °C. | 93.8% within only 40 s | SO | 4●− | [20] | [11] | |||||||
| Heat/PS | Ibuprofen (IBU) | [phosphate buffer] = 0.09 M, pH = 7.0, [IBU] = 20.36 μM, [PS] = 1.0 mM, T = 70 °C. | 100% with 20 min | SO | 4●− | and | ● | OH | [21] | [12] | ||||||||
| Heat/PS | 1-alkyl-3-methylimidazolium bromides (C4mimBr) | [C | 4 | mimBr] = 0.1 mM, [PS] = 10 mM, T = 60 °C, pH = 7, V = 100 mL. | 100% with 120 min | SO | 4●− | [22] | [13] | |||||||||
| Ultrasound/PS | 1,1,1-trichloroethane (TCA) | [TCA] = 25.0 mg/L, [PS] = 250.0 mg/L pH 7.0, T = 20 ± 2 °C, ultrasound: 400 kHz, 100 W. | 100% with 120 min | SO | 4●− | [23] | [14] | |||||||||||
| Electrolysis(boron-doped diamond anode)/PS | Ampicillin | [Ampicillin] = 1.1 mg/L, [PS] = 250 mg/L, current density (BDD anode) = 25 mAcm | −2 | . | 100% with 120 min | SO | 4●− | and | ● | OH | [24] | [15] | ||||||
| Fe | 0 | /PS | sulfamethoxazole (SMX) | [SMX] = 39.5 μM, [PS] = 1.0 mM, [Fe | 0 | ] = 2.23 mM; m (Fe | 0 | ) = 2.5 mg, pH = 3.52. | 100% with 30 min | SO | 4●− | [25] | [16] | |||||
| Fe | 2+ | /PS | Acetaminophen (ACT) | [ACT] = 0.05 mM, [Fe | 2+ | ] = 1 mM, [PS] = 0.8 mM, pH = 3, T = 20 °C. | 70% with 30 min | SO | 4●− | [26] | [17] | |||||||
| Fe(II)/PMS | 2-chlorobiphenyl (2-CB) | [2-CB] = 0.0212 mM; [Fe(II)] = 0.11 mM; [PMS] | 0 | = 0.11 mM. | 90% with 240 min | SO | 4●− | [27] | [18] | |||||||||
| Co | 2+ | /PMS | nuclear grade cationic IRN-77 resin | initial pH = 9, [Co | 2+ | ] = 4 mM, [PMS] = 60 mM T = 60 °C. | ∼90% COD removal (1000 mg/L) with 60 min | SO | 4●− | and | ● | OH | [28] | [19] | ||||
| Cu | 2+ | /PMS | Triclosan (TCS) | [TCS] = 9 mg/L (0.031 mM), initial pH = 7, molar ratio of oxidant to metal = 1:1, molar ratio of oxidant to triclosan = 5:1. | 95% with 10 min | SO | 4●− | [29] | [20] | |||||||||
| Ru | 3+ | /PMS | 2,4-dichlorophenol (2,4-DCP) | [2,4-DCP] = 0.311 mM, [RuCl | 3 | ·xH | 2 | O] = 2.553 mM, [KHSO | 5 | ] = 1.244 mM, pH = 7. | 98% in less than 1 min | SO | 4●− | [30] | [21] | |||
| Natural chalcopyrite/PMS | Bisphenol S | Bisphenol S = 25 μM, chalcopyrite = 2 g/L, PMS = 0.4 mM, initial pH = 6.2, T = 303 K. | 83% with 30 min | SO | 4•− | and | • | OH | [31] | [22] | ||||||||
