Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Beatrix Zheng and Version 5 by Beatrix Zheng.
Sirtfood is a new concept food that compounds diets that can target sirtuins (SIRTs). SIRTs are nicotinamide adenine dinucleotide (NAD+)-dependent deacylases and ADP-ribosyltransferases (enzymes). SIRTs are mediators of calorie restriction (CR) and their activation can achieve some effects similar to CR. SIRTs play essential roles in ameliorating obesity and age-related metabolic diseases. Food ingredients such as resveratrol, piceatannol, anthocyanidin, and quinine are potential modulators of SIRTs. SIRT modulators are involved in autophagy, apoptosis, aging, inflammation, and energy homeostasis. Sirtfood proponents believe that natural Sirtfood recipes exert significant health effects.
- Sirtfoods
- SIRT
- SIRT-modulating compound
- polyphenol
1. SIRT Genes
The first discovered silent information regulator 2 (Sirt2) gene prototype was the mating-type regulator 1 (MAR1); it was found in yeast and suppressed ribosomal DNA recombination, silenced genes, and regulated replicative lifespan [1]. Sirt2 proteins are universally present in all living organisms but become functionally complex in complex organisms [2]. The core of the SIRT structure consists of three parts: a large domain called the Rossman fold, characterized as the nicotinamide adenine dinucleotide (NAD+)-binding unit; a smaller Zn2+-binding motif domain; and an α-helical region that differentiates the seven mammalian SIRT family members [3]. SIRTs cleave acetyl groups from acetylated lysine in histones and other substrate proteins—resulting in condensed and inactive chromatin structures and gene silencing.
SIRT bioactivities are mainly controlled by dynamic changes in NAD+ levels and the NAD/NADH ratio, enabling cells to accept and donate electrons during essential reactions. NAD+ is deacetylated through a two-step reaction. In the first step, NAD+ is consumed to yield nicotinamide (NAM) and a 2- and 3-O-acetyl-ADP-ribose (OAADPr) mix. The second step releases deacetylated substrates [4]. The recognition of NAD+ has partly been due to the discovery of the mammalian SIRT family genes. SIRTs 1–7 genes’ differential subcellular localization, catalytic domain (CD) sequence, N/C-terminal do-main length, catalytic actions, and dependence on changes in NAD+ level allow for rapid regulation of many bioactivities in multiple tissues [1][5][6]; Different phytochemicals can modulate different SIRT members, and different SIRT members have different locations and substrates in cells, meanwhile, SIRT members have discrepancy functions and also have similar effects [7][8][9].
SIRTs are substrate-specific and are activated during CR. SIRTs are involved in cell proliferation, differentiation, DNA damage repair, genome stability, life extension, energy homeostasis, stress resistance, organ development, aging, cancer, tissue regeneration, inflammation, neuronal signaling, and circadian rhythms [5][6][10]; see Table 1. CR condition decreases cellular energy status, increases the AMP:ATP ratio, and activates the AMP-activated protein kinase (AMPK) pathway. There is growing interest in targeting SIRTs through diets, which promote health primarily by activating the NAD+/SIRT pathway and their downstream components [8][11]; see Figure 1.
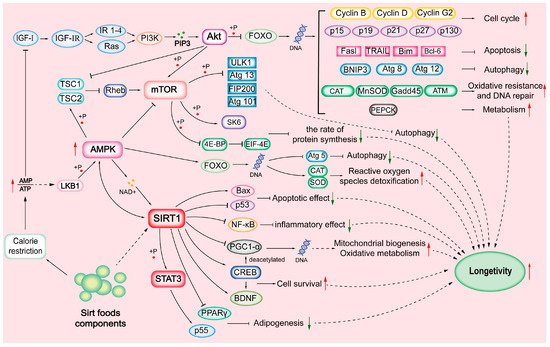
Figure 1.
Sirtuin (SIRT)-mediated mechanisms.
