Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Shuangyang Zou.
Halide perovskites are increasingly exploited as semiconducting materials in diverse optoelectronic applications, including light emitters, photodetectors, and solar cells. The halide perovskite can be easily processed in solution, making microfluidic synthesis possible.
- microfluidics
- halide perovskite
- doping
- nanomaterials
1. Introduction
The microfluidic chip that confines fluids in micron channels can scale the chemical reactions from extensive batch synthesis down to the microscale, exploiting the physical and chemical properties of liquids and gases at a microscale, significantly reducing the synthesis and analysis of volume reagents [1,2,3,4,5][1][2][3][4][5]. In nanocrystal (NC) synthetic processes, the batch synthesis strategies of NCs are almost always challenging due to rapid perovskite crystallization, the extensive precursor preparation, the difficulties associated with product purification, and the need for particle post-synthesis. It is envisioned that a microreactor platform consisting of flow-focusing microfluidics might be suitable to synthesize high-crystallinity and narrow-size-distribution NCs due to the ultrafast mixing and phase separation during the crystal nucleation and growth. The microfluidic chemical reactions can be precisely detected and explored by in situ spectroscopy [6,7,8,9,10][6][7][8][9][10] and more sufficient and continuous during the reaction on the micron scale. Therefore, there are at least two advantages to microfluidic synthesis. On the macroscopic level, a microreactor can be considered a powerful and effective platform for the mass synthesis of semiconductor nanomaterials. On the microscopic level, the microfluidic technique facilitates the simultaneous collection of both absorption and photoluminescence (PL) spectra of various luminescent materials synthesized in the liquid states, particularly that of halide perovskite nanocrystals.