| Eggshell-loaded CoFe | 2 | O | 4. | /PMS | Florfenicol (FF) | CoFe | 2 | O | 4 | /eggshell = 0.4 g/L, [PMS] = 0.96 mmol/L, [FF] = 10 mg/L, T = 30 °C, initial pH = 6.61. | 96.8% within 40 min | SO | 4•– | and | • | OH | [32] | [23] |
| CoFe layered double oxide/g-C | 3 | N | 4 | /PMS | paracetamol | [catalyst] = 0.2 g/L, [PMS] = 0.5 mM, [paracetamol] = 10 mg/L, temperature = 25 ± 0.5 °C, initial pH = 7 ± 0.2. | 100% in less than 10 min | SO | 4●− | [33] | [24] | |||||||
| Co | 3 | O | 4 | /PMS | Acid Orange 7 | [AO7] = 0.2 mM, [PMS] = 2 mM and [nano-Co | 3 | O | 4 | ] = 0.5 g/L, pH = 7. | 100% with 60 min | SO | 4●− | [34] | [25] | |||
| Co-MCM41 | Caffeine (CAF) | [CAF] = 0.05 mM, [PMS] = 0.2 mM, [catalyst] = 200 mg/L, pH = 7.10. | 100% with 15 min | SO | 4•– | and | • | OH | [35] | [26] | ||||||||
| Ag | 0.4 | -BiFeO | 3 | /PS | tetracycline (TC) | [catalysts] = 300 mg/L, [PS] = 5 mM, [TC] = 10 mg/L, pH = 4.5, T = 298 K. | 91% with 60 min | SO | 4●− | and | ● | OH | [36] | [27] | ||||
| S-doped α-Fe | 2 | O | 3 | /PS | carbamazepine (CBZ) | [CBZ] = 2 mg/L, [PS] | 0 | = 0.2 mM, [catalyst] = 0.2 g/L, T = 25 ± 1 °C and initial pH = 6.8 ± 0.5. | 93.13% with 30 min | SO | 4●− | [37] | [28] | |||||
| Fe | 3 | O | 4 | @Zn/Co-ZIFs/PMS | carbamazepine (CBZ) | [CBZ] = 5 mg/L, [catalyst] = 25 mg/L, [PMS] = 0.4 mM, initial pH = 6.8, T = 30 °C. | 100% with 30 min | SO | 4●− | [38] | [29] | |||||||
| Co | 3 | O | 4 | /C-BC/PMS | Bisphenol A (BPA) | [catalyst] = 0.3 g/L, [pollutant] = 20 mg/L, [PMS] = 1.0 mmol/L, [pH] = 7.0, [T] = 30 °C. | 100% within less than 30 min | SO | 4●− | [39] | [30] | |||||||
| ZnO/biochar/PS | tetracycline hydrochloride (TC) | [TC] = 0.05 g/L, [ZnO200/BC] = 0.1 g/L, [PS] = 1.0 mM, pH = 7.0 ± 0.1, T = 25 ± 2 °C. | 44.98% with 50 min | SO | 4●− | and | ● | OH | [40] | [31] | ||||||||
| microwave irradiation(MW)/CuO/PS | 2,4-dichlorophenol (2,4-DCP) | [PS] = 0.4 g/L, [CuO] = 40 mg/L, (2,4-DCP) = 50 mg/L, initial pH = 9, MW power intensity = 180 W. | >98% with 90 min | SO | 4●− | [41] | [32] | |||||||||||
| Ultrasound/Fe | 0 | /PS | Sulfadiazine (SD) | [SD] = 20 mg/L, [Fe | 0 | ] = 0.92 Mm, [PS] = 1.84 mM, US input power = 90 W, initial pH = 7, room temperature. | 99.1% with 60 min | SO | 4●− | [42] | [33] | |||||||
