Diffuse low-grade glioma (LGG) is a rare cerebral cancer, mostly involving young adults with an active life at diagnosis. If left untreated, LGG widely invades the brain and becomes malignant, generating neurological worsening and ultimately death. Early and repeat treatments for this incurable tumor, including maximal connectome-based surgical resection(s) in awake patients, enable postponement of malignant transformation while preserving quality of life owing to constant neural network reconfiguration. Due to considerable interindividual variability in terms of LGG course and consecutive cerebral reorganization, a multistage longitudinal strategy should be tailored accordingly in each patient. It is crucial to predict how the glioma will progress (changes in growth rate and pattern of migration, genetic mutation, etc.) and how the brain will adapt (changes in patterns of spatiotemporal redistribution, possible functional consequences such as epilepsy or cognitive decline, etc.). The goal is to anticipate therapeutic management, remaining one step ahead in order to select the optimal (re-)treatment(s) (some of them possibly kept in reserve), at the appropriate time(s) in the evolution of this chronic disease, before malignization and clinical worsening.
- low-grade glioma
- awake surgery
- brain mapping
- quality of life
- overall survival
- interindividual variability
- connectome
- neurocognition
1. Introduction
2. Predicting Oncological Interindividual Variability and Its Changes over Time
Distinct types of factors must be taken into consideration, including those related to the tumoral disease itself as well as external factors which may influence the course of glioma (Figure 1).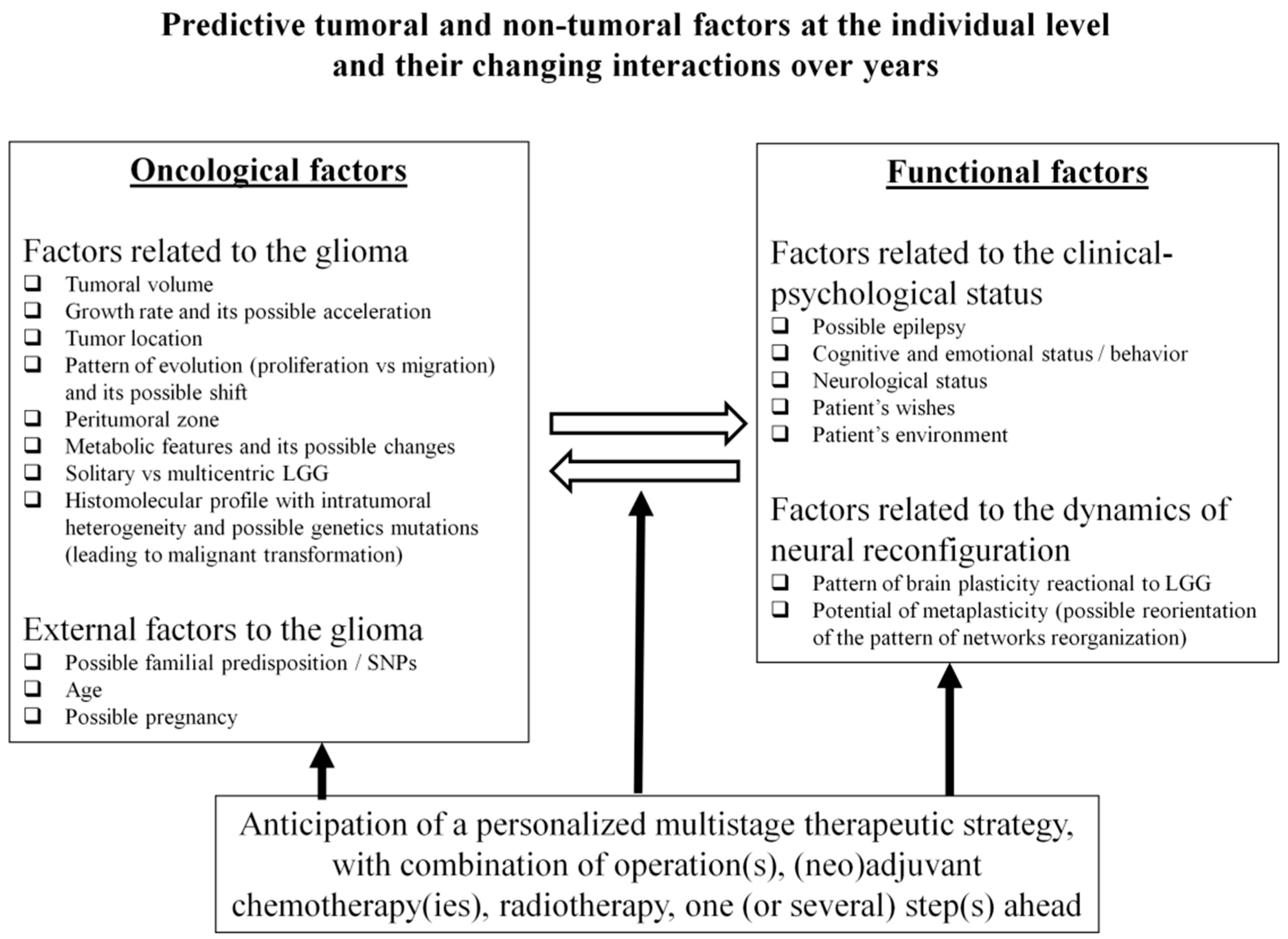
2.1. Factors Related to the Glioma
2.1.1. Tumoral Volume
The volume of LGG at diagnosis is highly variable, from 0.39 to 386 cc according to recent studies [4,27][4][26]. Notably, it has long been considered that a larger size of LGG is an adverse prognostic factor; however, only one dimension of the tumor was classically measured, with a poorer prognosis when the size was ≥6 cm [28,29,30][27][28][29]. More recently, it has been evidenced that a greater LGG volume (by definition, calculated in 3D) was significantly correlated with a higher risk of MT and with shorter OS [9,10,26][9][10][30]. This may be explained by the fact that a more voluminous glioma reflects a more prolonged natural history of the disease, with a higher number of tumoral cells and therefore an increased risk of mutational accumulations [31,32][31][32]. Such a hypothesis is supported by a decreased risk of MT as well as a significant survival benefit in incidental LGG compared with symptomatic LGG discovered later [3], although a higher volume at discovery of incidental LGG is also significantly associated with more progressive tumors [33]. Consequently, a screening policy has been proposed to diagnose and treat LGG earlier, as well as to optimize the opportunity to better understand the origin of these tumors [34]. In addition, during the follow-up of LGG patients already treated, it has also been suggested that further treatment(s) should be considered when the tumor volume re-increases over time, especially when it reaches a threshold of about 10–15 cc (even if the patient is asymptomatic at that time), in order to delay MT [7].2.1.2. Growth Rate
The velocity of a tumor’s spontaneous expansion, which can be plotted as a function of mean glioma diameter over time (computed from the volumes calculated by repeat MRIs) is predictive of long-term outcomes for LGG [5]. Indeed, the slope of the mean tumor diameter growth curve is an independent prognostic factor for malignant progression-free survival and for OS as a continuous predictor—that is, showing a linear relationship between OS and growth rate [35]. The relevant kinetics are very variable from one LGG to another at diagnosis, from less than 1 mm per year to 8 mm per year; over 8 mm/year, the LGG invokes a greater risk of MT [35]. Interestingly, this marker is independent of the molecular profile [35], in particular with regard to the IDH status [36]. Thus, identifying rapidly growing LGG during the pretherapeutic period, i.e., tumors at higher risk of worsening evolution, can be helpful to decide when to start treatment(s) [37], including for incidental LGG [38]. This is also true during the surveillance of LGG patients already treated, in order to determine when to consider further therapy. Especially because the growth rate is similar before and after surgery [39], acceleration of the velocity of the residual tumor in cases of incomplete resection may support earlier re-operation, even in asymptomatic patients [25]. Furthermore, re-growth after a period of stabilization following chemotherapy and/or radiotherapy can prompt consideration of further treatment [7]. The possibility of such changes favors deployment of a systematic control MRI every 3 to 6 months throughout life.2.1.3. Pattern of Migration Versus Proliferation and Tumor Location
