Bulk acoustic waves have been applied to microfluidic separations with many benefits, such as flexible placement of transducer, simple, and versatile setups. A BAW-based microfluidic device typically operates with bulk acoustic standing waves in a microchannel between two parallel opposite walls. BAW-based microfluidic separation techniques have been applied in separating various types of particles and biological samples based on their size, density and compressibility.
- Bulk acoustic waves
- Microbubble
- Separation
- Bioapplications
- Acoustic radiation force
- Acoustic streaming
1. DefiInitroduction
Bulk acoustic waves have been applied to microfluidic separations with many benefits, such as flexible placement of transducer, simple, and versatile setups. A BAW-based microfluidic device typically operates with bulk acoustic standing waves in a microchannel between two parallel opposite walls. A piepiezoelectric transducer can generate BAWs in a fluid-filled microchannel and resonance in the channel with acoustically contrasting materials, such as silicon, polydimethylsiloxane (PDMS), and glass. Compared with SAW-based separation devices, BAW-based devices usually work at a lower frequency and a longer wavelength, which allows for handling larger particles.[1]
2. Introduction
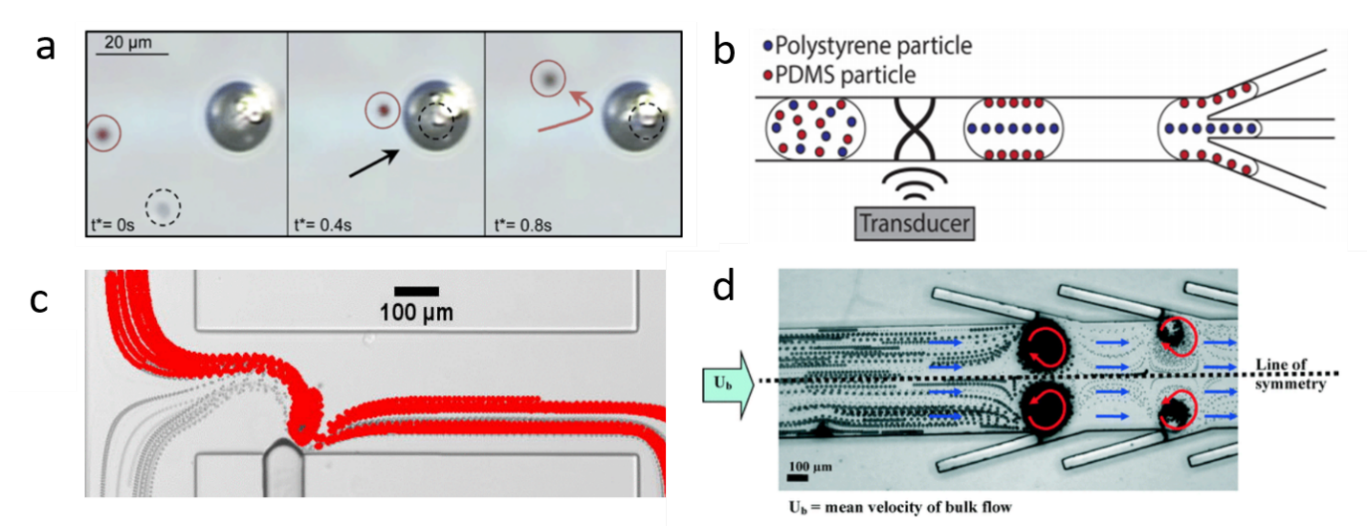
Using BAW-induced acoustic streaming and acoustic radiation force, Devendran et al. separated particles based on size differences.[2] Actuated by BAW, the acoustic streaming-induced drag force selectively delivered smaller particles to the target location, while the larger particles were dominated by acoustic radiation force. Dauson et al. demonstrated a robust “tilt-angle” BAW-based microfluidic device for the separation of different-sized particles.[3] Tilt angled lead zirconate titanate (PZT) induced primary acoustic radiation force on particles, which deflected particles from the straight path based on the size of the particles. BAW-based devices have also been implemented to separate sub-micron particles from micron particles. Due to the diameter difference, micron particles were dominated by primary acoustic radiation force and focused on the midline of the microchannel, while sub-micron particles were moved toward the sidewall via drag force. Using the same mechanism, they successfully separated bovine red blood cells and Escherichia coli (E. coli).[4] In addition to the size-based separation, BAW is also able to separate samples based on density or compressibility. Fornell et al. successfully separated polystyrene and PDMS particles with different acoustic contrast factors inside a water-in-oil droplet (Figure 1b).[5] Since acoustic radiation forces are proportional to acoustic contrast factors, the polystyrene particles were directed to the pressure nodes while the PDMS particles were moved to the pressure antinodes when BAW is on. By combining with a droplet splitter, these particles were separated in a continuous flow.