| granular activated carbon (GAC)/PMS | Acid Orange 7 (AO7) | [AO7] = 20 mg/L, [PMS]: [AO7] = 100:1, [GAC] = 1.0 g/L, without pH adjustment, T = 20 ± 0.5 °C. | 85% with 5 h | SO | 4●− | [43] | [34] | |||||||||||
| Fe | 3 | O | 4 | @Graphene oxide (GO)/PS | Rhodamine B (RhB) | [RhB] = 20 ppm, [Fe | 3 | O | 4 | @GO] = 500 mg/L, [PS] = 1.5 mM, pH = 4.34, T = 20 °C. | 89% with 120 min | SO | 4●− | [44] | [35] | |||
| alkali and CuO/PS | Cu-ethylenediamine tetraacetic acid (Cu(II)-EDTA) | [Cu(II)-EDTA] = 3.14 mM, [PS]/[Cu(II)-EDTA] = 15:1, [CuO] = 2 g/L, pH maintained at 11. | Nearly 100% with 120 min | SO | 4•− | , | • | OH and O | 2•− | [45] | [36] |
3. Singlet Oxygen-Based Advanced Oxidation
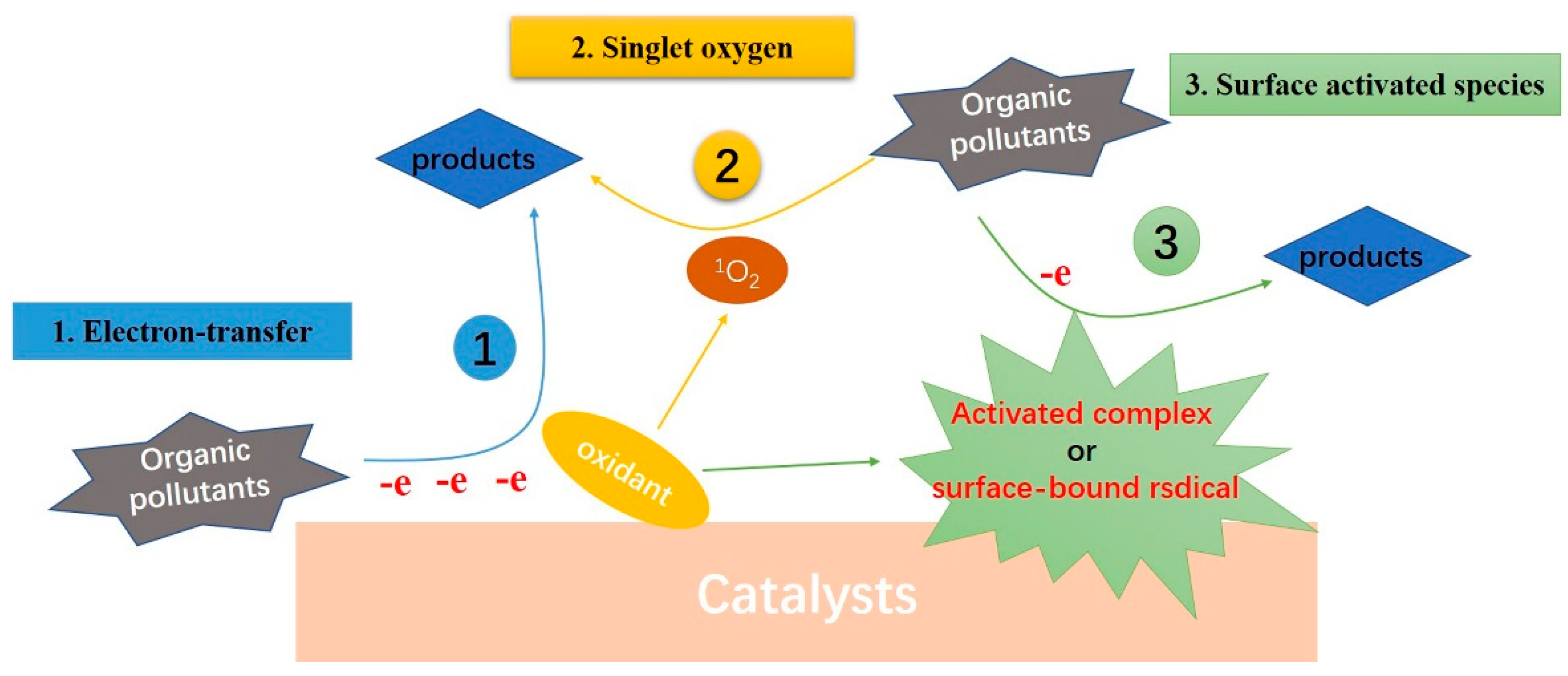
With the report of PMS and PS non-radical activation, people gradually have a strong interest in the process of non-radical oxidation. Non-radical reactive species are generally thought to be resistant to common free radical scavengers (e.g., methanol, ethanol, and tert-butanol), selective to electron-rich organic compounds, and particularly sensitive to organic substrates with mild redox potentials [8][37]. At present, non-radical oxidation processes have been found in a variety of reaction systems, especially in PMS and PS activation. However, the mechanism remains controversial. Based on current reports, carbon or metal catalysts mainly realize non-radical activation of oxidants (PMS, PS, H2O2, or O3) through three ways: electron transfer process, generation of activated complexes (or surface-bound radicals) and singlet oxygen participating in pollutant degradation, respectively, as shown in Figure 41. Among them, 1O2 shows significant advantages in the selective removal of organic pollutants, which has become the focus of research.
Figure 41.
Schematic diagram of organic pollutants degradation by non-radical AOPs (Oxidant can be PMS, PS, H
2
O
2
or O
3).
).
References
- Mirza-Aghayan, M.; Tavana, M.M.; Boukherroub, R. Direct oxidative synthesis of nitrones from aldehydes and primary anilines using graphite oxide and Oxone. Tetrahedron Lett. 2014, 55, 5471–5474.
