Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Jianwei Zhou.
JWA is a microtubule-associated protein and an environmental response gene. JWA has been identified as a potential therapeutic target for several cancers.
- JWA
- cancer therapy
- tumor growth
- angiogenesis
- metastasis
1. The Structure and Functions of JWA
JWA, also known as ADP ribosylation factor-like GTPase 6 interacting protein 5 (ARL6IP5, GenBank: AF070523, 1998), is a microtubule-associated protein and an environmental response gene [11,31,32,33][1][2][3][4]. The full-length cDNA open reading frame sequence is 564 bp, encoding 188 amino acids [11][1]. JWA proteins are highly conserved in humans and other mammals; for example, there is a 95% identity between human and mouse JWA proteins. Homologous genes of JWA, such as glutamate transport-associated protein 3-18 (GTRAP3-18), addicsin, and Jena-Muenchen 4 (JM4), have also been extensively studied for their structure and function [34,35,36][5][6][7]. The JWA gene is located on chromosome 3p14 and contains three exons and two introns. The JWA gene promoter sequence contains multiple response elements, such as TPA (12-O-tetradecanoyl-phorbol-13 acetate) response element (TRE), hemin response element (HRE), heat shock response element (HRE), stress response element (SRE) and ATRA response element (ARE) [11][1]. JWA belongs to the PRA1 domain family, member 3 (PRAF3), which contains a large prenylated Rab acceptor 1(PRA1) domain [37,38][8][9]. Protein structure analysis has shown that JWA is a very hydrophobic protein with three or four transmembrane domains, including protein kinase C (PKC) motifs (SDR-SLR, SDR: codons 18th–20th, SLR: codons 138th–140th) in both C-terminal and N-terminal domains (Figure 1A). JWA protein is located in the endoplasmic reticulum (Figure 1B). Furthermore, JWA binds to a variety of proteins (Figure 1C). The NH2 terminus of JWA is important for the binding between JWA and the receptor activator of NF-kB ligand (RANKL), thus inhibiting osteoclastogenesis [39][10]. The region between 103rd–117th aa of JWA protein is required for homodimer and heterodimer formation with ADP-ribosylation factor-like 6 interacting protein 1 (ARL6IP1) [40][11]. The region between 145th–188th aa of JWA is required for the formation of the addicsin-TR1 heterocomplex, involved in endoplasmic reticulum stress [41][12]. GTRAP3-18 was found to regulate the neuronal glutamate transporter excitatory amino acid carrier 1 (EAAC1) through direct protein-protein interactions [42,43][13][14]. In addition, JWA proteins also had post-translational modifications (Figure 1D). The PKC phosphorylation motif of JWA is responsible for the activation of the MEK-ERK pathway and cell migration [44,45][15][16]; in gastric cancer, JWA lysine 158 is a necessary site for RING Finger Protein 185 (RNF185) ubiquitination and degradation of JWA [46][17]. This suggests that JWA regulates self-protein stability mainly through protein phosphorylation and ubiquitination of its intracellular signaling molecules [32,37,45][3][8][16].