Quantum dot (QD) semiconductors are promising materials for various applications ranging from light-emitting diode (LED) displays to solar cells, biological sensing, and imaging [6,7,8][6][7][8]. Specifically as optoelectronic materials, perovskite nanocrystals have attracted much more attention due to their high PL quantum yields, high absorption/emission efficiency, long carrier lifetime, and tunable emission color over the entire visible region [9,10,11][9][10][11]. Lead halide perovskite structure can be characterized by the general formula ABX3 (X = Cl, Br, or I), where A and B represent two different cations. A-site cations can be inorganic or organic ions, such as cesium (Cs), formamidinium (FA), and methylammonium (MA), while B-site cation (Pb2+) could potentially be exchanged by dopant ions (Mn2+, Fe2+, Ce3+, Eu2+) [12,13,14,15,16,17,18][12][13][14][15][16][17][18]. Therefore, the hybrid organic−inorganic lead halide perovskite, such as CH3NH3PbX3; and all inorganic lead halide perovskite, such as CsPbX3, in the form of nanocrystals, thin films, microcrystals, and bulk single-crystals, show promising properties in LEDs [9[9][19],19], lasers [20], solar cells [21[21][22][23],22,23], gas sensors [24], etc.
2. Microfluidic Synthesis of Halide Perovskite
Generally, microfluidic devices have microchannels ranging from submicron to a few millimeters, as shown in Figure 1, which can move or analyze the tiny amount of liquid (droplet) in a single- or multi-phase flow.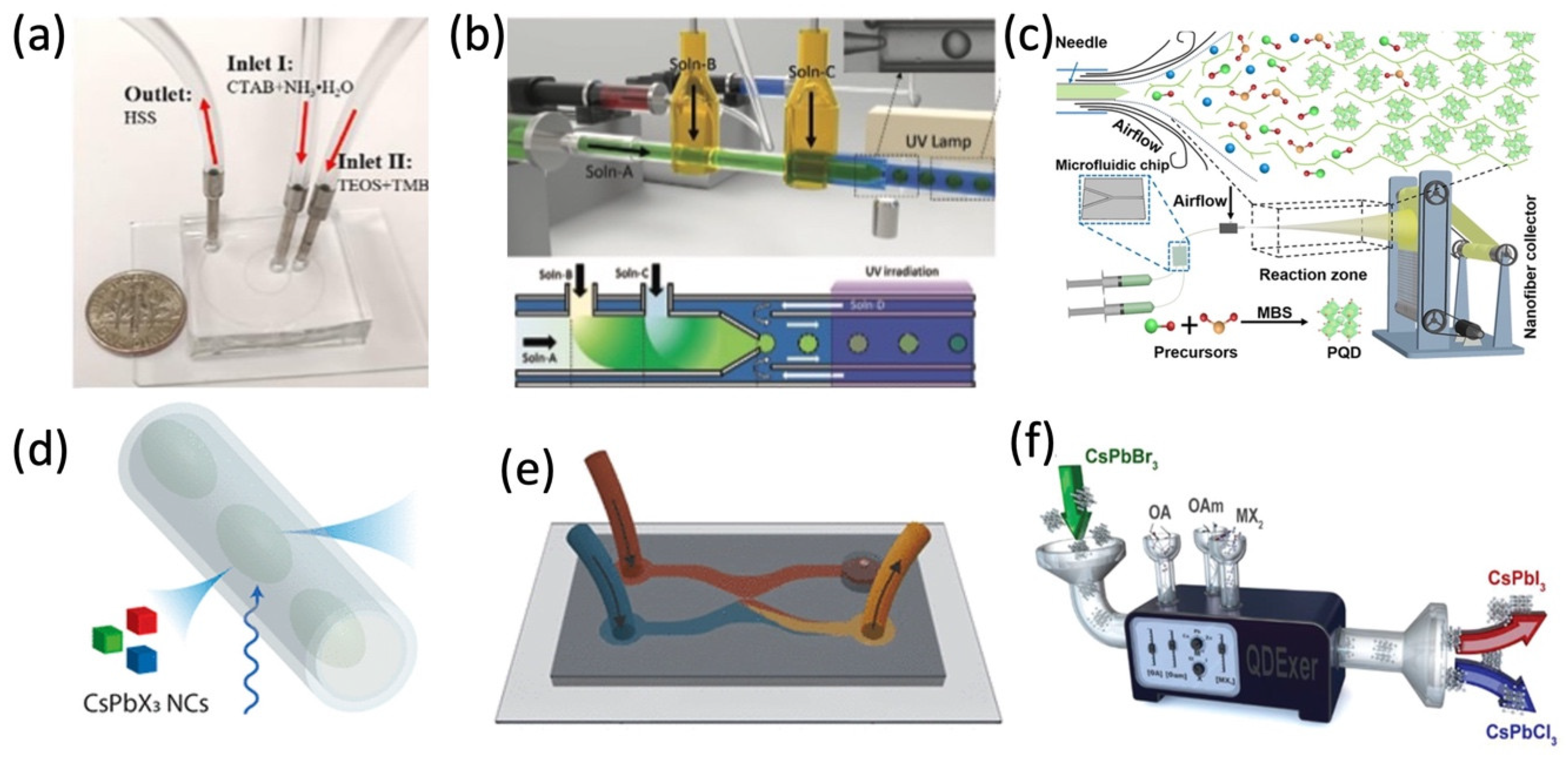
Figure 1. Various microfluidic syntheses of perovskite nanostructures and composite. (a) Microfluidic setup with a U.S. dime coin for comparison. Adapted with permission from Ref. [25]. Copyright 2019 Elsevier B.V. (b) Synthesis of perovskite composite microparticles. Adapted with permission from Ref. [26]. Copyright 2021 Wiley-VCH GmbH. (c) Formation of MAPbBr3 PQDs in nanofiber. Adapted with permission from Ref. [27]. Copyright 2022 Wiley-VCH GmbH. (d) Schematic of the PL dynamics of microfluidic droplet. Adapted with permission from Ref. [28]. Copyright 2020 American Chemical Society. (e) Microfluidic chips for synthesizing CsPbBr3. Adapted with permission from Ref. [29]. Copyright 2021 American Chemical Society. (f) QD anion exchange reaction in a continuous flow. Adapted with permission from Ref. [30]. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
Table 1.