In addition to variable glioma volumes and kinetics, LGG may exhibit distinct patterns of progression within the brain, i.e., a more proliferative “bulky” pattern versus a more diffuse “migratory” one. Interindividual variability may be considerable, ranging from a very focal tumor to a gliomatosis with bi-hemispheric dissemination [40]. Notably, in incidentally discovered focal LGG, the insular location was found to be a predictive factor of a more progressive tumor [33]. In a study of 1097 cases, a tumor located in a nonfrontal area was an independent factor of poor prognosis [10]. With its invasive profile, glioma has a high propension to migrate along the subcortical fibers, as shown radiologically [41,42][41][42] as well as pathologically: biopsy samples evidenced that the tumor cells followed the white matter tracts and were slightly more concentrated in the peripheral parts of those tracts [43]. It seems that the myelin status could play a pivotal role not only in LGG invasion in adults but also possibly in its origin in teenagers [44]. Biomathematical models have attempted to anticipate the profile of glioma evolution [45], knowing nonetheless that this pattern might change over time, maybe at least partly in relation to the therapeutic effects; for example, a bulky LGG before surgery may switch towards a predominantly diffuse pattern after incomplete resection [40]. Such a parameter is critical for treatment selection, because white matter connectivity represents the main limitation of neuroplasticity (see below). This could be a major problem for achieving massive surgical resection or for wide brain radiation therapy if the patient hopes to preserve an optimal QoL, particular in very diffuse LGG [23].2.1.4. The Peritumoral Zone
LGG is a heterogeneous and poorly circumscribed neoplasm with isolated tumor cells (ITC) that extend beyond the margins of the lesion depicted on MRI, as demonstrated by biopsy samples taken from within and beyond the “glioma core” (visible as a T2-FLAIR hypersignal on MRI); ITCs have been detected behind such signal abnormalities [43,46][43][46]. It is worth noting that the cycling tumor cell fraction was higher at the limits of the MRI-defined abnormalities than when closer to the center of the tumor, in 62.5% of patients [47]. This could explain the high risk of glioma relapse at the periphery of the surgical cavity, even following large resection [48]. Efforts to demarcate the glioma core from the surrounding healthy brain led to the definition of an intermediate region, the so-called peritumoral zone (PTZ) [49]. An important interindividual variability exists regarding this PTZ, as demonstrated by samples which found ITC from 10 to 20 mm around the glioma core [43[43][46],46], in agreement with the fact that the tumor core might be more bulky or more diffuse (see above). Interestingly, recent investigations have indicated that this interface between the glioma core and the healthy brain represents a specific metabolic and cellular entity. Such characteristics of the PTZ that are being increasingly explored through radiomics and radiogenomics [50,51][50][51] may play a pivotal role for decision making in the management of diffuse LGG.2.1.5. Metabolic Changes
While still a matter of debate from a radiological perspective, the occurrence of an enhancement during the course of LGG is usually associated with MT [52,53][52][53]. However, if the tumor has already become more aggressive when the (re)treatment is proposed, this means in essence that the opportunity for action has been missed [38]. Therefore, since the main oncological goal is to prevent MT, additional non-invasive metabolic information may be useful in order to predict when the LGG has a higher risk of degeneration. First, an increase of perfusion or diffusion value(s) obtained through sequential and multimodal MRI could be a predictor of changes in glioma behavior [54], possibly identifiable using new machine-learning classifiers [55], and might prompt earlier (re)treatment. In the same spirit, recent advances in PET scanning using tracers easily accessible in routine practice (such as F-DOPA) have enabled an increase in sensitivity for the detection of foci of MT within the LGG, before the onset of enhancement [56]. As mentioned, metabolic imaging could also be helpful to better investigate the PTZ [49]. This additional information could be of utmost interest for deciding the best timings of new therapies during follow-up of LGG patients.2.2. External Factors to the Glioma
2.2.1. Familial Predisposition
While the majority of gliomas are sporadic in origin, familial gliomas have been described, although these are exceptionally rare, especially in the form of LGG [69,70][57][58]. A potential heritable etiology for glioma families has been evoked; specifically, high-penetrance familial mutations and common low-penetrance susceptibility loci (e.g., single-nucleotide polymorphisms (SNPs)) may contribute to familial glioma risk [70,71][58][59]. Nonetheless, recent series have shown that familial gliomas, including LGG, showed similar genomic and molecular biomarker profiles to sporadic gliomas, consistent with the similarity in their clinical features [72,73][60][61]. However, identification of new susceptibility factors in familial LGG might help to elucidate the molecular pathogenesis of gliomas [73][61]. In practice, to increase the chances of earlier diagnosis of possible LGG, screening can be offered to relatives of gliomas patients, and such an “intentional discovery” may lead to more rapid treatment in the first period of the disease [74][62].2.2.2. Age