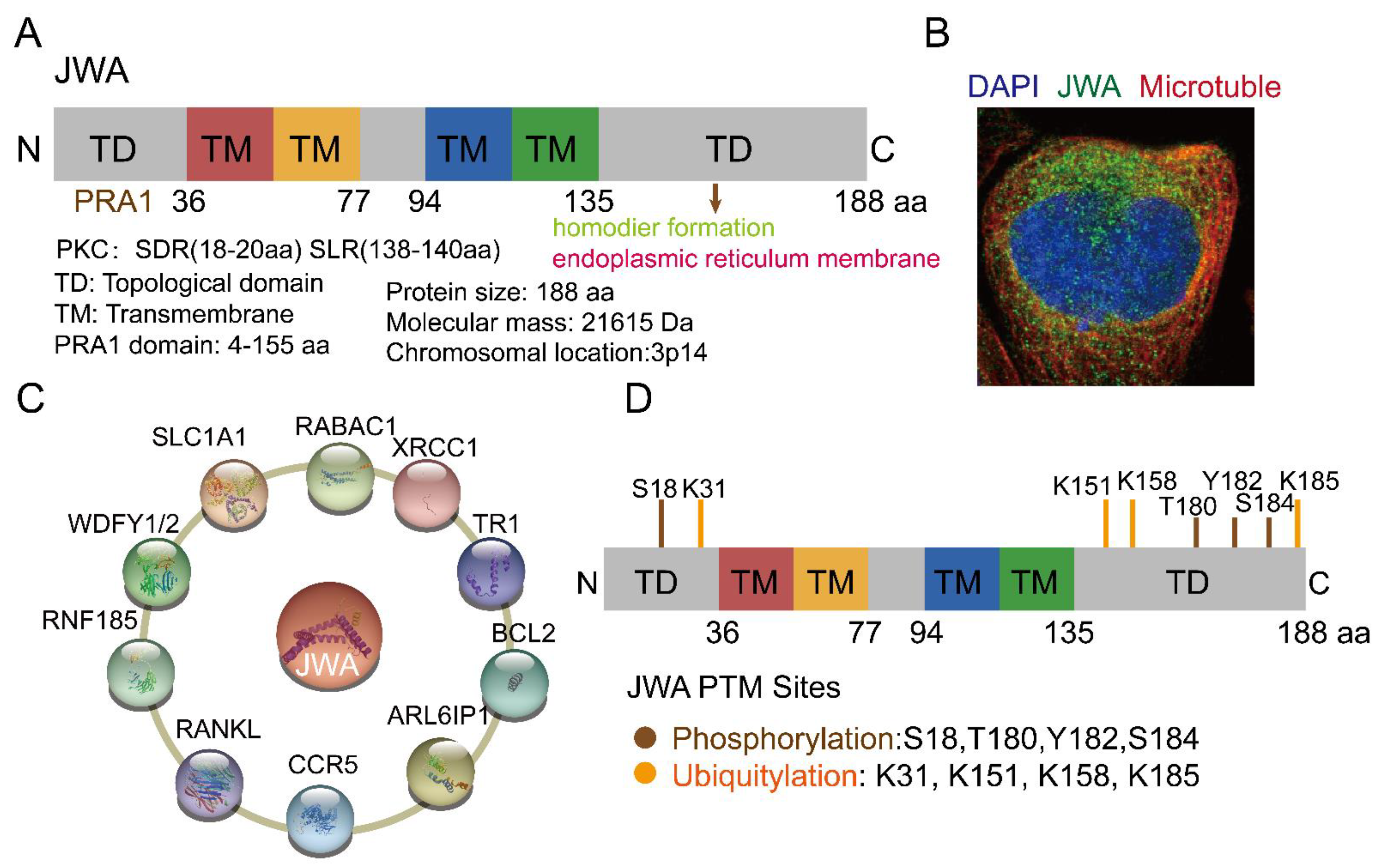
Figure 1. Structure, location and potential modification sites of JWA. (A) Domain structure of JWA. JWA consists of four transmembrane (TM) domains, two topological domains (TD) in N terminal (1–36 aa) and C terminal (135–188 aa). JWA also has protein kinase C (PKC) motifs (SDR-SLR) in both C-terminal and N-terminal domains. (B) The location of JWA in cells from The Human Protein Atlas (https://www.proteinatlas.org/ENSG00000144746-ARL6IP5/subcellular) (accessed on 2 August 2022)) [47][18]. The main location of JWA is the endoplasmic reticulum. Confocal imaging shows JWA (green), microtubules (red) and nucleus staining with DAPI (blue). (C) Binding protein of JWA. JWA binds to multiple proteins and participates in the regulation of signaling networks [22,35,38,39,40,41,46,48,49][6][9][10][11][12][17][19][20][21]. (D) Potential modification sites of JWA from the Phosphosite tool (http://www.phosphosite.org (accessed on 15 August 2022)) [50][22]. Among these, JWA K158 is required for its ubiquitination and degradation by E3 ubiquitin ligase RNF185. In addition, JWA has phosphorylation sites at the C- and N-terminal domains, which can stabilize its expression.