Microfluidic synthesis of nanostructured halide perovskite.
| Materials | Synthesis Temp. (°C) | Size | PL Peak Location (nm) | Year [Ref.] |
|---|---|---|---|---|
| CsPbBr3 QDs | RT | <10 nm | ~500 | 2017, Epps et al. [33] |
| CsPbBr3 QDs | RT | 10–20 nm | ~520 | 2019, Wei et al. [34] |
| CsPbBr3 NWs | 50 | 3–9 μm | 535 | 2021, Koryakina et al. [29] |
| CsPbBr3 NWs | 50 | ~4 nm (width) | ~475 | 2019, Zhang et al. [31] |
| MAPbI3 | 85 | 60 μm (width) | - | 2020, Khorramshahi et al. [35] |
| QD encapsulation | 37 | 500–700 nm | 430–625 | 2021, Bian et al. [32] |
| MAPbBr3 composite | - | 500 μm | ~530 | 2021, Kim et al. [26] |
| FAPb(I/Br)3 QDs | 120 | ~10 nm | 530–690 | 2017, Maceiczyk et al. [36] |
| Cs4PbBr6 MCs | 60–150 | >1 μm | 520 | 2018, Bao et al. [37] |
| CsPbX3 QDs | 130–220 | 8–12.5 nm | 470–690 | 2016, Lignos et al. [38,39,40][38][39][40] |
| CsPbX3 QDs | RT | <20 nm | 422–660 | 2019, Abdel-Latif et al. [30] |
| CsPbX3 NCs | RT | ~15 nm | ~520 | 2020, Lin et al. [41] |
| CsPbX3 NCs | 100–180 | <20 nm | 406–677 | 2022, Geng et al. [42] |
In the materials column of the table, QDs: quantum dots, NCs: nanocrystals, NWs: nanowires, MCs: microcrystals, and X = Br, I, Cl, respectively.
The microfluidic channel with controllable morphology and configuration could be efficiently designed and achieved, therefore, nanomaterials could be more precisely synthesized in the microfluidic channel. For example, Kim et al. reported the in situ reaction of metal halide perovskite nanoparticles by the ligand-assisted reprecipitation process (LARP) and encapsulation by ultraviolet light (UV) cross-linking polymerization, in which the stable, water-resistant light-emitting perovskite–polymer composite microparticles can be synthesized in a continuous one-step microfluidic reactor [26]. Tuning the reactant concentration and the flow rate in the microreactor, ranging from several nanometers to over one hundred nanometers, hollow spherical silica-based functional materials and the Cs4PbBr6 perovskite microcrystals (MCs) were synthesized by mixing two reactant flows, respectively [25,37][25][37]. With the microfluidic template in Figure 2a,c, well-aligned and uniform heterojunctions of MAPbI3 and organic semiconductors (OSC) in the silicon nanowire patterns can be grown. In Figure 2b,d,f, different morphologies (1D, 2D) of halide perovskite have already been successfully synthesized via solution methods [17[17][43][44],43,44], which are difficult to batch produce and industrially apply in comparison to microfluidic synthesis. In Figure 2e, the halide exchange reactions are realized in a modular microfluidic platform called Quantum Dot Exchanger, which offers a unique time- and material-efficient approach for studies of solution phase-processed colloidal nanocrystals [30,45,46][30][45][46]. Perovskite precursor solutions could be simultaneously pumped into the microfluidic device. By changing the ratio of different perovskite precursor solutions, a series of perovskite QDs can be precipitated and encapsulated in ethyleneglycol dimethacrylate (EGDMA) resin [32]. The microfluidic synthesis makes chemical composition tuning and doping in perovskite more available.
