Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shuangyang Zou and Version 3 by Conner Chen.
Halide perovskites are increasingly exploited as semiconducting materials in diverse optoelectronic applications, including light emitters, photodetectors, and solar cells. The halide perovskite can be easily processed in solution, making microfluidic synthesis possible.
- microfluidics
- halide perovskite
- doping
- nanomaterials
1. Introduction
The microfluidic chip that confines fluids in micron channels can scale the chemical reactions from extensive batch synthesis down to the microscale, exploiting the physical and chemical properties of liquids and gases at a microscale, significantly reducing the synthesis and analysis of volume reagents [1][2][3][4][5][1,2,3,4,5]. In nanocrystal (NC) synthetic processes, the batch synthesis strategies of NCs are almost always challenging due to rapid perovskite crystallization, the extensive precursor preparation, the difficulties associated with product purification, and the need for particle post-synthesis. It is envisioned that a microreactor platform consisting of flow-focusing microfluidics might be suitable to synthesize high-crystallinity and narrow-size-distribution NCs due to the ultrafast mixing and phase separation during the crystal nucleation and growth. The microfluidic chemical reactions can be precisely detected and explored by in situ spectroscopy [6][7][8][9][10][6,7,8,9,10] and more sufficient and continuous during the reaction on the micron scale. Therefore, there are at least two advantages to microfluidic synthesis. On the macroscopic level, a microreactor can be considered a powerful and effective platform for the mass synthesis of semiconductor nanomaterials. On the microscopic level, the microfluidic technique facilitates the simultaneous collection of both absorption and photoluminescence (PL) spectra of various luminescent materials synthesized in the liquid states, particularly that of halide perovskite nanocrystals.
Quantum dot (QD) semiconductors are promising materials for various applications ranging from light-emitting diode (LED) displays to solar cells, biological sensing, and imaging [6][7][8][6,7,8]. Specifically as optoelectronic materials, perovskite nanocrystals have attracted much more attention due to their high PL quantum yields, high absorption/emission efficiency, long carrier lifetime, and tunable emission color over the entire visible region [9][10][11][9,10,11]. Lead halide perovskite structure can be characterized by the general formula ABX3 (X = Cl, Br, or I), where A and B represent two different cations. A-site cations can be inorganic or organic ions, such as cesium (Cs), formamidinium (FA), and methylammonium (MA), while B-site cation (Pb2+) could potentially be exchanged by dopant ions (Mn2+, Fe2+, Ce3+, Eu2+) [12][13][14][15][16][17][18][12,13,14,15,16,17,18]. Therefore, the hybrid organic−inorganic lead halide perovskite, such as CH3NH3PbX3; and all inorganic lead halide perovskite, such as CsPbX3, in the form of nanocrystals, thin films, microcrystals, and bulk single-crystals, show promising properties in LEDs [9][19][9,19], lasers [20], solar cells [21][22][23][21,22,23], gas sensors [24], etc.
2. Microfluidic Synthesis of Halide Perovskite
Generally, microfluidic devices have microchannels ranging from submicron to a few millimeters., For various microfluidic syntheses of perovskite nanostructures and compositeas shown in Figure 1, which can move or analyze the tiny amount of liquid (droplet) in a single- or multi-phase flow.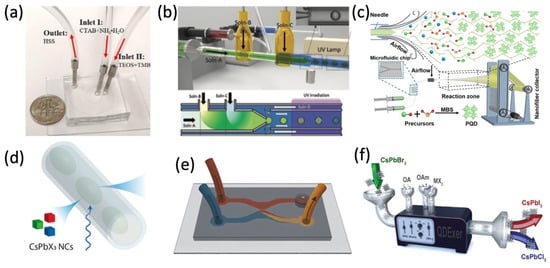
Figure 1. Various microfluidic syntheses of perovskite nanostructures and composite. (a) Microfluidic setup with a U.S. dime coin for comparison. Adapted with permission from Ref. [25]. Copyright 2019 Elsevier B.V. (b) Synthesis of perovskite composite microparticles. Adapted with permission from Ref. [26]. Copyright 2021 Wiley-VCH GmbH. (c) Formation of MAPbBr3 PQDs in nanofiber. Adapted with permission from Ref. [27]. Copyright 2022 Wiley-VCH GmbH. (d) Schematic of the PL dynamics of microfluidic droplet. Adapted with permission from Ref. [28]. Copyright 2020 American Chemical Society. (e) Microfluidic chips for synthesizing CsPbBr3. Adapted with permission from Ref. [29]. Copyright 2021 American Chemical Society. (f) QD anion exchange reaction in a continuous flow. Adapted with permission from Ref. [30]. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
Table 1.