2. SIRTs and Oxidative Stress
Normal cellular functions generate reactive oxygen species (ROS) in the mitochondria. Excessive production and accumulation of ROS impair cellular viability through DNA structure damage and oxidations of protein, fatty acids, and lipids [4][6]. However, SIRT1 and SIRT3 activations are involved in mitohormesis [12]. Here, mitochondrial activities are regulated by deacetylating relevant proteins to stimulate mitohormetic responses—small harmful ROS activations that increase mitochondria number and actions to combat injury. In a recent study, the oral administration of piceatannol (10 mg/kg BW/day) attenuated hepatic and renal mitochondrial oxidative stresses in male albino rats. Piceatannol induces the production of antioxidative response elements (SOD, CAT, GSH-Px, and GR) and suppresses the expression levels of pro-inflammatory (TNF-α, IL 6) and apoptotic (cytochrome c, caspase-3) biomarkers. Piceatannol can exert antioxidation effects through SIRT1/p38/AMPK/PGC-1α pathway activation [13]. Genistein treatment suppresses the production levels of reactive oxygen species (ROS) and malondialdehyde (MDA) in human umbilical vein endothelial cells (HUVECs). Furthermore, genistein upregulates the expression levels of SOD, CAT, glutathione, and GSH-Px in the cells. Zhang et al. [14] found that genistein-mediated oxidative damage protection was via SIRT1 stimulation, which deacetylated FOXO3a. Poljsak and Milisay [15] suggested that NAD+’s role as a signaling molecule influenced transcription factors, helped SIRTs convert ROS, and influenced direct apoptotic response to ROS production. Diet modulators stimulate multiple SIRT family genes and offer protection against oxidative stress.
3. SIRTs and Apoptosis
Apoptosis maintains cell balance in the human body. The process genetically detects abnormal cells and initiates their death [16][17]. The increased permeabilization of the mitochondrial outer membrane, caspase-activating molecules, caspase-independent death effectors, and the disruption of ATP production trigger programmed cell death. Once started, cell death becomes inevitable [18]. The apoptosis process involves cell turnover, new structure development, the immune system, hormone-dependent atrophy, embryonic development, chemical-induced death, and the progression of several diseases [18]. SIRT activation can regulate the apoptosis process. Quercetin treatment alleviates isoniazid-induced hepatotoxicity through the SIRT1/ERK signaling pathway in HepG2 cells. Quercetin reversed isoniazid-induced SIRT1 inhibition, upregulated Bcl-2 expression levels, downregulated Bax, cleaved caspase-3, and cleaved caspase-9 expression levels in [19].
Liang et al. [20] found that lipid accumulation was reduced due to combined genistein and daidzein stimulating colon cancer cell apoptosis. The perilipin-1, ADRP, and Tip-47 family protein expression levels were downregulated by a genistein–daidzein mixture. Meanwhile, expression levels of PPARγ, Fas, FABP, glycerol-3-phosphate acyltransferase, and microsomal TG transfer protein were significantly induced. The genistein–daidzein mixture induced cell apoptosis through significantly increasing FOXO3a and caspase-8 expression and decreasing P13k expression. The activation of the SIRT3 gene decreased stress-induced apoptosis via Bcl2-53 and JNK regulation. Studies showed that the SIRT3 gene suppressed tumor cells via ROS repression and DNA protection [21]. SIRT3-null mice could develop mammary tumors after one year; meanwhile, SIRT3 expression levels of breast cancers were decreased in humans.