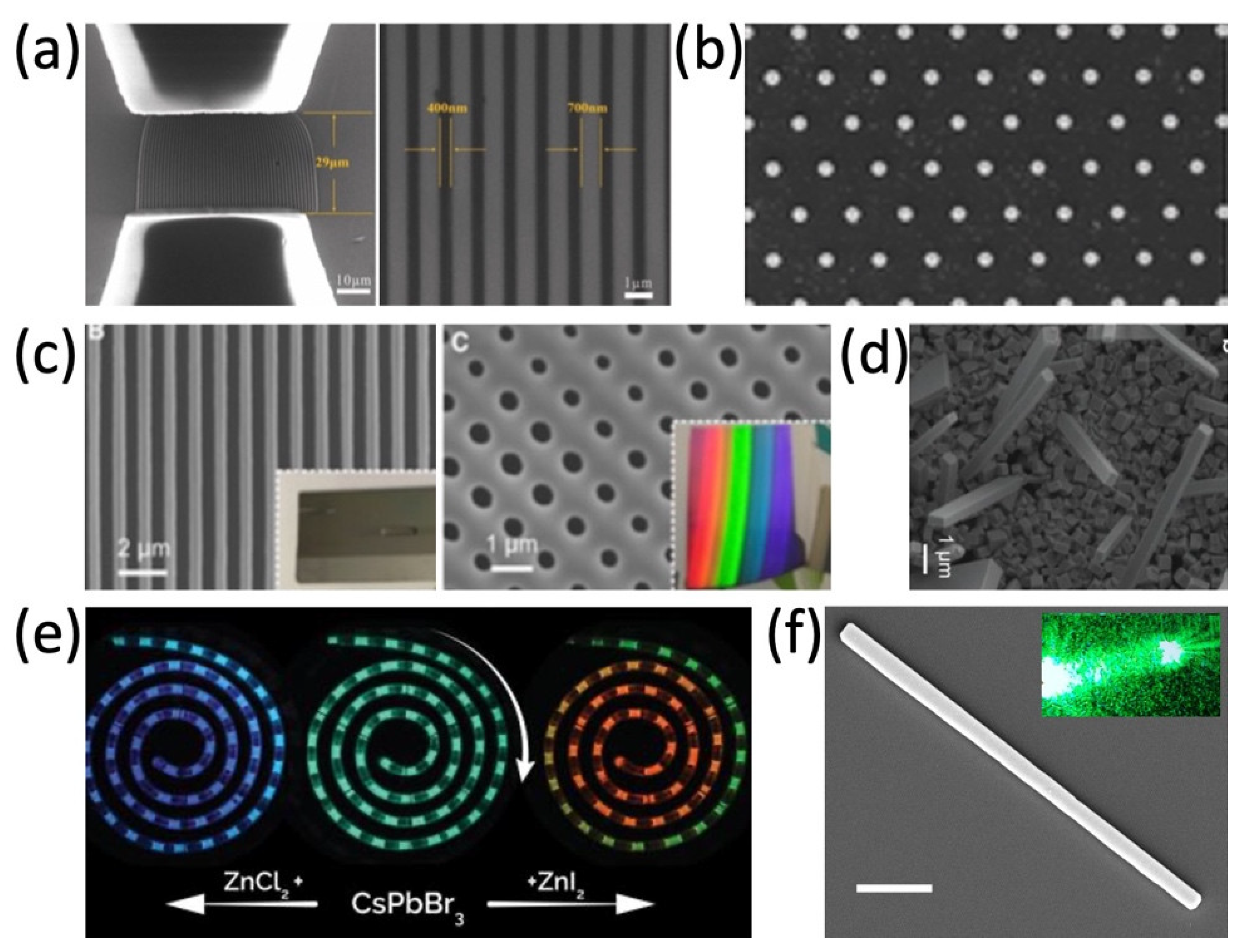

Figure 2. (a) SEM images of the silicon nanowire template for microfluidic synthesis. Adapted with permission from Ref. [45]. Copyright 2020 The Royal Society of Chemistry. (b) SEM image of the CH3NH3PbX3 platelet array. Adapted with permission from Ref. [43]. Copyright 2016 American Chemical Society. (c) Periodic parallel lines and surface of grating-patterned Si substrate, respectively. Adapted under a creative commons license from Ref. [46] (www.creativecommons.org/licenses/by-nc-nd/4.0/ (accessed on 14 September 2022)). Copyright 2020 The Authors. (d) SEM image of the CsPbI3 NWs. Adapted with permission from Ref. [44]. Copyright 2016 American Chemical Society. (e) Continuous anion exchange reactions of CsPbBr3 QDs. Adapted with permission from Ref. [30]. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) SEM image of the Fe-doped CsPb(Cl/Br)3 NW. Adapted with permission from Ref. [17]. Copyright 2018 American Chemical Society.
References
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373.
