Crosslinking strategies have been extensively explored in order to design novel hydrogels for bone tissue engineering. Lately, the fabrication of hydrogels with the help of enzyme-mediated crosslinking approaches has been extensively explored. This approach has resulted in promising outcomes with convincing prospects. Enzymes are required in minimal quantity and are very efficient in their actions, as they increase the reaction rate without being expended during the course of the reaction process. The efficiency of an enzyme is defined by the number of substrate molecules converted into products per unit of enzyme, which is also known as turnover number (k cat). The high efficiency of enzyme-based reactions comes from the precise specificity, which ensures the conversion of a particular type of substrate to products. MSo far, many enzymes have been explored in order to prepare biomimetic hydrogels for bone tissue engineering. The details of every enzyme-based crosslinking approach are discussed in the following sections.
- crosslinking
- enzyme
- hydrogels
- polymers
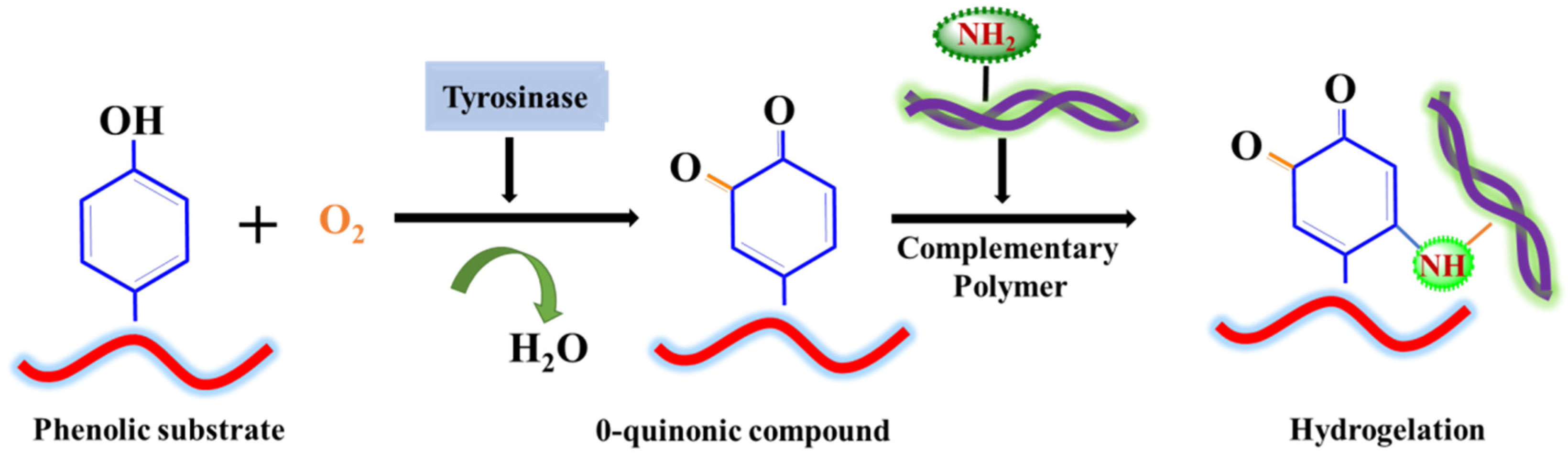
1. Tyrosinase
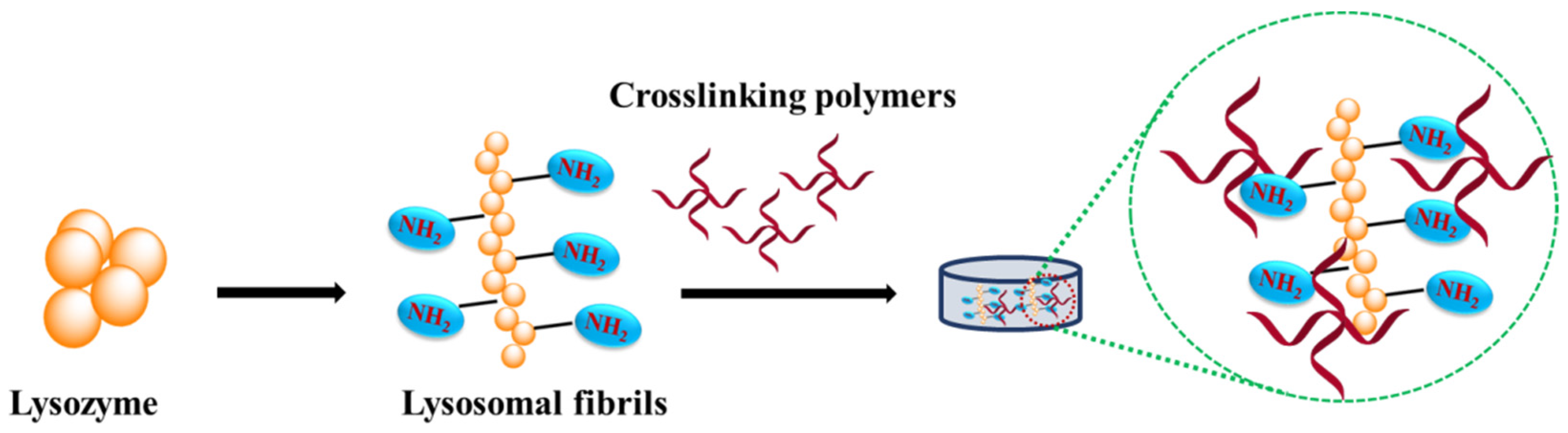
2. Lysozyme
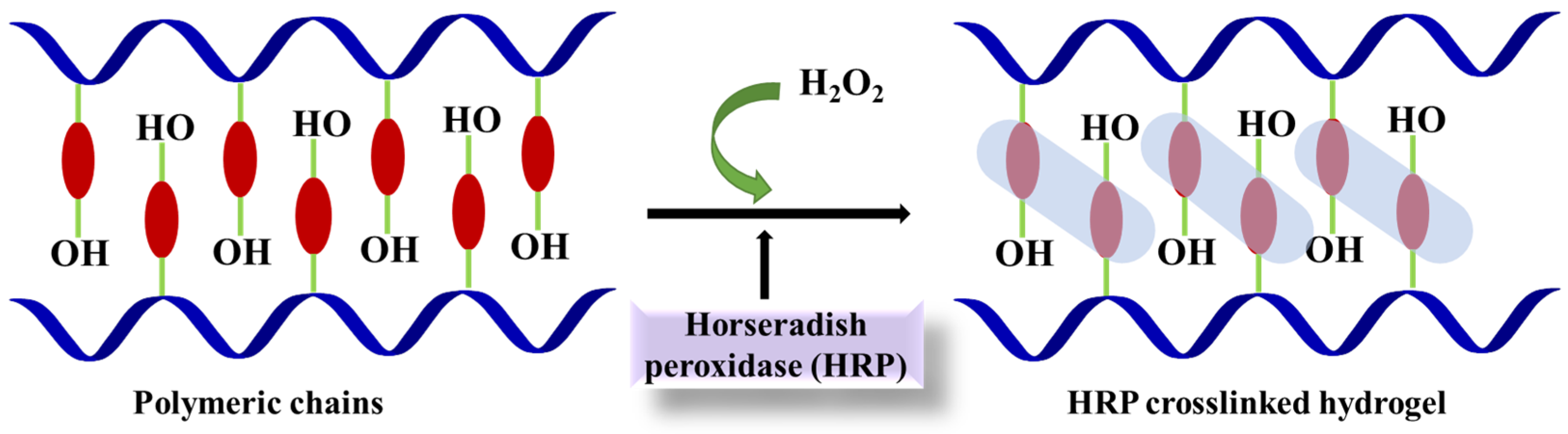
3. Horseradish Peroxidase
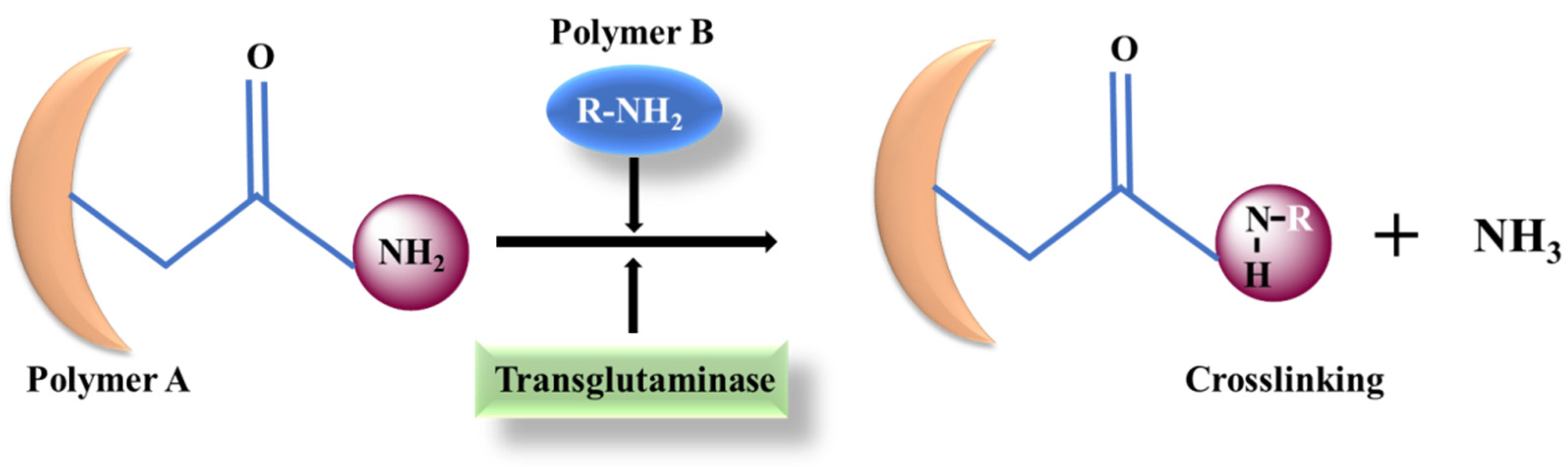