- Anipsitakis, G.P.; Tufano, T.P.; Dionysiou, D.D. Chemical and microbial decontamination of pool water using activated potassium peroxymonosulfate. Water Res. 2008, 42, 2899–2910.
- Xiao, G.; Xu, T.; Faheem, M.; Xi, Y.; Zhou, T.; Moryani, H.; Bao, J.; Du, J. Evolution of Singlet Oxygen by Activating Peroxydisulfate and Peroxymonosulfate: A Review. Int. J. Environ. Res. Public Health 2021, 18, 3344.
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62.
- House, D.A. Kinetics and Mechanism of Oxidations by Peroxydisulfate. Chem. Rev. 1962, 62, 185–203.
- Tang, L.; Ma, X.Y.; Wang, Y.; Zhang, S.; Zheng, K.; Wang, X.C.; Lin, Y. Removal of trace organic pollutants (pharmaceuticals and pesticides) and reduction of biological effects from secondary effluent by typical granular activated carbon. Sci. Total Environ. 2020, 749, 141611.
- Bu, Z.; Hou, M.; Li, Z.; Dong, Z.; Zeng, L.; Zhang, P.; Wu, G.; Li, X.; Zhang, Y.; Pan, Y. Fe3+/Fe2+ cycle promoted peroxymonosulfate activation with addition of boron for sulfamethazine degradation: Efficiency and the role of boron. Sep. Purif. Technol. 2022, 298, 121596.
- Ding, Y.; Zhu, L.; Wang, N.; Tang, H. Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Appl. Catal. B Environ. 2012, 129, 153–162.
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314.
- Acero, J.L.; Benítez, F.J.; Real, F.J.; Rodríguez, E. Degradation of selected emerging contaminants by UV-activated persulfate: Kinetics and influence of matrix constituents. Sep. Purif. Technol. 2018, 201, 41–50.
- Dong, Z.-Y.; Xu, B.; Hu, C.-Y.; Zhang, T.-Y.; Tang, Y.-L.; Pan, Y.; El-Din, M.G.; Xian, Q.-M.; Gao, N.-Y. The application of UV-C laser in persulfate activation for micropollutant removal: Case study with iodinated X-ray contrast medias. Sci. Total Environ. 2021, 779, 146340.
- Ghauch, A.; Tuqan, A.M.; Kibbi, N. Ibuprofen removal by heated persulfate in aqueous solution: A kinetics study. Chem. Eng. J. 2012, 197, 483–492.
- Ren, T.-L.; Ma, X.-W.; Wu, X.-Q.; Yuan, L.; Lai, Y.-L.; Tong, Z.-H. Degradation of imidazolium ionic liquids in a thermally activated persulfate system. Chem. Eng. J. 2021, 412, 128624.
- Li, B.; Li, L.; Lin, K.; Zhang, W.; Lu, S.; Luo, Q. Removal of 1,1,1-trichloroethane from aqueous solution by a sono-activated persulfate process. Ultrason. Sonochem. 2013, 20, 855–863.
- Frontistis, Z.; Mantzavinos, D.; Meriç, S. Degradation of antibiotic ampicillin on boron-doped diamond anode using the combined electrochemical oxidation—Sodium persulfate process. J. Environ. Manag. 2018, 223, 878–887.
- Ayoub, G.; Ghauch, A. Assessment of bimetallic and trimetallic iron-based systems for persulfate activation: Application to sulfamethoxazole degradation. Chem. Eng. J. 2014, 256, 280–292.
- Wang, S.; Wu, J.; Lu, X.; Xu, W.; Gong, Q.; Ding, J.; Dan, B.; Xie, P. Removal of acetaminophen in the Fe2+/persulfate system: Kinetic model and degradation pathways. Chem. Eng. J. 2018, 358, 1091–1100.
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179.
- Hafeez, M.A.; Hong, S.J.; Jeon, J.; Lee, J.; Singh, B.K.; Hyatt, N.C.; Walling, S.A.; Heo, J.; Um, W. Co2+/PMS based sulfate-radical treatment for effective mineralization of spent ion exchange resin. Chemosphere 2022, 287, 132351.
- Nfodzo, P.; Choi, H. Triclosan decomposition by sulfate radicals: Effects of oxidant and metal doses. Chem. Eng. J. 2011, 174, 629–634.
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712.