3. Microbubble-Based Separation
In recent years, acoustically excited bubbles have attracted more and more interest due to their great potential for manipulating objects and fluids in microfluidic applications, which is typically excited by BAWs with relatively low frequencies (kHz).[6][7][8][9] As mentioned in the theory section, when a bubble is actuated by acoustic waves, the objects near the bubble will experience both secondary radiation force and drag force. Secondary radiation force tends to trap the objects, while microstreaming-induced drag force can transport particles. With ingenious design in force control, many acoustic bubble-based BAW devices have been developed for size or density-based separation for biomedical applications. For example, Rogers et al. demonstrated a density-based method for the separation of same-sized silica beads and polystyrene particles using acoustic bubbles.[10] Due to different densities, the silica beads were trapped by the secondary radiation force, while polystyrene particles, dominated by drag force, were repelled and transported along the streamlines (Figure 1a). This finding shows the acoustic bubble-based microfluidic device has a great potential to be implemented in sorting uniformed and low-concentrated biological samples. Similarly, aided by a syringe pump, size-selective trapping and release were realized via tuning the strength of a Poiseuille flow and a bubble-induced microstreaming, which allows sorting particles of desired size (Figure 1c).[11] Later, an improved acoustic bubble-based separator termed lateral cavity acoustic transducers (LCATs) is demonstrated, enabling simultaneous self-pumping and size-based cell separation (Figure 1d).[12] Compared with current bulk lab-based sorting methods, this method is more flexible to integrate with upstream and downstream sample preparation and analysis systems. Our group developed an acoustic bubble array for trapping and releasing a live animal—Caenorhabditis elegans (C. elegans).[13] Dominated by secondary radiation force, the C. elegans were trapped by the bubble array at a certain frequency and voltage. Gradually decreasing the applied voltage to the actuator, C. elegans were released in the order from the biggest to the smallest. This study shows the controllability of the bubble-based device in size-selective trapping and releasing microorganisms. Acoustic bubbles could also be employed in enhancing the separation effect. Zhou et al. integrated an acoustic bubble with pinched flow fractionation (PFF) to enhance particle separation performance, which overcomes the limitation of the conventional PFF method.[14] Xie et al. presented a method for enhancing mass transfer in a liquid–liquid extraction process, which has the potential to be further applied in biochemical separation.[15]
Figure 1. (a) At 217 kHz, 5 µm silica particles are trapped by acoustic bubbles, while 5 µm polystyrene particles follow with streamline due to the drag force. Reprinted with permission from reference [10]. (b) BAW-based separation of polystyrene and polydimethylsiloxane (PDMS) particles with different acoustic contrast factors. Reprinted with permission from reference [5]. (c) Size-sensitive sorting mixture of 5 µm and 2.5 µm particles by acoustic bubble. Reprinted with permission from reference [11]. (d) Angled lateral cavity acoustic transducers (LCATs) produce a directional flow from inlet to outlet, large-sized particles are selectively trapped and small-sized particles are transported to the outlet. Reprinted with permission from reference [12].