- Colin, A.; Squires, T.M.; Bocquet, L. Soft Matter Principles of Microfluidics. Soft Matter 2012, 8, 10527–10529.
- Park, J.I.; Saffari, A.; Kumar, S.; Günther, A.; Kumacheva, E. Microfluidic Synthesis of Polymer and Inorganic Particulate Materials. Annu. Rev. Mater. Res. 2010, 40, 415–443.
- Convery, N.; Gadegaard, N. 30 Years of Microfluidics. Micro Nano Eng. 2019, 2, 76–91.
- Fallahi, H.; Zhang, J.; Phan, H.P.; Nguyen, N.T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines 2019, 10, 830.
- Kovalenko, M.v.; Manna, L.; Cabot, A.; Hens, Z.; Talapin, D.v.; Kagan, C.R.; Klimov, V.I.; Rogach, A.L.; Reiss, P.; Milliron, D.J.; et al. Prospects of Nanoscience with Nanocrystals. ACS Nano 2015, 9, 1012–1057.
- Klimov, V.I.; Mikhailovsky, A.A.; Xu, S.; Malko, A.; Hollingsworth, J.A.; Leatherdale, C.A.; Eisler, H.-J.; Bawendi, M.G. Optical Gain and Stimulated Emission in Nanocrystal Quantum Dots. Science (1979) 2000, 290, 314–317.
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55.
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167.
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542.
- Wang, Y.; Zhao, H.; Piotrowski, M.; Han, X.; Ge, Z.; Dong, L.; Wang, C.; Pinisetty, S.K.; Balguri, P.K.; Bandela, A.K.; et al. Cesium Lead Iodide Perovskites: Optically Active Crystal Phase Stability to Surface Engineering. Micromachines 2022, 13, 1318.
- Eperon, G.E.; Ginger, D.S. B-Site Metal Cation Exchange in Halide Perovskites. ACS Energy Lett. 2017, 2, 1190–1196.
- Wu, Z.; Zhang, Q.; Li, B.; Shi, Z.; Xu, K.; Chen, Y.; Ning, Z.; Mi, Q. Stabilizing the CsSnCl3 Perovskite Lattice by B-Site Substitution for Enhanced Light Emission. Chem. Mater. 2019, 31, 4999–5004.
- Zhang, Y.; Fan, C.; Tang, J.; Huang, G.; Qiang, X.; Fu, Y.; Zhou, W.; Wu, J.; Huang, S. Systematic Microwave-Assisted Postsynthesis of Mn-Doped Cesium Lead Halide Perovskites with Improved Color-Tunable Luminescence and Stability. Nanomaterials 2022, 12, 2535.
- Zheng, F.; Chen, W.; Bu, T.; Ghiggino, K.P.; Huang, F.; Cheng, Y.; Tapping, P.; Kee, T.W.; Jia, B.; Wen, X. Triggering the Passivation Effect of Potassium Doping in Mixed-Cation Mixed-Halide Perovskite by Light Illumination. Adv. Energy Mater. 2019, 9, 1901016.
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961.
- Zou, S.; Yang, G.; Yang, T.; Zhao, D.; Gan, Z.; Chen, W.; Zhong, H.; Wen, X.; Jia, B.; Zou, B. Template-Free Synthesis of High-Yield Fe-Doped Cesium Lead Halide Perovskite Ultralong Microwires with Enhanced Two-Photon Absorption. J. Phys. Chem. Lett. 2018, 9, 4878–4885.
- Xia, Z.; Xu, Z.; Chen, M.; Liu, Q. Recent Developments in the New Inorganic Solid-State LED Phosphors. Dalton Trans. 2016, 45, 11214–11232.
- Ren, A.; Wang, H.; Zhang, W.; Wu, J.; Wang, Z.; Penty, R.V.; White, I.H. Emerging Light-Emitting Diodes for next-Generation Data Communications. Nat. Electron. 2021, 4, 559–572.
- Zhang, Q.; Shang, Q.; Su, R.; Do, T.T.H.; Xiong, Q. Halide Perovskite Semiconductor Lasers: Materials, Cavity Design, and Low Threshold. Nano Lett. 2021, 21, 1903–1914.