Older age has been correlated with poorer oncological outcomes in LGG patients, even though the cut-off may vary, e.g., 40 years [28,29,30][27][28][29] versus 55 years [10]. Nevertheless, even if the prognosis seems to be directly linked to age per se, it cannot be rule out that glioma discovery in a younger patient may mean that the diagnosis was made at an earlier stage of the tumoral disease—therefore, with lesser volume and fewer mutational changes. Furthermore, LGG mainly affects young adults, explaining why screening policy design has suggested the application of MRI in a selected population before 40 years [75][63]. In clinical routine, beyond the age at diagnosis and during the years (or even decades) of LGG management, practitioners be aware that the risk of MT is potentially increasing as the patient becomes older, justifying continuation of regular surveillance even in cases of long-term tumor stabilization [40].3. Predicting Neural Interindividual Variability and Its Changes over Time
Although linked, factors related to the clinical–psychological status of the patient and those related to the dynamics of neural network reconfiguration are here considered separately.3.1. Factors Related to the Clinical–Psychological Status of the Patient
3.1.1. Epilepsy
Seizure is the first symptom in LGG, leading to diagnosis in the vast majority of patients [104][64]. An epileptic symptomatology is linked to a better oncological outcome [10]. Interestingly, computational models have evidenced that the onset of seizures corresponds with a time point which may already represent the overcompensation stage of cerebral plasticity, depending on the tumor growth rate and its pattern of progression, in particular in the event of massive invasion of the white matter tracts [105][65]. Translation of these results into the clinical situation is another argument in favor of early surgery in asymptomatic patients, before the occurrence of epilepsy [74][62]. Indeed, seizures can have a negative impact on daily life, in particular by preventing driving (and indirectly employment) for medico-legal reasons [91,106][66][67]. Furthermore, epilepsy is mainly elicited by the diffusion of the tumoral cells at the periphery of the LGG (and not by the glioma core itself) [107][68]. This explains why larger surgical resection has a higher impact on epilepsy. Indeed, postoperative seizure control is more likely when EOR is ≥91% and/or residual tumor volume is ≤19 cc [108][69]. This also supports the suggestion of supratotal resection (with removal of the PTZ) for functional reasons (in addition to the improvement of oncological outcomes), i.e., with an optimization of QoL as a result of freedom from epilepsy [49]. Notably, important inter-individual variability has been observed, with about 15% of LGG patients experiencing intractable seizures, notably in temporal and/or paralimbic gliomas. In this situation, it has been proposed to remove the hippocampus, even if not invaded by the tumor according to preoperative MRI, since this can result in significant improvement of epilepsy control [109][70]. During follow-up, the reappearance of seizures may be correlated with LGG relapse, and may prompt clinicians to propose reoperation prior to MT [25]. When further resection is not possible due to diffusion within critical structures, for example within the Rolandic area which is very epileptogenic [110][71], adjuvant medical treatment can have an impact on seizures [111][72].3.1.2. Cognitive and Emotional Status
Because LGG patients are generally young and enjoy active lives at diagnosis, presenting no or only slight deficit at so-called “standard neurological examination”, it has been claimed in the literature that these patients do not exhibit any significant functional disturbances [6]. However, important variations are found across patients in terms of their neurocognitive status. In fact, a recent cohort including 157 LGG patients who benefited from extensive neuropsychological evaluation before treatment showed that 55.4% of them had already experienced cognitive decline, in particular with respect to language, verbal episodic memory, psychomotor speed, attention, and executive functions (phonological and categorical fluency) [4]. Interestingly, neurocognitive impairments have also been found in patients with incidental LGG [112][73].3.1.3. Neurological Status
Due to earlier diagnosis of LGG, moderate or severe neurological deficits at clinical examination (e.g., hemiparesis or aphasia) are usually rare [17]. Beyond possible episodes of transient worsening which might be elicited by repeat seizures, permanent impairments that arise are generally due to voluminous gliomas with mass effect and/or to MT, with acceleration of the neoplasm kinetics. In other words, with more “prophylactic management”, such major deteriorations should cease to occur before the last stage of the disease, thus giving the opportunity for LGG patients to enjoy active lives for many years or even decades [7].3.1.4. Patients’ Needs
Definition of QoL is eminently variable from one human being to another. Indeed, beyond the fact that patients do not want to experience hemiplegia or aphasia, especially following surgical resection, their expectations are very different according to their lifestyles: do they work? (if yes, what kind of employment they have, and do they want to resume their professional activities postoperatively and/or during medical treatment?); what are their hobbies? (e.g., do they practice sports, art, etc.?); what is their socio-cultural environment? (e.g., do they speak multiple languages?) [127][74], do they need to drive? (if yes, what about possible medico-legal issues in case of visual field deficit and/or seizures?) [106][67]. On the basis of these individual wishes, with the aim of enabling each patient to develop long-term projects (such as getting married, having a baby, buying a house, etc.), and also with due consideration given to the neurocognitive assessment at diagnosis (i.e., the presence or otherwise