4. SIRTs and Autophagy
Autophagy functions as a cellular housekeeper process: it degrades the bulk of defective organelles, aging cells, pathogens, and proteins in eukaryotes. The autophagy process directs the formation of a double-membrane cytoplasmic vesicle and autophagosomes come from the cell’s endoplasmic reticulum [22]. The formed vesicle engulfs target substances, fuses to the lysosome, and degrades them. The efficiency and maintenance of the autophagy process enhance lifespan. Energy-deficient conditions activate the AMPK pathway while stress conditions activate the ULK1 cascade; these regulate NAD+ levels and metabolism and promote autophagy [23]. CR induces SIRT1 activation and p53 deacetylation, which regulate autophagy. In studies by Yang et al. [24], resveratrol treatment could activate SIRT1 to prevent osteoporosis in aging rats. In vivo results showed that resveratrol-mediated SIRT1 and P13k/Akt/mTOR signaling pathways could significantly improve bone quality and protected osteoblasts in rats with osteoporosis.
SIRT1 induces autophagy via two modes. The first deacetylates light chain 3 (LC3), which binds to autophagy proteins (Atg 7 and Atg 8) [25]. The alternative model is REGy, which prevents SIRT1 from binding and deacetylating autophagy complex components [26]. SIRT2 binds to forkhead box O1 (FOXO1) and deacetylates it, interacting with Atg 7 [27][28]. SIRT2 KO mice manifest altered mitochondrial protein acetylation, reduced ATP production, defective mitophagy, increased oxidative stress, and increased p62, PINK1/Parkin, and ubiquitin-protein expression levels [28][29]. Mitochondrial functions are regulated by the SIRT3-activated AMPK/PGC-1α pathway. The SIRT3 promotes autophagy by upregulating mTOC1 expression and MnSOD production [30][31]. The overexpression of SIRT6 induces autophagy [32]. The autophagy process declines with age, resulting in certain disease conditions [22]. However, experts opine that targeting SIRTs could regulate these age-related degenerative diseases.
5. SIRTs and Aging
Aging is a functional decline of multi-cellular homeostasis pathways—including events such as genome fidelity, nutrient sensing, and proteostasis [33]. Aging ultimately culminates in the G1 phase arrest of the cell cycle process of continuously proliferating cells in response to either metabolic, genotoxic, or oncogene-induced stresses. Function decline puts cells at risk of malignant transformation via secreted degradative proteases, growth factors, and inflammatory cytokines. These secretions could compromise the microenvironments of non-senescent cells and promote cell cycle arrest, which are related with increases in senescence-associated β-galactosidase activity and DNA damage [2][34]. Pizarro et al. [35] evaluated the effect of resveratrol on neuroblastoma cell B65 in vitro. They found that resveratrol could inhibit cell proliferation and arrested cancer cells at the S phase. Resveratrol plays an anti-aging role by increasing expression of SIRT1. Similarly, calorie restriction conditions could also stimulate SIRT1 expression, whose levels coincided with NAD+ availability and reduced DNA damage in non-senescent cells, as discussed in [36]. Yousefzadeh and colleagues [37] studied the anti-aging effect of fisetin in aging mice. They found that fisetin could reduce senescence-related markers and improved tissue homeostasis; fisetin could also suppress age-related pathological alterations and extended the lifespan of mice.
The SIRT family proteins are a prominent and promising target and diagnostic tool in aging and anti-aging studies. Lee [27] stated that the upregulated expressions of SIRT1 could delay aging and increased lifespan. Elevated expression of SIRT2 is also a biomarker of senescence in cells [38]. SIRT3 gene deficiency could increase cellular ROS levels and damaged DNA molecules [39]. Additionally, the lack of the SIRT6 gene could promote the expression of glucose transporter type (GLUT) 1 and 4 transporters, which could cause hypoglycemia and premature death in mice [40]. Conversely, the overexpression of SIRT6 could increase IGF-1 binding proteins and change the phosphorylation levels of IGF-1 signaling components, which inhibits the IGF-1 pathway. These changes facilitate glucose tolerance, decrease fat accumulation, and extend lifespans of male mice [41]. Other investigators found that increased expression of SIRT7 mRNA is found in metabolically active tissues, while decreased expression is correlated with age [6]. Further, SIRT7-deficient cells display increased replication stress and impaired DNA repair [42].