4. Transglutaminase (TG)
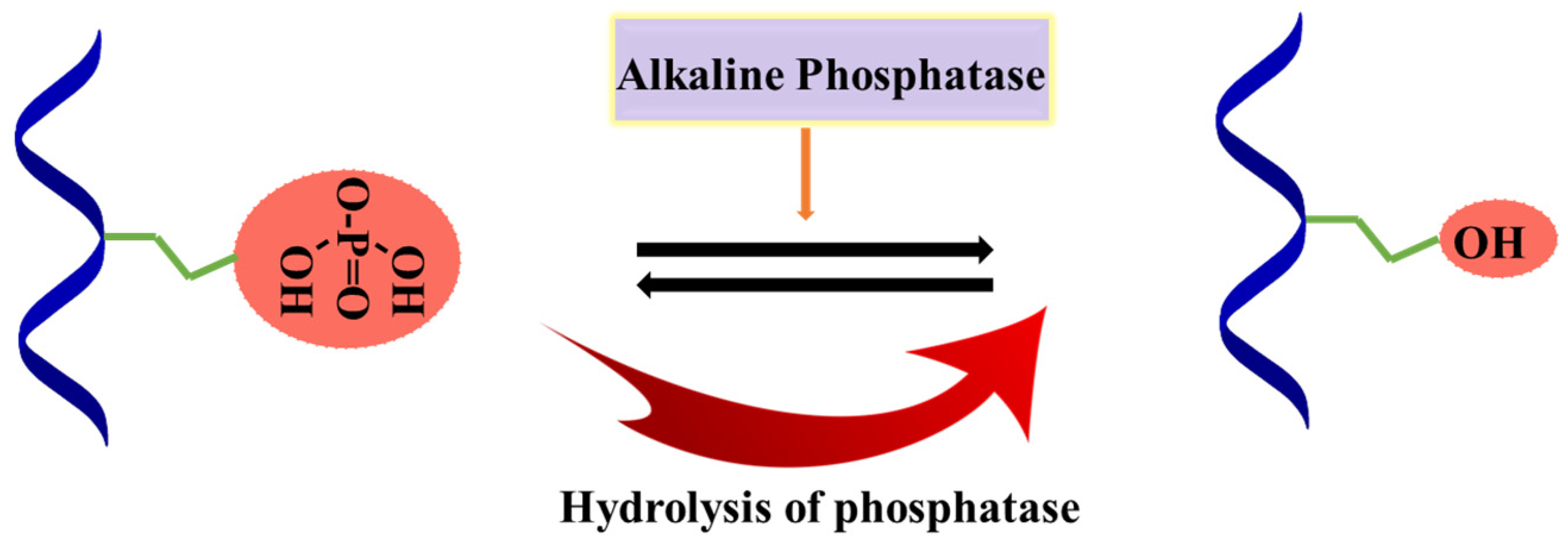
5. Alkaline Phosphatase (ALP)
References
- Choi, S.; Ahn, H.; Kim, S.-H. Tyrosinase-mediated hydrogel crosslinking for tissue engineering. J. Appl. Polym. Sci. 2022, 139, 51887.
- Song, W.; Ko, J.; Choi, Y.H.; Hwang, N.S. Recent advancements in enzyme-mediated crosslinkable hydrogels: In vivo-mimicking strategies. APL Bioeng. 2021, 5, 021502.
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841.
- Chen, S.; Gil, C.J.; Ning, L.; Jin, L.; Perez, L.; Kabboul, G.; Tomov, M.L.; Serpooshan, V. Adhesive Tissue Engineered Scaffolds: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 683079.