of some degrees of disturbance), it is possible to elaborate tailored management “à la carte”, beginning with a selection of optimal tasks to be performed during awake surgery [128][75]. Moreover, therapeutic strategies should be re-adapted over time, not only according to the LGG course, but also taking into account possible changes in the patient’s priorities. An example of such an intra-individual variability could be when a patient would like to preserve executive functions during the first surgery because he or she was employed at the time, but sparing higher-order cognitive capacities is no longer absolutely mandatory a few years later because the patient has retired in the meantime. Therefore, a clear and extensive explanation of the principles of chronic tumoral disease should be provided to the patient and his or her relatives after the diagnosis. They should understand that management necessitates surveillance with constant anticipation of a specific lifelong therapeutic strategy, to give them the opportunity to make choices for their current and future life by thinking one step ahead—which increases the chance of finding a better psychological equilibrium [7,23][7][23].3.2. Factors Related to the Dynamics of the Neural Network Reconfiguration
3.2.1. Patterns of Neuroplasticity
The structural anatomy of the brain is highly variable across healthy individuals, especially at the cortical level [129][76], while variations are less pronounced at the level of the white matter tracts [18]. Furthermore, advances in non-invasive functional imaging methods which permit the investigation of the functional connectivity have resulted in the development of a large database demonstrating between-subject variability in the distribution of neural networks [130,131][77][78]. Recent models of neurocognition employed in basic neuroscience have rejected the classical localizationist dogma (one cerebral site underpinning one specific function), and go beyond a simple network organization of the central nervous system (one cerebral circuit underpinning one specific function) by evidencing the critical role of dynamic interplay within and across neural networks which allows behaviors to be constantly adapted to the surrounding world [132][79]. According to this meta-networking framework (based on a network of networks), complex cognitive abilities are made possible by the activation and coordination (combination or competition) of large-scale neural circuits involving domain-specific networks (e.g., movement or language circuits), and the activity of a multiple-demand system recruited during the performance of a wide range of cognitive-demanding activities with the aim of maintaining fluid intelligence [132,133][79][80]. Alongside this flexible and ever-changing physiological organization of the functional connectome, inter-individual anatomo-functional variability is significantly increased in brain-damaged patients, particularly in the event of slowly evolving lesions such as LGG [134][81]. These mechanisms of neuroplastic functional reshaping permit neurological compensation during LGG growth (explaining why the vast majority of patients are active at diagnosis), at least to some extent, considering that over half of LGG patients already experience some degree of cognitive disturbance at the first neuropsychological evaluation, as previously mentioned [4]. Maps of neuroplasticity have evidenced that potential for cortical reallocation is high (except for input such as the primary visual cortex and output such as the primary motor cortex), whereas axonal connectivity represents the main limit of functional reshaping [135,136,137][82][83][84]. Thus, as in healthy subjects, variability across LGG patients is greater for cortical than subcortical reorganization [17]. This implies that various dynamic processes of neural reconfiguration may be mobilized from one patient to another, such as peritumoral rearrangement, or recruitment of remote structures in the ipsilesional hemisphere and/or the contralateral side [21,22][21][22]. Inter-patient differences in such patterns of redistribution are strongly correlated with LGG characteristics, i.e., the volume of the tumor, the kinetics of the glioma (as plasticity is linked to the time course of the disease, with less compensation in more rapidly evolving lesions [134][81]), and to the severity of brain invasion, with less plastic potential in more diffuse tumors which migrate more widely along the white matter pathways [44,138][44][85]. These connectomal considerations play a pivotal role when selecting the optimal therapeutic attitude, knowing that better prediction of the individual processes of neural reconfiguration may be valuable for anticipating the next treatment(s), thereby avoiding missing the opportunity for action from an oncological point of view.References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251.
- Darlix, A.; Gozé, C.; Rigau, V.; Bauchet, L.; Taillandier, L.; Duffau, H. The etiopathogenesis of diffuse low-grade gliomas. Crit. Rev. Oncol. Hematol. 2017, 109, 51–62.
- Pallud, J.; Fontaine, D.; Duffau, H.; Mandonnet, E.; Sanai, N.; Taillandier, L.; Peruzzi, P.; Guillevin, R.; Bauchet, L.; Bernier, V.; et al. Natural history of incidental World Health Organization grade II gliomas. Ann. Neurol. 2010, 68, 727–733.
- Lemaitre, A.L.; Herbet, G.; Ng, S.; Moritz-Gasser, S.; Duffau, H. Cognitive preservation following awake mapping-based neurosurgery for low-grade gliomas: A longitudinal, within-patient design study. Neuro-Oncology 2022, 24, 781–793.