References
- Ivo Leibacher; Peter Reichert; Jürg Dual; Microfluidic droplet handling by bulk acoustic wave (BAW) acoustophoresis. Lab on a Chip 2014, 15, 2896-2905, 10.1039/c5lc00083a.
- Citsabehsan Devendran; Ian Gralinski; Adrian Neild; Separation of particles using acoustic streaming and radiation forces in an open microfluidic channel. Microfluidics and Nanofluidics 2014, 17, 879-890, 10.1007/s10404-014-1380-4.
- Erin R. Dauson; Kelvin B. Gregory; David W. Greve; Gregory P. Healy; Irving J. Oppenheim; Mechanically robust microfluidics and bulk wave acoustics to sort microparticles. Health Monitoring of Structural and Biological Systems 2016 2016, 9805, 98051, 10.1117/12.2214394.
- Gayatri P. Gautam; Rubi Gurung; Frank A. Fencl; Menake E. Piyasena; Separation of sub-micron particles from micron particles using acoustic fluid relocation combined with acoustophoresis. Analytical and Bioanalytical Chemistry 2018, 410, 6561-6571, 10.1007/s00216-018-1261-x.
- Anna Fornell; Kevin Cushing; Johan Nilsson; Maria Tenje; Binary particle separation in droplet microfluidics using acoustophoresis. Applied Physics Letters 2018, 112, 063701, 10.1063/1.5020356.
- Lin, Y.; Gao, Y.; Wu, M.; Zhou, R.; Chung, D.; Caraveo, G.; Xu, J. Acoustofluidic stick-and-play micropump built on foil for single-cell trapping. Lab Chip 2019, 19, 3045–3053. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, C.; Gao, Y.; Wu, M.; Yazdi, A.A.; Xu, J. Acoustofluidic micromixer on lab-on-a-foil devices. Sens. Actuators B Chem. 2019, 287, 312–319. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Juluri, B.K.; Huang, T.J. A fast microfluidic mixer based on acoustically driven sidewall-trapped microbubbles. Microfluid. Nanofluid. 2009, 7, 727. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef]
- Rogers, P.; Neild, A. Selective particle trapping using an oscillating microbubble. Lab Chip 2011, 11, 3710–3715. [Google Scholar] [CrossRef]
- Wang, C.; Jalikop, S.V.; Hilgenfeldt, S. Size-sensitive sorting of microparticles through control of flow geometry. Appl. Phys. Lett. 2011, 99, 034101. [Google Scholar] [CrossRef]
- Patel, M.V.; Nanayakkara, I.A.; Simon, M.G.; Lee, A.P. Cavity-induced microstreaming for simultaneous on-chip pumping and size-based separation of cells and particles. Lab Chip 2014, 14, 3860–3872. [Google Scholar] [CrossRef]
- Xu, Y.H.; Hashmi, A.; Yu, G.; Lu, X.N.; Kwon, H.J.; Chen, X.L.; Xu, J. Microbubble array for on-chip worm processing (vol 102, 023702, 2013). Appl. Phys. Lett. 2013, 102, 023702. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, C. Acoustic bubble enhanced pinched flow fractionation for microparticle separation. J. Micromech. Microeng. 2015, 25, 084005. [Google Scholar] [CrossRef]
- Xie, Y.L.; Chindam, C.; Nama, N.; Yang, S.K.; Lu, M.Q.; Zhao, Y.H.; Mai, J.D.; Costanzo, F.; Huang, T.J. Exploring bubble oscillation and mass transfer enhancement in acoustic-assisted liquid-liquid extraction with a microfluidic device. Sci. Rep. 2015, 5, 12572. [Google Scholar] [CrossRef]
- Fornell, A.; Cushing, K.; Nilsson, J.; Tenje, M. Binary particle separation in droplet microfluidics using acoustophoresis. Appl. Phys. Lett. 2018, 112, 063701. [Google Scholar] [CrossRef]