Microfluidic synthesis of nanostructured halide perovskite.
| Materials | Synthesis Temp. (°C) | Size | PL Peak Location (nm) | Year [Ref.] |
|---|---|---|---|---|
| CsPbBr3 QDs | RT | <10 nm | ~500 | 2017, Epps et al. [33] |
| CsPbBr3 QDs | RT | 10–20 nm | ~520 | 2019, Wei et al. [34] |
| CsPbBr3 NWs | 50 | 3–9 μm | 535 | 2021, Koryakina et al. [29] |
| CsPbBr3 NWs | 50 | ~4 nm (width) | ~475 | 2019, Zhang et al. [31] |
| MAPbI3 | 85 | 60 μm (width) | - | 2020, Khorramshahi et al. [35] |
| QD encapsulation | 37 | 500–700 nm | 430–625 | 2021, Bian et al. [32] |
| MAPbBr3 composite | - | 500 μm | ~530 | 2021, Kim et al. [26] |
| FAPb(I/Br)3 QDs | 120 | ~10 nm | 530–690 | 2017, Maceiczyk et al. [36] |
| Cs4PbBr6 MCs | 60–150 | >1 μm | 520 | 2018, Bao et al. [37] |
| CsPbX3 QDs | 130–220 | 8–12.5 nm | 470–690 | 2016, Lignos et al. [38][39][40][38,39,40] |
| CsPbX3 QDs | RT | <20 nm | 422–660 | 2019, Abdel-Latif et al. [30] |
| CsPbX3 NCs | RT | ~15 nm | ~520 | 2020, Lin et al. [41] |
| CsPbX3 NCs | 100–180 | <20 nm | 406–677 | 2022, Geng et al. [42] |
In the materials column of the table, QDs: quantum dots, NCs: nanocrystals, NWs: nanowires, MCs: microcrystals, and X = Br, I, Cl, respectively.
The microfluidic channel with controllable morphology and configuration could be efficiently designed and achieved, therefore, nanomaterials could be more precisely synthesized in the microfluidic channel. For example, Kim et al. reported the in situ reaction of metal halide perovskite nanoparticles by the ligand-assisted reprecipitation process (LARP) and encapsulation by ultraviolet light (UV) cross-linking polymerization, in which the stable, water-resistant light-emitting perovskite–polymer composite microparticles can be synthesized in a continuous one-step microfluidic reactor [26]. Tuning the reactant concentration and the flow rate in the microreactor, ranging from several nanometers to over one hundred nanometers, hollow spherical silica-based functional materials and the Cs4PbBr6 perovskite microcrystals (MCs) were synthesized by mixing two reactant flows, respectively [25][37][25,37]. With the microfluidic template in Figure 2a,c, well-aligned and uniform heterojunctions of MAPbI3 and organic semiconductors (OSC) in the silicon nanowire patterns can be grown. DIn Figure 2b,d,f, different morphologies (1D, 2D) of halide perovskite have already been successfully synthesized via solution methods [17][43][44][17,43,44], which are difficult to batch produce and industrially apply in comparison to microfluidic synthesis. In continuous anion Figure 2exchange reactions of CsPbBr3 QDs, the halide exchange reactions are realized in a modular microfluidic platform called Quantum Dot Exchanger, which offers a unique time- and material-efficient approach for studies of solution phase-processed colloidal nanocrystals [30][45][46][30,45,46]. Perovskite precursor solutions could be simultaneously pumped into the microfluidic device. By changing the ratio of different perovskite precursor solutions, a series of perovskite QDs can be precipitated and encapsulated in ethyleneglycol dimethacrylate (EGDMA) resin [32]. The microfluidic synthesis makes chemical composition tuning and doping in perovskite more available.
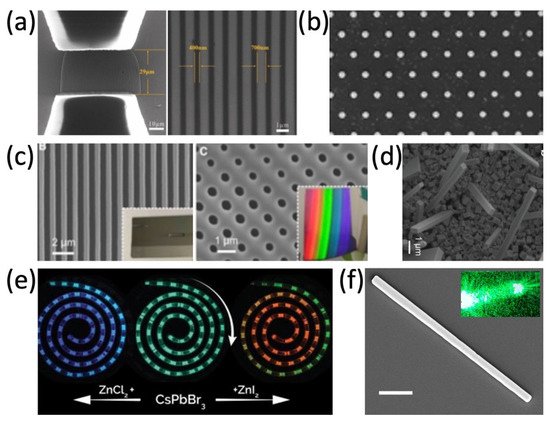

Figure 2. (a) SEM images of the silicon nanowire template for microfluidic synthesis. Adapted with permission from Ref. [45]. Copyright 2020 The Royal Society of Chemistry. (b) SEM image of the CH3NH3PbX3 platelet array. Adapted with permission from Ref. [43]. Copyright 2016 American Chemical Society. (c) Periodic parallel lines and surface of grating-patterned Si substrate, respectively. Adapted under a creative commons license from Ref. [46] (www.creativecommons.org/licenses/by-nc-nd/4.0/ (accessed on 14 September 2022)). Copyright 2020 The Authors. (d) SEM image of the CsPbI3 NWs. Adapted with permission from Ref. [44]. Copyright 2016 American Chemical Society. (e) Continuous anion exchange reactions of CsPbBr3 QDs. Adapted with permission from Ref. [30]. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) SEM image of the Fe-doped CsPb(Cl/Br)3 NW. Adapted with permission from Ref. [17]. Copyright 2018 American Chemical Society.