- Mandonnet, E.; Delattre, J.Y.; Tanguy, M.L.; Swanson, K.R.; Carpentier, A.F.; Duffau, H.; Cornu, P.; Van Effenterre, R.; Alvord, E.C., Jr.; Capelle, L. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann. Neurol. 2003, 53, 524–528.
- Smits, A.; Jakola, A.S. Clinical Presentation, Natural History, and Prognosis of Diffuse Low-Grade Gliomas. Neurosurg. Clin. N. Am. 2019, 30, 35–42.
- Duffau, H.; Taillandier, L. New Concepts in the Management of Diffuse Low-Grade Glioma: Proposal of a Multistage and Individualized Therapeutic Approach. Neuro-Oncology 2015, 17, 332–342.
- Chaichana, K.L.; McGirt, M.J.; Laterra, J.; Olivi, A.; Quiñones-Hinojosa, A. Recurrence and Malignant Degeneration After Resection of Adult Hemispheric Low-Grade Gliomas. J. Neurosurg. 2010, 112, 10–17.
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; Vandenberg, S.; McDermott, M.W.; Berger, M.S. Role of Extent of Resection in the Long-Term Outcome of Low-Grade Hemispheric Gliomas. J. Clin. Oncol. 2008, 26, 1338–1345.
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Baron, M.H.; Kujas, M.; et al. Spontaneous and Therapeutic Prognostic Factors in Adult Hemispheric World Health Organization Grade II Gliomas: A Series of 1097 Cases. J. Neurosurg. 2013, 118, 1157–1168.
- Jakola, A.S.; Skjulsvik, A.J.; Myrmel, K.S.; Sjåvik, K.; Unsgård, G.; Torp, S.H.; Aaberg, K.; Berg, T.; Dai, H.Y.; Johnsen, K.; et al. Surgical Resection Versus Watchful Waiting in Low-Grade Gliomas. Ann. Oncol. 2017, 28, 1942–1948.
- Hamdan, N.; Duffau, H. Extending the Multistage Surgical Strategy for Recurrent Initially Low-Grade Gliomas: Functional and Oncological Outcomes in 31 Consecutive Patients Who Underwent a Third Resection Under Awake Mapping. J. Neurosurg. 2021, 136, 1035–1044.
- Ius, T.; Ng, S.; Young, J.S.; Tomasino, B.; Polano, M.; Ben-Israel, D.; Kelly, J.J.P.; Skrap, M.; Duffau, H.; Berger, M.S. The benefit of early surgery on overall survival in incidental low-grade glioma patients: A multicenter study. Neuro-Oncology 2022, 24, 624–638.
- Pedeutour-Braccini, Z.; Burel-Vandenbos, F.; Gozé, C.; Roger, C.; Bazin, A.; Costes-Martineau, V.; Duffau, H.; Rigau, V. Microfoci of Malignant Progression in Diffuse Low-Grade Gliomas: Towards the Creation of an Intermediate Grade in Glioma Classification? Virchows. Arch. 2015, 466, 433–444.
- Roodakker, K.R.; Alhuseinalkhudhur, A.; Al-Jaff, M.; Georganaki, M.; Zetterling, M.; Berntsson, S.G.; Danfors, T.; Strand, R.; Edqvist, P.H.; Dimberg, A.; et al. Region-by-Region Analysis of PET, MRI, and Histology in En Bloc-Resected Oligodendrogliomas Reveals Intra-Tumoral Heterogeneity. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 569–579.
- Kirby, A.J.; Lavrador, J.P.; Bodi, I.; Vergani, F.; Bhangoo, R.; Ashkan, K.; Finnerty, G.T. Multicellular “Hotspots” Harbor High-Grade Potential in Lower-Grade Gliomas. Neurooncol. Adv. 2021, 3, vdab026.
- Duffau, H. (Ed.) Diffuse Low-Grade Gliomas in Adults, 2nd ed.; Springer: London, UK, 2017.
- Duffau, H. A two-level model of interindividual anatomo-functional variability of the brain and its implications for neurosurgery. Cortex 2017, 86, 303–313.
- Gerin, C.; Pallud, J.; Grammaticos, B.; Mandonnet, E.; Deroulers, C.; Varlet, P.; Capelle, L.; Taillandier, L.; Bauchet, L.; Duffau, H.; et al. Improving the time-machine: Estimating date of birth of grade II gliomas. Cell. Prolif. 2012, 45, 76–90.
- Duffau, H. Lessons from brain mapping in surgery for low-grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol. 2005, 4, 476–486.
- Almairac, F.; Deverdun, J.; Cochereau, J.; Coget, A.; Lemaitre, A.L.; Moritz-Gasser, S.; Duffau, H.; Herbet, G. Homotopic redistribution of functional connectivity in insula-centered diffuse low-grade glioma. Neuroimage Clin. 2021, 29, 102571.
- Duffau, H. Functional Mapping before and after Low-Grade Glioma Surgery: A New Way to Decipher Various Spatiotemporal Patterns of Individual Neuroplastic Potential in Brain Tumor Patients. Cancers 2020, 12, 2611.
- Duffau, H. Dynamic Interplay between Lower-grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy. Cancers 2021, 13, 4759.
- Duffau, H. Brain connectomics applied to oncological neuroscience: From a traditional surgical strategy focusing on glioma topography to a meta-network approach. Acta Neurochir. 2021, 163, 905–917.
- Duffau, H. Repeated Awake Surgical Resection(s) for Recurrent Diffuse Low-Grade Gliomas: Why, When, and How to Reoperate? Front. Oncol. 2022, 12, 947933.