- Sousa, M.P.; Mano, J.F. Cell-Adhesive Bioinspired and Catechol-Based Multilayer Freestanding Membranes for Bone Tissue Engineering. Biomimetics 2017, 2, 19.
- Mishra, D.; Bhunia, B.; Banerjee, I.; Datta, P.; Dhara, S.; Maiti, T.K. Enzymatically crosslinked carboxymethyl–chitosan/gelatin/nano-hydroxyapatite injectable gels for in situ bone tissue engineering application. Mater. Sci. Eng. C 2011, 31, 1295–1304.
- Sharma, A.; Desando, G.; Petretta, M.; Chawla, S.; Bartolotti, I.; Manferdini, C.; Paolella, F.; Gabusi, E.; Trucco, D.; Ghosh, S.; et al. Investigating the Role of Sustained Calcium Release in Silk-Gelatin-Based Three-Dimensional Bioprinted Constructs for Enhancing the Osteogenic Differentiation of Human Bone Marrow Derived Mesenchymal Stromal Cells. ACS Biomater. Sci. Eng. 2019, 5, 1518–1533.
- Chameettachal, S.; Midha, S.; Ghosh, S. Regulation of Chondrogenesis and Hypertrophy in Silk Fibroin-Gelatin-Based 3D Bioprinted Constructs. ACS Biomater. Sci. Eng. 2016, 2, 1450–1463.
- Das, S.; Pati, F.; Chameettachal, S.; Pahwa, S.; Ray, A.R.; Dhara, S.; Ghosh, S. Enhanced Redifferentiation of Chondrocytes on Microperiodic Silk/Gelatin Scaffolds: Toward Tailor-Made Tissue Engineering. Biomacromolecules 2013, 14, 311–321.
- Lim, S.; Jeong, D.; Ki, M.-R.; Pack, S.P.; Choi, Y.S. Tyrosinase-mediated rapid and permanent chitosan/gelatin and chitosan/gelatin/nanohydroxyapatite hydrogel. Korean J. Chem. Eng. 2021, 38, 98–103.
- Tabatabaei, F.; Rasoulianboroujeni, M.; Yadegari, A.; Tajik, S.; Moharamzadeh, K.; Tayebi, L. Osteo-mucosal engineered construct: In situ adhesion of hard-soft tissues. Mater. Sci. Eng. C 2021, 128, 112255.
- Pangburn, S.H.; Trescony, P.V.; Heller, J. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials 1982, 3, 105–108.
- Chen, X.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005, 40, 123–132.
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200.
- Kim, S.; Fan, J.; Lee, C.S.; Lee, M. Dual Functional Lysozyme-Chitosan Conjugate for Tunable Degradation and Antibacterial Activity. ACS Appl. Bio. Mater. 2020, 3, 2334–2343.
- Kim, S.; Cui, Z.K.; Koo, B.; Zheng, J.; Aghaloo, T.; Lee, M. Chitosan-Lysozyme Conjugates for Enzyme-Triggered Hydrogel Degradation in Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 41138–41145.
- Proctor, V.A.; Cunningham, F.E. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit. Rev. Food Sci. Nutr. 1988, 26, 359–395.
- Tan, H.; Jin, D.; Qu, X.; Liu, H.; Chen, X.; Yin, M.; Liu, C. A PEG-Lysozyme hydrogel harvests multiple functions as a fit-to-shape tissue sealant for internal-use of body. Biomaterials 2019, 192, 392–404.
- Hu, S.; Lu, Q.; Xu, Y. Biosensors based on direct electron transfer of protein. In Electrochemical Sensors, Biosensors and Their Biomedical Applications; Zhang, X., Ju, H., Wang, J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 531–581.
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259.
- Lee, F.; Bae, K.H.; Kurisawa, M. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed. Mater. 2015, 11, 014101.
- Carnes, M.E.; Gonyea, C.R.; Mooney, R.G.; Njihia, J.W.; Coburn, J.M.; Pins, G.D. Horseradish Peroxidase-Catalyzed Crosslinking of Fibrin Microthread Scaffolds. Tissue Eng. Part C Methods 2020, 26, 317–331.
- Shoji, S.; Uchida, K.; Satio, W.; Sekiguchi, H.; Inoue, G.; Miyagi, M.; Takata, K.; Yokozeki, Y.; Takaso, M. Acceleration of bone union by in situ-formed hydrogel containing bone morphogenetic protein-2 in a mouse refractory fracture model. J. Orthop. Surg. Res 2020, 15, 426.