- Trifiletti, V.; Degousée, T.; Manfredi, N.; Fenwick, O.; Colella, S.; Rizzo, A. Molecular Doping for Hole Transporting Materials in Hybrid Perovskite Solar Cells. Metals 2020, 10, 14.
- Wu, H.T.; Cheng, Y.T.; Leu, C.C.; Wu, S.H.; Shih, C.F. Improving Two-Step Prepared CH3NH3PbI3 Perovskite Solar Cells by Co-Doping Potassium Halide and Water in PbI2 Layer. Nanomaterials 2019, 9, 666.
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface Engineering of Highly Efficient Perovskite Solar Cells. Science (1979) 2014, 345, 542–546.
- Xie, L.; Zan, J.; Yang, Z.; Wu, Q.; Chen, X.; Ou, X.; Lin, C.; Chen, Q.; Yang, H. A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays. Front. Chem. 2021, 9, 682006.
- Hao, N.; Nie, Y.; Xu, Z.; Closson, A.B.; Usherwood, T.; Zhang, J.X.J. Microfluidic Continuous Flow Synthesis of Functional Hollow Spherical Silica with Hierarchical Sponge-like Large Porous Shell. Chem. Eng. J. 2019, 366, 433–438.
- Kim, K.H.; Park, J.K.; Im, S.H.; Park, B.J. Waterproof Light-Emitting Metal Halide Perovskite–Polymer Composite Microparticles Prepared via Microfluidic Device. Part. Part. Syst. Charact. 2021, 38, 2100006.
- Cheng, R.; Liang, Z.B.; Zhu, L.; Li, H.; Zhang, Y.; Wang, C.F.; Chen, S. Fibrous Nanoreactors from Microfluidic Blow Spinning for Mass Production of Highly Stable Ligand-Free Perovskite Quantum Dots. Angew. Chem.—Int. Ed. 2022, 61, e202204371.
- Lignos, I.; Maceiczyk, R.M.; Kovalenko, M.V.; Stavrakis, S. Tracking the Fluorescence Lifetimes of Cesium Lead Halide Perovskite Nanocrystals During Their Synthesis Using a Fully Automated Optofluidic Platform. Chem. Mater. 2020, 32, 27–37.
- Koryakina, I.G.; Naumochkin, M.; Markina, D.I.; Khubezhov, S.A.; Pushkarev, A.P.; Evstrapov, A.A.; Makarov, S.v.; Zyuzin, M.v. Single-Step Microfluidic Synthesis of Halide Perovskite Nanolasers in Suspension. Chem. Mater. 2021, 33, 2777–2784.
- Abdel-Latif, K.; Epps, R.W.; Kerr, C.B.; Papa, C.M.; Castellano, F.N.; Abolhasani, M. Facile Room-Temperature Anion Exchange Reactions of Inorganic Perovskite Quantum Dots Enabled by a Modular Microfluidic Platform. Adv. Funct Mater. 2019, 29, 1900712.
- Zhang, Z.; Liu, Y.; Geng, C.; Shi, S.; Zhang, X.; Bi, W.; Xu, S. Rapid Synthesis of Quantum-Confined CsPbBr3 Perovskite Nanowires Using a Microfluidic Reactor. Nanoscale 2019, 11, 18790–18796.
- Bian, F.; Sun, L.; Wang, Y.; Zhang, D.; Li, Z.; Zhao, Y. Microfluidic Generation of Barcodes with in Situ Synthesized Perovskite Quantum Dot Encapsulation. Sci. China Chem. 2021, 64, 1540–1546.
- Epps, R.W.; Felton, K.C.; Coley, C.W.; Abolhasani, M. Automated Microfluidic Platform for Systematic Studies of Colloidal Perovskite Nanocrystals: Towards Continuous Nano-Manufacturing. Lab Chip 2017, 17, 4040–4047.
- Wei, Z.; Chen, Y.; Lin, P.; Yan, Q.; Fan, Y.; Cheng, Z. Synthesis and Encapsulation of All Inorganic Perovskite Nanocrystals by Microfluidics. J. Mater. Sci. 2019, 54, 6841–6852.