- Rossi, M.; Gay, L.; Ambrogi, F.; Conti Nibali, M.; Sciortino, T.; Puglisi, G.; Leonetti, A.; Mocellini, C.; Caroli, M.; Cordera, S.; et al. Association of Supratotal Resection with Progression-Free Survival, Malignant Transformation, and Overall Survival in Lower-Grade Gliomas. Neuro-Oncology 2021, 23, 812–826.
- Karim, A.B.; Maat, B.; Hatlevoll, R.; Menten, J.; Rutten, E.H.; Thomas, D.G.; Mascarenhas, F.; Horiot, J.C.; Parvinen, L.M.; van Reijn, M.; et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 549–556.
- van den Bent, M.J.; Afra, D.; de Witte, O.; Ben Hassel, M.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.O.; Collette, L.; Piérart, M.; Mirimanoff, R.; et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet. 2005, 366, 985–990.
- Pignatti, F.; van den Bent, M.; Curran, D.; Debruyne, C.; Sylvester, R.; Therasse, P.; Afra, D.; Cornu, P.; Bolla, M.; Vecht, C.; et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J. Clin. Oncol. 2002, 20, 2076–2084.
- Obara, T.; Blonski, M.; Brzenczek, C.; Mézières, S.; Gaudeau, Y.; Pouget, C.; Gauchotte, G.; Verger, A.; Vogin, G.; Moureaux, J.-M.; et al. Adult Diffuse Low-Grade Gliomas: 35-Year Experience at the Nancy France Neurooncology Unit. Front. Oncol. 2020, 10, 574679.
- Suzuki, H.; Aoki, K.; Chiba, K.; Sato, Y.; Shiozawa, Y.; Shiraishi, Y.; Shimamura, T.; Niida, A.; Motomura, K.; Ohka, F.; et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015, 47, 458–468.
- Mazor, T.; Chesnelong, C.; Pankov, A.; Jalbert, L.E.; Hong, C.; Hayes, J.; Smirnov, I.V.; Marshall, R.; Souza, C.F.; Shen, Y.; et al. Clonal expansion and epigenetic reprogramming following deletion or amplification of mutant IDH1. Proc. Natl. Acad. Sci. USA 2017, 114, 10743–10748.
- Boetto, J.; Ng, S.; Duffau, H. Predictive Evolution Factors of Incidentally Discovered Suspected Low-Grade Gliomas: Results From a Consecutive Series of 101 Patients. Neurosurgery 2021, 88, 797–803.
- Mandonnet, E.; de Witt Hamer, P.; Pallud, J.; Bauchet, L.; Whittle, I.; Duffau, H. Silent diffuse low-grade glioma: Toward screening and preventive treatment? Cancer 2014, 120, 1758–1762.
- Pallud, J.; Blonski, M.; Mandonnet, E.; Audureau, E.; Fontaine, D.; Sanai, N.; Bauchet, L.; Peruzzi, P.; Frénay, M.; Colin, P.; et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro-Oncology 2013, 15, 595–606.
- Gozé, C.; Blonski, M.; Le Maistre, G.; Bauchet, L.; Dezamis, E.; Page, P.; Varlet, P.; Capelle, L.; Devaux, B.; Taillandier, L.; et al. Imaging growth and isocitrate dehydrogenase 1 mutation are independent predictors for diffuse low-grade gliomas. Neuro-Oncology 2014, 16, 1100–1109.
- Pallud, J.; Taillandier, L.; Capelle, L.; Fontaine, D.; Peyre, M.; Ducray, F.; Duffau, H.; Mandonnet, E. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma: A plea for systematic measurement of growth rates. Neurosurgery 2012, 71, 729–739.
- Pallud, J.; Capelle, L.; Taillandier, L.; Badoual, M.; Duffau, H.; Mandonnet, E. The silent phase of diffuse low-grade gliomas. Is it when we missed the action? Acta Neurochir. 2013, 155, 2237–2242.
- Mandonnet, E.; Pallud, J.; Fontaine, D.; Taillandier, L.; Bauchet, L.; Peruzzi, P.; Guyotat, J.; Bernier, V.; Baron, M.H.; Duffau, H.; et al. Inter- and intrapatients comparison of WHO grade II glioma kinetics before and after surgical resection. Neurosurg. Rev. 2010, 33, 91–96.
- Ferracci, F.X.; Michaud, K.; Duffau, H. The landscape of postsurgical recurrence patterns in diffuse low-grade gliomas. Crit. Rev. Oncol. Hematol. 2019, 138, 148–155.
- Mandonnet, E.; Capelle, L.; Duffau, H. Extension of paralimbic low grade gliomas: Toward an anatomical classification based on white matter invasion patterns. J. Neurooncol. 2006, 78, 179–185.
- Latini, F.; Fahlström, M.; Beháňová, A.; Sintorn, I.M.; Hodik, M.; Staxäng, K.; Ryttlefors, M. The Link between Gliomas Infiltration and White Matter Architecture Investigated with Electron Microscopy and Diffusion Tensor Imaging. Neuroimage Clin. 2021, 31, 102735.
- Zetterling, M.; Roodakker, K.R.; Berntsson, S.G.; Edqvist, P.H.; Latini, F.; Landtblom, A.M.; Pontén, F.; Alafuzoff, I.; Larsson, E.M.; Smits, A. Extension of Diffuse Low-Grade Gliomas beyond Radiological Borders as Shown by the Coregistration of Histopathological and Magnetic Resonance Imaging Data. J. Neurosurg. 2016, 125, 1155–1166.
- Duffau, H. White Matter Tracts and Diffuse Lower-Grade Gliomas: The Pivotal Role of Myelin Plasticity in the Tumor Pathogenesis, Infiltration Patterns, Functional Consequences and Therapeutic Management. Front. Oncol. 2022, 12, 855587.