Functionally, JWA is involved in a wide range of biological processes, including proliferation, differentiation, apoptosis, migration, angiogenesis, DNA damage repair, and drug resistance [22,44,51,52,53,54,55,56,57][15][19][23][24][25][26][27][28][29]. When environmental physicochemical factors (cold and heat stimuli) act on cells, the JWA gene responds rapidly and increases its expression to inhibit oxidative stress and repair DNA damage [12,13,57,58][29][30][31][32]. In addition, JWA also plays an important role in neuroprotection and osteoblast development [39,42][10][13]. Due to its multiple biological functions, JWA is closely associated with a variety of diseases, including neurodegenerative diseases and cancer [14,15,21,59,60][33][34][35][36][37]. JWA is a novel tumor suppressor gene, which can inhibit the growth and metastasis of malignant tumors and reverse drug resistance by regulating different signaling networks.
2. Anticancer Strategies Targeting JWA
Chemotherapeutic drugs have long been used to kill tumor cells by promoting apoptosis and inhibiting proliferation; however, chemotherapy is unable to distinguish between tumor cells and normal cells, leading to serious toxic side effects in the body. Compared with traditional chemotherapy drugs, targeted drugs can specifically target cancer cells without obviously affecting normal cells, and have high efficiency and low toxicity [116][38]. Targeted drugs can be broadly classified into two categories: small molecules and large molecules (e.g., monoclonal antibodies, peptides, antibody-drug couples and nucleic acids) [117,118][39][40].
2.1. JWA Peptide—JP1 and JP3
According to the potential functioning fragments in the coding region of the JWA gene identified in mechanistic evidence, several JWA functional mimic polypeptide fragments have been designed with regular modifications in both ends of the polypeptide fragments to delay their rapid degradation. The characteristics of these polypeptides are modified with the phosphate groups for serine (S), threonine (T) or tyrosine (Y) and designed as small kinase molecules. The subcutaneous tumor-bearing mouse models with the A375 cell line are firstly used for in vivo screening of its anticancer activities by intra-tumoral injection. PJP1 contains seven amino acids from the JWA coding region and shows the best anti-proliferative effect among those candidate fragments. Based on the data of the intra-tumoral injection mouse model, the PJP1 is further modified to form an intergrin targeted peptide (named JP1) by the amino acid triplet Arg-Gly-ASP (RGD) [119,120][41][42]. Subsequently, JP1 has shown precise targeting and anticancer activities in subcutaneous tumor-bearing mice by intraperitoneal injection (Figure 2A). In addition, the combination of JP1 and dacarbazine show synergistic inhibition of the tumor-bearing growth of melanoma cells and alleviate dacarbazine-induced liver injury in mice. Furthermore, in a mouse tail vein injection model of the passive metastasis of melanoma, JP1 intraperitoneal injection significantly inhibits lung metastasis in mice, and it prolongs the survival of lung metastasis in melanoma mice. To simulate the development of clinical cancer metastasis, an active metastasis model of lung metastasis with B16F10 was constructed. JP1 was found to significantly inhibit active metastasis when the drug was continued after tumor removal [26][43]. The results from multiple animal models show that JP1 has a potent anti-metastatic effect and has some clinical application prospects.