6. SIRTs and Inflammation
Acute inflammation mends and restores cellular functions after a challenge, but chronic inflammation results in endothelial dysfunction, pro-inflammatory cytokine recruitment, adhesion molecules, matrix-degrading enzymes at the inflamed sites, and disease conditions [43]. NAD+ precursors have anti-inflammatory effects. SIRT transcription/protein and NAD+ levels are persistently reduced in tissues with chronic inflammation [44][45], making their increased activation an important anti-inflammatory biomarker. Quercetin alleviated diabetes-induced atherosclerosis-related inflammatory and oxidative stress in male Wistar rats. After two weeks of administering quercetin (30 mg/kg/day), they improved the lipid profile, vascular oxidative stress markers, and inflammatory cytokines in rats’ tissues. Quercetin ameliorated inflammatory and oxidative conditions through the AMPK/SIRT1/NF-kB pathway [46]. Additionally, quercetin relieved oxLDL, which caused endothelial oxidative injuries by stimulating the SIRT1 and AMPK/NF-kB/NADH oxidase/ATK/endothelial NO synthase signaling pathways [47].
Another study used piceatannol and resveratrol (10 mg/kg/day) in a high-fat diet-induced inflammation intervention experiment with male C57BL/6J mice. Results showed that SIRT modulators improved glucose and glycemic levels via increasing insulin receptor and AMPK levels in liver cells after four weeks [32]. Piceatannol administration increased the expression levels of SIRT1, SIRT3, SIRT6, PGC-1α, and forkhead box O1 genes. Resveratrol administration decreased the expression levels of IL-1β and IL-6 genes. SIRT modulators significantly decreased TNF-α expression levels. Nuclear-localized SIRTs (1, 2, 6, and 7) suppressed the activity and expression of inflammatory factor NF-κB by activating the cAMP/PKA/AMPK/SIRT1 signaling pathway [44][48]. Suppressed NF-κB activation could decrease the expressions of COX-2, iNOS, TNF-α, IL-1β, IL-6, and IL-8 but could increase the expressions of antioxidant and anti-apoptotic genes [45]. The activation of SIRTs suppresses pro-inflammatory cytokines and anti-apoptotic gene expression levels by deacetylating the NF-κB pathway p65 subunit [45].
7. SIRTs and Viral Infection
Viruses depend on host cells’ metabolism and compartments for energy, genetic components, replication, maturation, and dissemination. Reduced NAD+ cellular levels influence immune cell activation and infection advancement. Koyuncu et al. [49] demonstrated that small interfering RNA (siRNA)-mediated knockdown of individual SIRTs and drug-mediated inhibitory actions on SIRT enzymes increased viral progeny production in infected humans’ cells. They concluded that all SIRTs have a broad range of anti-viral effects. p53 and transcription factor c-Myc are associated with viral infections. SIRT1 activation of p53 leads to the apoptosis of infected cells. At the same time, deactivating SIRT2 degrades the c-Myc transcription factor and curbs viral nucleic acid biosynthesis. SIRT1 controls ACE2 receptor expression, which can be used for viral attachment. Dietary SIRT1 inhibitors deregulate ACE2 receptor proteins and inhibit viral attachment [10].
Pre-treating MERS-CoV-infected Vero E6 cells with resveratrol decreased nucleocapsid protein expression and downregulated apoptosis in a dose-dependent manner [50]. The N protein is essential for MERS-CoV replication. Recently, Saeedi-Boroujeni and Mahmoudian-Sani [51] reported that quercetin administration ameliorated COVID-19-positive-associated inflammatory conditions. Quercetin could suppress the NLRP3 inflammasome through SIRT1 activation and generated active forms of pro-inflammatory cytokines IL-1β and IL-18. SIRT1, SIRT2, and SIRT6 could inhibit viral infection-associated pro-inflammatory cytokine storm through deacetylating the NF-κB p65 subunit. Natural modulators of SIRTs are similar to broad-spectrum antibiotics: (1) they can effectively prevent attachment, entry, and multiplication of many DNA and RNA viruses, (2) they are non-discriminatory against mutated variants because they predominantly interact with host cells and receptors, and (3) they suppress the expression of infection-associated pro-inflammatory cytokines [10][52].