- Mandonnet, E. Biomathematical Modeling of DLGG. In Diffuse Low-Grade Gliomas in Adults, 2nd ed.; Duffau, H., Ed.; Springer: London, UK, 2017; pp. 651–664.
- Pallud, J.; Varlet, P.; Devaux, B.; Geha, S.; Badoual, M.; Deroulers, C.; Page, P.; Dezamis, E.; Daumas-Duport, C.; Roux, F.-X. Diffuse Low-Grade Oligodendrogliomas Extend beyond MRI-Defined Abnormalities. Neurology 2010, 74, 1724–1731.
- Gerin, C.; Pallud, J.; Deroulers, C.; Varlet, P.; Oppenheim, C.; Roux, F.X.; Chrétien, F.; Thomas, S.R.; Grammaticos, B.; Badoual, M. Quantitative Characterization of the Imaging Limits of Diffuse Low-Grade Oligodendrogliomas. Neuro-Oncology 2013, 15, 1379–1388.
- Duffau, H. Long-Term Outcomes after Supratotal Resection of Diffuse Low-Grade Gliomas: A Consecutive Series with 11-Year Follow-Up. Acta Neurochir. 2016, 158, 51–58.
- Silva, M.; Vivancos, C.; Duffau, H. The Concept of «Peritumoral Zone» in Diffuse Low-Grade Gliomas: Oncological and Functional Implications for a Connectome-Guided Therapeutic Attitude. Brain Sci. 2022, 12, 504.
- Abrol, S.; Kotrotsou, A.; Salem, A.; Zinn, P.O.; Colen, R.R. Radiomic Phenotyping in Brain Cancer to Unravel Hidden Information in Medical Images. Top. Magn. Reson. Imaging 2017, 26, 43–53.
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and Radiogenomics in Gliomas: A Contemporary Update. Br. J. Cancer 2021, 125, 641–657.
- Pallud, J.; Capelle, L.; Taillandier, L.; Fontaine, D.; Mandonnet, E.; Guillevin, R.; Bauchet, L.; Peruzzi, P.; Laigle-Donadey, F.; Kujas, M.; et al. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro-Oncology 2009, 11, 176–182.
- Krigers, A.; Demetz, M.; Grams, A.E.; Thomé, C.; Freyschlag, C.F. The diagnostic value of contrast enhancement on MRI in diffuse and anaplastic gliomas. Acta Neurochir. 2022, 164, 2035–2040.
- Chen, I.E.; Swinburne, N.; Tsankova, N.M.; Hefti, M.M.; Aggarwal, A.; Doshi, A.H.; Hormigo, A.; Delman, B.N.; Nael, K. Sequential Apparent Diffusion Coefficient for Assessment of Tumor Progression in Patients with Low-Grade Glioma. AJNR Am. J. Neuroradiol. 2018, 39, 1039–1046.
- Hashido, T.; Saito, S.; Ishida, T. Radiomics-Based Machine Learning Classification for Glioma Grading Using Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2021, 45, 606–613.
- Girard, A.; Le Reste, P.J.; Metais, A.; Carsin Nicol, B.; Chiforeanu, D.C.; Bannier, E.; Campillo-Gimenez, B.; Devillers, A.; Palard-Novello, X.; Le Jeune, F. Combining 18F-DOPA PET and MRI with perfusion-weighted imaging improves delineation of high-grade subregions in enhancing and non-enhancing gliomas prior treatment: A biopsy-controlled study. J. Neurooncol. 2021, 155, 287–295.
- Sadetzki, S.; Bruchim, R.; Oberman, B.; Armstrong, G.N.; Lau, C.C.; Claus, E.B.; Barnholtz-Sloan, J.S.; Il’yasova, D.; Schildkraut, J.; Johansen, C.; et al. Gliogene Consortium. Description of selected characteristics of familial glioma patients-results from the Gliogene Consortium. Eur. J. Cancer. 2013, 49, 1335–1345.
- Osorio, J.A.; Hervey-Jumper, S.L.; Walsh, K.M.; Clarke, J.L.; Butowski, N.A.; Prados, M.D.; Berger, M.S. Familial gliomas: Cases in two pairs of brothers. J. Neurooncol. 2015, 121, 135–140.
- Mukherjee, S.; Stroberg, E.; Wang, F.; Morales, L.; Shan, Y.; Rao, A.; Huang, J.H.; Wu, E.; Fonkem, E. SMARCB1 Gene Mutation Predisposes to Earlier Development of Glioblastoma: A Case Report of Familial GBM. J. Neuropathol. Exp. Neurol. 2020, 79, 562–565.
- Lu, J.; Burnett, M.G.; Shpak, M.A. Comparative Study of the Molecular Characteristics of Familial Gliomas and Other Cancers. Cancer Genom. Proteom. 2016, 13, 467–474.
- Jacobs, D.I.; Fukumura, K.; Bainbridge, M.N.; Armstrong, G.N.; Tsavachidis, S.; Gu, X.; Doddapaneni, H.V.; Hu, J.; Jayaseelan, J.C.; Muzny, D.M.; et al. Elucidating the molecular pathogenesis of glioma: Integrated germline and somatic profiling of a familial glioma case series. Neuro-Oncology 2018, 20, 1625–1633.
- Duffau, H. Higher-Order Surgical Questions for Diffuse Low-Grade Gliomas: Supramaximal Resection, Neuroplasticity, and Screening. Neurosurg. Clin. N. Am. 2019, 30, 119–128.