- Khorramshahi, F.; Takshi, A. Microfluidic Approach for Lead Halide Perovskite Flexible Phototransistors. Electron. 2020, 9, 1852.
- MacEiczyk, R.M.; Dümbgen, K.; Lignos, I.; Protesescu, L.; Kovalenko, M.v.; Demello, A.J. Microfluidic Reactors Provide Preparative and Mechanistic Insights into the Synthesis of Formamidinium Lead Halide Perovskite Nanocrystals. Chem. Mater. 2017, 29, 8433–8439.
- Bao, Z.; Wang, H.C.; Jiang, Z.F.; Chung, R.J.; Liu, R.S. Continuous Synthesis of Highly Stable Cs4PbBr6 Perovskite Microcrystals by a Microfluidic System and Their Application in White-Light-Emitting Diodes. Inorg. Chem. 2018, 57, 13071–13074.
- Lignos, I.; Stavrakis, S.; Nedelcu, G.; Protesescu, L.; deMello, A.J.; Kovalenko, M.V. Synthesis of Cesium Lead Halide Perovskite Nanocrystals in a Droplet-Based Microfluidic Platform: Fast Parametric Space Mapping. Nano Lett. 2016, 16, 1869–1877.
- Lignos, I.; Protesescu, L.; Emiroglu, D.B.; MacEiczyk, R.; Schneider, S.; Kovalenko, M.v.; DeMello, A.J. Unveiling the Shape Evolution and Halide-Ion-Segregation in Blue-Emitting Formamidinium Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform. Nano Lett. 2018, 18, 1246–1252.
- Lignos, I.; Morad, V.; Shynkarenko, Y.; Bernasconi, C.; Maceiczyk, R.M.; Protesescu, L.; Bertolotti, F.; Kumar, S.; Ochsenbein, S.T.; Masciocchi, N.; et al. Exploration of Near-Infrared-Emissive Colloidal Multinary Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform. ACS Nano 2018, 12, 5504–5517.
- Lin, P.; Chen, H.; Wei, Z.; Lin, Y.; Lin, J.; Chen, Y.; Cheng, Z. Continuous-Flow Synthesis of Doped All-Inorganic Perovskite Nanocrystals Enabled by a Microfluidic Reactor for Light-Emitting Diode Application. Sci. China Mater. 2020, 63, 1526–1536.
- Geng, Y.; Guo, J.; Ling, S.D.; Wu, X.; Liu, H.; Chen, Z.; Chen, S.; Xu, J. A Nano-Liter Droplet-Based Microfluidic Reactor Serves as Continuous Large-Scale Production of Inorganic Perovskite Nanocrystals. Sci. China Mater. 2022, 65, 2746–2754.
- Liu, X.; Niu, L.; Wu, C.; Cong, C.; Wang, H.; Zeng, Q.; He, H.; Fu, Q.; Fu, W.; Yu, T.; et al. Periodic Organic–Inorganic Halide Perovskite Microplatelet Arrays on Silicon Substrates for Room-Temperature Lasing. Adv. Sci. 2016, 3, 1600137.
- Fu, Y.; Zhu, H.; Stoumpos, C.C.; Ding, Q.; Wang, J.; Kanatzidis, M.G.; Zhu, X.; Jin, S. Broad Wavelength Tunable Robust Lasing from Single-Crystal Nanowires of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). ACS Nano 2016, 10, 7963–7972.
- Chen, P.A.; Guo, J.; Nouri, M.; Tao, Q.; Li, Z.; Li, Q.; Du, L.; Chen, H.; Dong, Z.; Chang, L.; et al. Microfluidic Solution-Processed Organic and Perovskite Nanowires Fabricated for Field-Effect Transistors and Photodetectors. J. Mater. Chem. C Mater. 2020, 8, 2353–2362.
- Xin, B.; Pak, Y.; Shi, M.; Mitra, S.; Zheng, X.; Bakr, O.M.; Roqan, I.S. Micropump Fluidic Strategy for Fabricating Perovskite Microwire Array-Based Devices Embedded in Semiconductor Platform. Cell Rep. Phys. Sci. 2021, 2, 100304.
More