8. SIRTs and Energy Homeostasis
The body auto-maintains its serum glucose balance through gluconeogenesis. SIRTs regulate hepatic gluconeogenesis and fatty acid oxidative pathways by targeting and deacetylating the PGC-1α protein [53]. A study by Higashida et al. [54] on muscular mitochondrial biogenesis showed that a high concentration of resveratrol lowered ATP concentration and activated the AMPK/SIRT1 pathway in the skeletal muscle of mice. Resveratrol improved the mice’s running endurance through SIRT1-mediated deacetylation, activating PGC-1α and increasing mitochondrial proteins. Resveratrol administration could increase SIRT1 expression and suppressed PGC-1α expression. It could also lower mitochondrial biogenesis and attenuated tesaglitazar-induced cardiac dysfunction in mice models [55]. Overexpression of the SIRT2 gene could inhibit preadipocyte differentiation, while decreased expression promotes adipogenesis in 3T3-L1 cells [56].
A lack of SIRT3 results in fatty acid disorders and lowers ATP levels during fasting of mice because SIRT3 influences ATP formation and adaptive thermogenesis [57]. SIRTs regulate the expression and maturation of adipocytes. They also modulate plasma glucose levels, mitochondrial energy capacity, insulin secretion, insulin tissue sensitivity, and cholesterol/lipid homeostasis [58][59]. The activations of PPARs and PGC-1α modulated by SIRT1 significantly affect fat mobilization and fatty acid oxidation [60]. The combined effect of berberine and resveratrol resulted in one-fold higher lipid metabolism via significantly increased low-density lipoprotein receptor expression in HepG2 cells [61]. Resveratrol showed better synergistic results with other Sirtfood components.
9. SIRTs and Cancer
One-third of all cancer-induced deaths can be averted by lifestyle change—including the consumption of proper nutrition, according to Danaei et al. [62]. However, multi-risk factors increase cancer cases and plague cancer studies. Cancerous cells propagate out of control and cause dysfunctions in nearby normal cells. SIRTs are the primary metabolic and stress sensors. SIRT2 and SIRT6 suppress oncogenic factors, while the SIRT1 gene has bifunctional actions [63][64]. A retrospective study by Chao et al. [65] surmised that resveratrol activated SIRT1 in human chondrosarcoma cells, decreased cell viability, and induced apoptosis dose-dependently. The mechanism of resveratrol exerting its underlying inhibitory effect was the deacetylation of the p65 subunit of the NF-kB factor. Resveratrol activated SIRT-mediated Akt, PI3K/Akt, NF-κB, and ER signaling pathways to elicit its anti-cancer function [66].
Genistein could suppress tyrosine kinases and regulated Atk/MEK signaling pathways, which caused cell cycle arrest and inhibited the proliferation of cancer cells [67]. Additionally, SIRT modulators ameliorate cellular stress through their high antioxidative effects. They activate the Nrf2 pathway to scavenge radicals and produce antioxidative enzymes [68]. Biological evidence implicates mitochondrial SIRTs (mtSIRTs) as essential regulators that control cancer and tumor cell progression or ‘onco-metabolism’. SIRTs 3, 4, and 5 target and alter metabolic and mitochondrial energetics of cancer cells. These genes elicit pathways that control cell proliferation, apoptosis, cell cycle progression, inflammation, angiogenesis, invasion, and metastasis [63]. The multi-functions of SIRT modulators make them suitable natural therapeutics.
References
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13.
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476.
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol. Biochem. 2016, 72, 371–380.