- Mandonnet, E.; Taillandier, L.; Duffau, H. Proposal of screening for diffuse low-grade gliomas in the population from 20 to 40 years. Presse Med. 2017, 46, 911–920.
- Pallud, J.; Audureau, E.; Blonski, M.; Sanai, N.; Bauchet, L.; Fontaine, D.; Mandonnet, E.; Dezamis, E.; Psimaras, D.; Guyotat, J.; et al. Epileptic Seizures in Diffuse Low-Grade Gliomas in Adults. Brain 2014, 137, 449–462.
- Szalisznyo, K.; Silverstein, D.N.; Duffau, H.; Smits, A. Pathological neural attractor dynamics in slowly growing gliomas supports an optimal time frame for white matter plasticity. PLoS. ONE 2013, 8, e69798.
- Ng, S.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Return to Work Following Surgery for Incidental Diffuse Low-Grade Glioma: A Prospective Series With 74 Patients. Neurosurgery 2020, 87, 720–729.
- Vanacôr, C.; Duffau, H. Analysis of Legal, Cultural, and Socioeconomic Parameters in Low-Grade Glioma Management: Variability Across Countries and Implications for Awake Surgery. World Neurosurg. 2018, 120, 47–53.
- Pallud, J.; Le Van Quyen, M.; Bielle, F.; Pellegrino, C.; Varlet, P.; Cresto, N.; Baulac, M.; Duyckaerts, C.; Kourdougli, N.; Chazal, G.; et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci. Transl. Med. 2014, 6, 244ra89.
- Still, M.E.H.; Roux, A.; Huberfeld, G.; Bauchet, L.; Baron, M.H.; Fontaine, D.; Blonski, M.; Mandonnet, E.; Guillevin, R.; Guyotat, J.; et al. Extent of Resection and Residual Tumor Thresholds for Postoperative Total Seizure Freedom in Epileptic Adult Patients Harboring a Supratentorial Diffuse Low-Grade Glioma. Neurosurgery 2019, 85, E332–E339.
- Ghareeb, F.; Duffau, H. Intractable epilepsy in paralimbic Word Health Organization Grade II gliomas: Should the hippocampus be resected when not invaded by the tumor? J. Neurosurg. 2012, 116, 1226–1234.
- Schucht, P.; Ghareeb, F.; Duffau, H. Surgery for low-grade glioma infiltrating the central cerebral region: Location as a predictive factor for neurological deficit, epileptological outcome, and quality of life. J. Neurosurg. 2013, 119, 318–323.
- Sherman, J.H.; Moldovan, K.; Yeoh, H.K.; Starke, R.M.; Pouratian, N.; Shaffrey, M.E.; Schiff, D. Impact of Temozolomide Chemotherapy on Seizure Frequency in Patients with Low-Grade Gliomas. J. Neurosurg. 2011, 114, 1617–1621.
- Cochereau, J.; Herbet, G.; Duffau, H. Patients With Incidental WHO Grade II Glioma Frequently Suffer From Neuropsychological Disturbances. Acta Neurochir. 2016, 158, 305–312.
- Sellier, A.; Moritz-Gasser, S.; Lemaitre, A.L.; Herbet, G.; Duffau, H. Presence of a translator in the operating theater for awake mapping in foreign patients with low-grade glioma: A surgical experience based on 18 different native languages. J. Neurosurg. 2020, 135, 496–504.
- Duffau, H. New Philosophy, Clinical Pearls, and Methods for Intraoperative Cognition Mapping and Monitoring “a La Carte” in Brain Tumor Patients. Neurosurgery 2021, 88, 919–930.
- Ono, M.; Kubik, S.; Abernathey, C.D. Atlas of the Cerebral Sulci; Thieme Medical Publishers: Stuttgart, Germany, 1990.
- Naveau, M.; Doucet, G.; Delcroix, N.; Petit, L.; Zago, L.; Crivello, F.; Jobard, G.; Mellet, E.; Tzourio-Mazoyer, N.; Mazoyer, B.; et al. A novel group ICA approach based on multi-scale individual component clustering. Application to a large sample of fMRI data. Neuroinformatics 2012, 10, 269–285.
- Mazoyer, B.; Mellet, E.; Perchey, G.; Zago, L.; Crivello, F.; Jobard, G.; Delcroix, N.; Vigneau, M.; Leroux, G.; Petit, L.; et al. BIL&GIN: A neuroimaging, cognitive, behavioral, and genetic database for the study of human brain lateralization. NeuroImage 2015, 124, 1225–1231.
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 100, 1181–1228.
- Woolgar, A.; Duncan, J.; Manes, F.; Fedorenko, E. The Multiple-Demand System But Not the Language System Supports Fluid Intelligence. Nat. Hum. Behav. 2018, 2, 200–204.
- Desmurget, M.; Bonnetblanc, F.; Duffau, H. Contrasting acute and slow-growing lesions: A new door to brain plasticity. Brain 2007, 130, 898–914.
- Ius, T.; Angelini, E.; Thiebaut de Schotten, M.; Mandonnet, E.; Duffau, H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: Towards a “minimal common brain”. Neuroimage 2011, 56, 992–1000.
- Herbet, G.; Maheu, M.; Costi, E.; Lafargue, G.; Duffau, H. Mapping neuroplastic potential in brain-damaged patients. Brain 2016, 139, 829–844.
- Sarubbo, S.; Tate, M.; De Benedictis, A.; Merler, S.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: An original functional atlas of the human brain. Neuroimage 2020, 205, 116237.
- Picart, T.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Iterative Surgical Resections of Diffuse Glioma With Awake Mapping: How to Deal With Cortical Plasticity and Connectomal Constraints? Neurosurgery 2019, 85, 105–116.