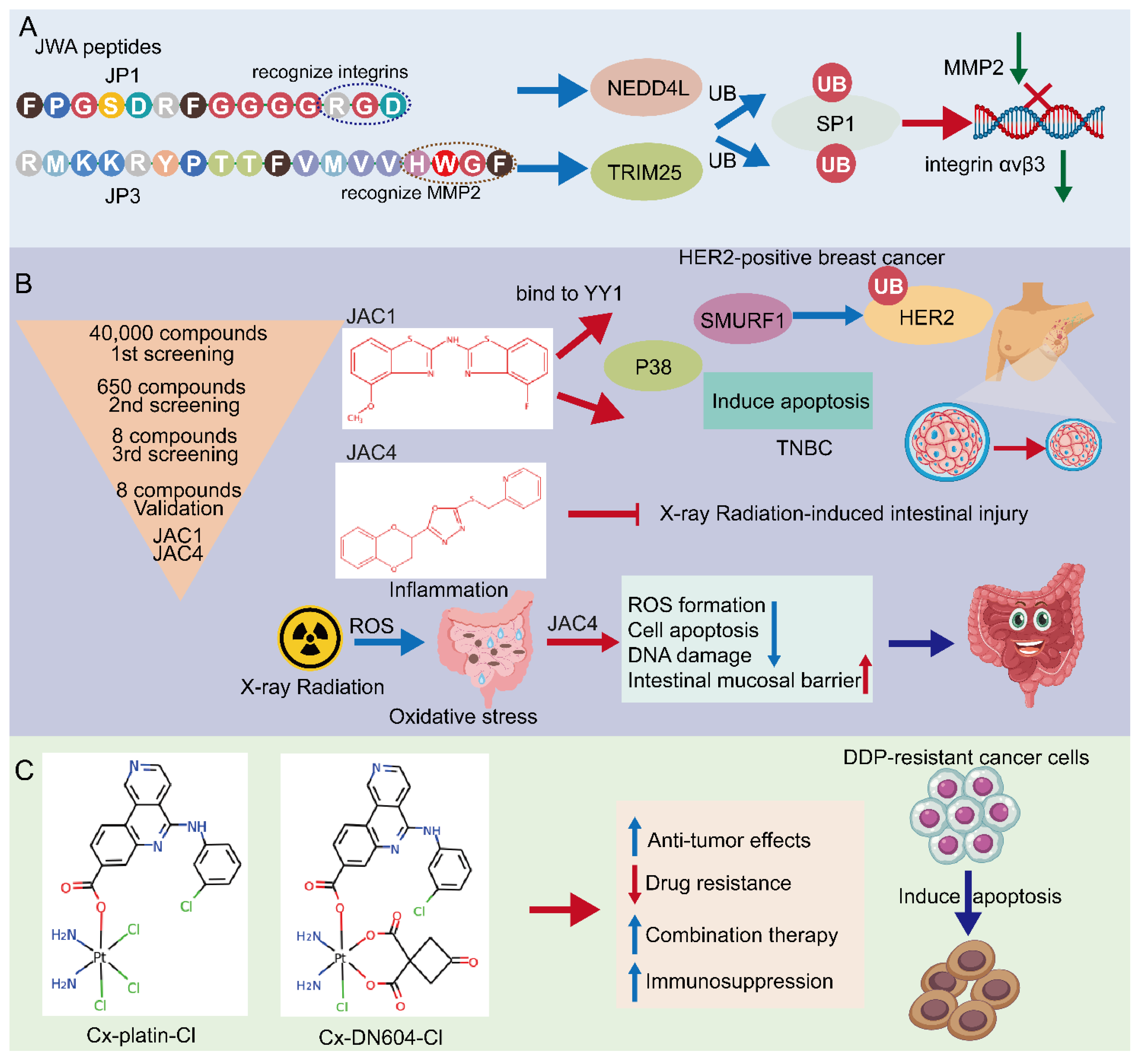
Figure 2. Potential approaches for JWA targeting in cancers. (A) Screening of JWA-targeted peptides: mechanistic study of anti-metastatic peptide JP1 and anti-angiogenetic peptide JP3. (B) Screening of JWA agonists: JAC1 has pro-apoptotic effect in HER2-positive breast cancer and TNBC. Moreover, JAC4 can attenuate X-ray radiation-induced intestinal epithelium injury by JWA-mediated anti-oxidation/inflammation signaling. (C) Emerging JWA-targeted Pt (IV) conjugated with CK2 inhibitor CX-4945: CX-platin-Cl and CX-DN604-Cl can overcome chemo-immune-resistance.