- Zhong, L.; Mostoslavsky, R. Fine tuning our cellular factories: Sirtuins in mitochondrial biology. Cell Metab. 2011, 13, 621–626.
- Poulose, N.; Raju, R. Sirtuin regulation in aging and injury. BBA-Mol. Basis Dis. 2015, 1852, 2442–2455.
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Sign. 2018, 28, 643–661.
- Pallauf, K.; Giller, K.; Huebbe, P.; Rimbach, G. Nutrition and healthy ageing: Calorie restriction or polyphenol-rich “MediterrAsian” diet? Oxid. Medi. Cell. Longev. 2013, 2013, 707421.
- Karaman, B.M.; Sippl, W.; Ntie-Kang, F. Natural products as modulators of Sirtuins. Molecules 2020, 25, 3287.
- Schiedel, M.; Robaa, D.; Rumpf, T.; Sippl, W.; Jung, M. The current state of NAD+-dependent histone deacetylases (Sirtuins) as novel therapeutic targets. Med. Res. Rev. 2017, 38, 147–200.
- Alqarni, M.H.; Foudah, A.I.; Muharram, M.M.; Labrou, N.E. The pleiotropic function of human sirtuins as modulators of metabolic pathways and viral infections. Cells 2021, 10, 460.
- Gertz, M.; Nguyen, G.T.; Fischer, F.; Suenkel, B.; Schlicker, C.; Fränzel, B.; Tomaschewski, J.; Aladini, F.; Becker, C.; Wolters, D.; et al. A molecular mechanism for direct sirtuin activation by resveratrol. PLoS ONE 2012, 7, e49761.
- Palmeira, C.M.; Teodoro, J.S.; Amorim, J.A.; Steegborn, C.; Sinclair, D.A.; Rolo, A.P. Mitohormesis and metabolic health: The interplay between ROS, cAMP and sirtuins. Free Radic. Biol. Med. 2019, 141, 483–491.
- Moustafa, E.M.; Rashed, E.R.; Rashed, R.R.; Omar, N.N. Piceatannol promotes hepatic and renal AMPK/SIRT1/PGC-1α mitochondrial pathway in rats exposed to reserpine or gamma-radiation. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211016194.
- Zhang, H.P.; Zhao, Z.X.; Pang, X.F.; Yang, J.; Yu, H.X.; Zhang, Y.H.; Zhou, H.; Zhao, J.H. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol. Lett. 2017, 277, 115–122.
- Poljsak, B.; Milisav, I. NAD+ as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity, and health span. Rejuvenation Res. 2016, 19, 406–415.
- D’Arcy, M. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592.
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213.
- Cikla-Suzgun, P.; Kucukguzel, S.G. Recent advances in apoptosis: The role of hydrazones. Mini Rev. Med. Chem. 2019, 19, 1427–1442.
- Zhang, Y.M.; Zhang, W.R.; Tao, L.N.; Zhai, J.H.; Gao, H.; Song, Y.Q.; Qu, X.Y. Quercetin protected against isoniazide-induced HepG2 cell apoptosis by activating SIRT1/ERK pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22369.
- Liang, Y.S.; Qi, W.T.; Guo, W.; Wang, C.L.; Hu, Z.B.; Li, A.K. Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets. Food Nutr. Res. 2018, 2018, 62.
- Kim, H.S.; Patel, K.; Muldoon-Jacobs, K.; Bisht, K.S.; Aykin-Burns, N.; Pennington, J.D.; van der Meer, R.; Nguyen, P.; Savage, J.; Owens, K.M.; et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 2010, 17, 41–52.
- Rajendran, R.; Garva, R.; Krstic-Demonacos, M.; Demonacos, C. Sirtuins: Molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J. Biomed. Biotechnol. 2011, 2011, 368276.
- Zhang, D.X.; Zhang, J.P.; Hu, J.Y.; Huang, Y.S. The potential regulatory roles of NAD+ and its metabolism in autophagy. Metabolism 2016, 65, 454–462.