- Jo, B.S.; Lee, Y.; Suh, J.S.; Park, Y.S.; Lee, H.J.; Lee, J.-Y.; Cho, J.; Lee, G.; Chung, C.P.; Park, K.D.; et al. A novel calcium-accumulating peptide/gelatin in situ forming hydrogel for enhanced bone regeneration. J. Biomed. Mater. Res. Part A 2018, 106, 531–542.
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720.
- Oliveira, I.M.; Gonçalves, C.; Shin, M.E.; Lee, S.; Reis, R.L.; Khang, G.; Oliveira, J.M. Enzymatically crosslinked tyramine-gellan gum hydrogels as drug delivery system for rheumatoid arthritis treatment. Drug Deliv. Transl. Res. 2021, 11, 1288–1300.
- Yokoyama, K.; Nio, N.; Kikuchi, Y. Properties and applications of microbial transglutaminase. Appl. Microbiol. Biotechnol. 2004, 64, 447–454.
- Savoca, M.P.; Tonoli, E.; Atobatele, A.G.; Verderio, E.A.M. Biocatalysis by Transglutaminases: A Review of Biotechnological Applications. Micromachines 2018, 9, 562.
- Kuwahara, K.; Yang, Z.; Slack, G.C.; Nimni, M.E.; Han, B. Cell delivery using an injectable and adhesive transglutaminase-gelatin gel. Tissue Eng. Part C Methods 2010, 16, 609–618.
- Spurlin, T.A.; Bhadriraju, K.; Chung, K.H.; Tona, A.; Plant, A.L. The treatment of collagen fibrils by tissue transglutaminase to promote vascular smooth muscle cell contractile signaling. Biomaterials 2009, 30, 5486–5496.
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, e2497.
- Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290.
- Sun, C.-K.; Ke, C.-J.; Lin, Y.-W.; Lin, F.-H.; Tsai, T.-H.; Sun, J.-S. Transglutaminase Cross-Linked Gelatin-Alginate-Antibacterial Hydrogel as the Drug Delivery-Coatings for Implant-Related Infections. Polymers 2021, 13, 414.
- La Gatta, A.; Tirino, V.; Cammarota, M.; La Noce, M.; Stellavato, A.; Pirozzi, A.V.A.; Portaccio, M.; Diano, N.; Laino, L.; Papaccio, G.; et al. Gelatin-biofermentative unsulfated glycosaminoglycans semi-interpenetrating hydrogels via microbial-transglutaminase crosslinking enhance osteogenic potential of dental pulp stem cells. Regen. Biomater. 2021, 8, rbaa052.
- Dennis, J.E.; Esterly, K.; Awadallah, A.; Parrish, C.R.; Poynter, G.M.; Goltry, K.L. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells 2007, 25, 2575–2582.
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716.
- Horwitz, E.M. MSC: A coming of age in regenerative medicine. Cytotherapy 2006, 8, 194–195.
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334.
- Vallmajo-Martin, Q.; Broguiere, N.; Millan, C.; Zenobi-Wong, M.; Ehrbar, M. PEG/HA Hybrid Hydrogels for Biologically and Mechanically Tailorable Bone Marrow Organoids. Adv. Funct. Mater. 2020, 30, 1910282.
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010, 77, 4–12.
- Schnepp, Z.A.C.; Gonzalez-McQuire, R.; Mann, S. Hybrid Biocomposites Based on Calcium Phosphate Mineralization of Self-Assembled Supramolecular Hydrogels. Adv. Mater. 2006, 18, 1869–1872.
- Douglas, T.E.; Messersmith, P.B.; Chasan, S.; Mikos, A.G.; de Mulder, E.L.; Dickson, G.; Schaubroeck, D.; Balcaen, L.; Vanhaecke, F.; Dubruel, P.; et al. Enzymatic mineralization of hydrogels for bone tissue engineering by incorporation of alkaline phosphatase. Macromol. Biosci. 2012, 12, 1077–1089.
- Su, H.; Koo, J.M.; Cui, H. One-component nanomedicine. J. Control. Release 2015, 219, 383–395.
- Yuan, D.; Shi, J.; Du, X.; Huang, Y.; Gao, Y.; Baoum, A.A.; Xu, B. The enzyme-instructed assembly of the core of yeast prion Sup35 to form supramolecular hydrogels. J. Mater. Chem. B 2016, 4, 1318–1323.
- Dias, M.R.; Fernandes, P.R.; Guedes, J.M.; Hollister, S.J. Permeability analysis of scaffolds for bone tissue engineering. J. Biomech. 2012, 45, 938–944.
- Akbar, I. Permeability study of functionally graded scaffold based on morphology of cancellous bone. Malays. J. Med. Health Sci. 2021, 17, 60–66.