- Peng, J.; Zhou, H.; Liu, W.; Ao, Z.; Ji, H.; Liu, Y.; Su, S.; Yao, G.; Lai, B. Insights into heterogeneous catalytic activation of peroxymonosulfate by natural chalcopyrite: pH-dependent radical generation, degradation pathway and mechanism. Chem. Eng. J. 2020, 397, 125387.
- Gao, Y.; Han, Y.; Liu, B.; Gou, J.; Feng, D.; Cheng, X. CoFe2O4 nanoparticles anchored on waste eggshell for catalytic oxidation of florfenicol via activating peroxymonosulfate. Chin. Chem. Lett. 2022, 33, 3713–3720.
- Shen, Y.; de Vidales, M.J.M.; Gorni, G.; Gómez-Herrero, A.; Fernández-Martínez, F.; Dos Santos-García, A.J. Enhanced performance and recyclability for peroxymonosulfate activation via g-C3N4 supported CoFe layer double oxide. Chem. Eng. J. 2022, 444, 136610.
- Chen, X.; Chen, J.; Qiao, X.; Wang, D.; Cai, X. Performance of nano-Co3O4/peroxymonosulfate system: Kinetics and mechanism study using Acid Orange 7 as a model compound. Appl. Catal. B Environ. 2008, 80, 116–121.
- Qi, F.; Chu, W.; Xu, B. Catalytic degradation of caffeine in aqueous solutions by cobalt-MCM41 activation of peroxymonosulfate. Appl. Catal. B Environ. 2013, 134–135, 324–332.
- Ouyang, M.; Li, X.; Xu, Q.; Tao, Z.; Yao, F.; Huang, X.; Wu, Y.; Wang, D.; Yang, Q.; Chen, Z.; et al. Heterogeneous activation of persulfate by Ag doped BiFeO3 composites for tetracycline degradation. J. Colloid Interface Sci. 2020, 566, 33–45.
- Deng, J.; Ye, C.; Cai, A.; Huai, L.; Zhou, S.; Dong, F.; Li, X.; Ma, X. S-doping α-Fe2O3 induced efficient electron-hole separation for enhanced persulfate activation toward carbamazepine oxidation: Experimental and DFT study. Chem. Eng. J. 2021, 420, 129863.
- Wu, Z.; Wang, Y.; Xiong, Z.; Ao, Z.; Pu, S.; Yao, G.; Lai, B. Core-shell magnetic Fe3O4@Zn/Co-ZIFs to activate peroxymonosulfate for highly efficient degradation of carbamazepine. Appl. Catal. B Environ. 2020, 277, 119136.
- Chen, X.-L.; Li, F.; Zhang, M.; Liu, B.; Chen, H.; Wang, H. Highly dispersed and stabilized Co3O4/C anchored on porous biochar for bisphenol A degradation by sulfate radical advanced oxidation process. Sci. Total Environ. 2021, 777, 145794.
- Xu, R.; Li, M.; Zhang, Q. Collaborative optimization for the performance of ZnO/biochar composites on persulfate activation through plant enrichment-pyrolysis method. Chem. Eng. J. 2021, 429, 132294.
- Ghorbanian, Z.; Asgari, G.; Samadi, M.T.; Seid-Mohammadi, A. Removal of 2,4 dichlorophenol using microwave assisted nanoscale zero-valent copper activated persulfate from aqueous solutions: Mineralization, kinetics, and degradation pathways. J. Mol. Liq. 2019, 296, 111873.
- Zou, X.; Zhou, T.; Mao, J.; Wu, X. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe0/persulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44.
- Zhang, J.; Shao, X.; Shi, C.; Yang, S. Decolorization of Acid Orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon. Chem. Eng. J. 2013, 232, 259–265.
- Pervez, N.; He, W.; Zarra, T.; Naddeo, V.; Zhao, Y. New Sustainable Approach for the Production of Fe3O4/Graphene Oxide-Activated Persulfate System for Dye Removal in Real Wastewater. Water 2020, 12, 733.
- Hong, Y.; Luo, Z.; Zhang, N.; Qu, L.; Zheng, M.; Suara, M.A.; Chelme-Ayala, P.; Zhou, X.; El-Din, M.G. Decomplexation of Cu(II)-EDTA by synergistic activation of persulfate with alkali and CuO: Kinetics and activation mechanism. Sci. Total Environ. 2022, 817, 152793.
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B Environ. 2018, 224, 973–982.
More