- Yang, X.H.; Jiang, T.L.; Wang, Y.; Guo, L. The role and mechanism of SIRT1 in resveratrol-regulated osteoblast autophagy in osteoporosis rats. Sci. Rep. 2019, 9, 18424.
- Liu, T.; Ma, X.R.; Ouyang, T.X.; Chen, H.P.; Lin, J.; Liu, J.; Xiao, Y.; Yu, J.; Huang, Y.Y. SIRT1 reverses senescence via enhancing autophagy and attenuates oxidative stress-induced apoptosis through promoting p53 degradation. Int. J. Biol. Macromol. 2018, 117, 225–234.
- Dong, S.X.; Jia, C.F.; Zhang, S.P.; Fan, G.J.; Li, Y.B.; Shan, P.P.; Sun, L.H.; Xiao, W.Z.; Li, L.; Zheng, Y.; et al. The REGγ proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab. 2013, 18, 380–391.
- Lee, I.H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Exp. Mol. Med. 2019, 51, 1–11.
- Inoue, T.; Nakayama, Y.; Li, Y.; Matsumori, H.; Takahashi, H.; Kojima, H.; Wanibuchi, H.; Katoh, M.; Oshimura, M. SIRT2 knockdown increases basal autophagy and prevents postslippage death by abnormally prolonging the mitotic arrest that is induced by microtubule inhibitors. FEBS J. 2014, 281, 2623–2637.
- Liu, G.; Park, S.H.; Imbesi, M.; Nathan, W.J.; Zou, X.; Zhu, Y.; Jiang, H.; Parisiadou, L.; Gius, D. Loss of NAD-dependent protein deacetylase sirtuin-2 alters mitochondrial protein acetylation and dysregulates mitophagy. Antioxid. Redox Signal. 2017, 26, 849–863.
- Cho, C.S.; Lombard, D.B.; Lee, J.H. SIRT3 as a regulator of hepatic autophagy. Hepatology 2017, 66, 700–702.
- Li, S.T.; Dou, X.B.; Ning, H.; Song, Q.; Wei, W.; Zhang, X.M.; Shen, C.; Li, J.X.; Sun, C.H.; Song, Z.Y. Sirtuin 3 acts as a negative regulator of autophagy dictating hepatocyte susceptibility to lipotoxicity. Hepatology 2017, 66, 936–952.
- Lee, H.J.; Kang, M.G.; Cha, H.Y.; Kim, Y.M.; Lim, Y.; Yang, S.J. Effects of piceatannol and resveratrol on sirtuins and hepatic inflammation in high-fat diet-fed mice. J. Med. Food 2019, 22, 833–840.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2020, 22, 119–141.
- Pizarro, J.G. Resveratrol inhibits proliferation and promotes apoptosis of neuroblastoma cells: Role of sirtuin 1. Neurochem. Res. 2011, 36, 187–194.
- Hwang, J.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110.
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28.
- Anwar, T.; Khosla, S.; Ramakrishna, G. Increased expression of SIRT2 is a novel marker of cellular senescence and is dependent on wild type p53 status. Cell Cycle 2016, 15, 1883–1897.
- Jing, E.; Emanuelli, B.; Hirschey, M.D.; Boucher, J.; Lee, K.Y.; Lombard, D.; Verdin, E.M.; Kahn, C.R. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2011, 108, 14608–14613.
- Zhong, L.; D’Urso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.D.; Nir, T.; et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 2010, 140, 280–293.
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221.
- Vazquez, B.N.; Thackray, J.K.; Simonet, N.G.; Kane-Goldsmith, N.; Martinez-Redondo, P.; Nguyen, T.; Bunting, S.; Vaquero, A.; Tischfield, J.A.; Serrano, L. SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair. EMBO J. 2016, 35, 1488–1503.
- Vachharajani, V.T.; Liu, T.F.; Wang, X.F.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins link inflammation and metabolism. J. Immunol. Res. 2016, 2016, 8167273.
- Mendes, K.L.; Lelis, D.F.; Santos, S. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev. 2017, 38, 98–105.
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948.
- Zhang, F.W.; Feng, J.; Zhang, J.Y.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 280.
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917.
- Chen, M.L.; Yi, L.; Jin, X.; Liang, X.Y.; Zhou, Y.; Zhang, T.; Xie, Q.; Zhou, X.; Chang, H.; Fu, Y.J.; et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 2013, 9, 2033–2045.
- Koyuncu, E.; Budayeva, H.G.; Miteva, Y.V.; Ricci, D.P.; Silhavy, T.J.; Shenk, T.; Cristea, I.M. Sirtuins are evolutionarily conserved viral restriction factors. mBio 2014, 5, e2214–e2249.
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144.
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-inflammatory potential of quercetin in COVID-19 treatment. J. Inflamm. 2021, 18, 3.
- Okeke, E.S.; Ita, R.E.; Egong, E.J.; Udofia, L.E.; Mgbechidinma, C.L.; Akan, O.D. Metaproteomic insights into fermented fish and vegetable products and associated microbes. Food Chem. Mol. Sci. 2021, 3, 100045.
- Rodgers, J.T.; Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA 2007, 104, 12861–12866.
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013, 11, e1001603.
- Kalliora, C.; Kyriazis, I.D.; Oka, S.; Lieu, M.J.; Yue, Y.J.; Area-Gomez, E.; Pol, C.J.; Tian, Y.; Mizushima, W.; Chin, A.; et al. Dual PPARα/γ activation inhibits SIRT1-PGC-1α axis and causes cardiac dysfunction. JCI Insight 2019, 4, e129556.
- Jing, E.; Gesta, S.; Kahn, C.R. SIRT2 regulates adipocyte differentiation through FOXO1 acetylation/deacetylation. Cell Metab. 2007, 6, 105–114.
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125.
- Kelly, G.S. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: Part 2. Altern. Med. Rev. 2010, 15, 313–338.
- Liang, F.; Kume, S.; Koya, D. SIRT1 and insulin resistance. Nat. Rev. Endocrinol. 2009, 5, 367–373.
- Rodgers, J.T.; Lerin, C.; Gerhart-Hines, Z.; Puigserver, P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008, 582, 46–53.
- Zhu, X.F.; Yang, J.Y.; Zhu, W.J.; Yin, X.X.; Yang, B.B.; Wei, Y.H.; Guo, X.F. Combination of berberine with resveratrol improves the lipid-lowering efficacy. Int. J. Mol. Sci. 2018, 19, 3903.
- Danaei, G.; Hoorn, S.V.; Lopez, A.D.; Murray, C.J.L.; Ezzati, M. Comparative risk assessment collaborating group (Cancers). Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793.
- Bosch-Presegué, L.; Vaquero, A. The dual role of sirtuins in cancer. Genes Cancer 2011, 2, 648–662.
- George, J.; Ahmad, N. Mitochondrial sirtuins in cancer: Emerging roles and therapeutic potential. Cancer Res. 2016, 76, 2500–2506.
- Chao, S.C.; Chen, Y.J.; Huang, K.H.; Kuo, K.L.; Yang, T.H.; Huang, K.Y.; Wang, C.C.; Tang, C.H.; Yang, R.S.; Liu, S.H. Induction of sirtuin-1 signaling by resveratrol induces human chondrosarcoma cell apoptosis and exhibits antitumor activity. Sci. Rep. 2017, 7, 3180.
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; Ramírez de Molina, A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599.
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A potent anti-breast cancer agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517.
- Thomas, R.; Butler, E.; Macchi, F.; Williams, M. Phytochemicals in cancer prevention and management. Brit. J. Med. Pract. 2015, 8, a815.
More