Mechanistically, JP1 targets and enters melanoma cells with high expression of integrin αvβ3 through RGD, then interacts with MEK1/2 and further activates the E3 ubiquitinase NEDD4L (neural precursor cell expressed developmentally downregulated 4-like), accelerates the degradation of SP1 via its K685, and finally exerts a transcriptional inhibitory effect on integrin αvβ3 in targeted A375 cells [26][43]. Integrins are cell adhesion and signaling proteins that play an important role in tumor cell stemness, epithelial cell plasticity, metastasis, and therapeutic resistance [121][44]. Inhibitors of integrins are currently used in the treatment of cardiovascular disease and inflammatory bowel disease, however, there are significant challenges in cancer [122][45]. JP1 is an effective peptide for integrin inhibitors, with potential translational applications. Furthermore, in safety testing, no significant changes in body weight are observed in mice after JP1 intraperitoneal intervention, In fact, the mice treated with JP1 are usually show obvious improvements in the indicators of liver function, kidney function and myocardial kinases, suggesting that JP1 has no significant toxicity in the mouse model. No significant adverse effects were observed in healthy crab-eating monkeys injected intravenously with JP1 at a high dose (150 mg/kg) for 14 days (IV, qd), suggesting the biosafety of JP1 [26][43].
Novel peptides containing histidine-tryptophan-phenylalanine (HWGF) motif specifically recognize MMP2 [123,124][46][47]. JWA has shown obvious inhibition of angiogenesis via downregulation of the expression of MMP2 in gastric cancer cells [17][48]. PJP3 peptide shows an effective tumor suppressive role in subcutaneous tumor-bearing mouse models of gastric cancer cells (Figure 2A); HWGF linked PJP3 (named as JP3) further enhanced its MMP targeting properties. Mechanistically, as an MMP2 targeting peptide, JP3 inhibits angiogenesis through TRIM25 (Tripartite motif containing 25)/SP1/MMP2 signaling, and plays a therapeutic role in gastric cancer [27][49]. In addition, JP3 competitively inhibits the binding of XRCC1 and CK2 in cisplatin-resistant gastric cancer cells, and JP3 in combination with cisplatin synergistically promotes DNA damage and apoptosis gastric cancer. At the same time, JP3 also protects normal cells from cisplatin-induced DNA damage and apoptosis through the ERK/Nrf2 signaling pathway [28][50]. Therefore, both JP1 and JP3 may be used in combination with other chemotherapeutic drugs in cancer cells, which not only has a synergistic anti-cancer effect, but also reduces damage to normal organs through anti-oxidative stress signal pathways, which can address two objectives in cancer treatment.
2.2. Small Molecular JWA Agonists
Small molecule targeted drugs have advantages compared with large molecule drugs in terms of pharmacokinetic (PK) properties, cost, patient compliance, drug storage, and transport [125,126][51][52]. Many studies have shown the deficiency or down regulation of JWA expression in cancer tissues compared with the adjacent normal tissues. Therefore, the agonists screening of the JWA gene at a transcriptional level may be an important way to find JWA gene-based anti-cancer compound candidates. A reporter gene plasmid for the JWA promoter fragment has been successfully constructed and the high throughput assays have been completed in HBE cells. Obviously, this reporter gene plasmid design philosophy is based on maintaining the homeostasis in normal cells and not cancer cells. Both JWA agonist compounds JAC1 and JAC4 are finally selected from multiple candidates based on their lipid-water partition coefficient, water solubility, molecular weight, and activation intensity in HBE cells. Ren et al. recently reported that in HER2-positive breast cancer cells, JAC1 inhibits cell proliferation through the JWA/P38/SMURF1/HER2 signaling pathway [29][53]. The triple-negative breast cancer (TNBC) is the most aggressive of breast cancer cells. Zhai et al. found that JAC1 effectively suppresses TNBC proliferation and induction of apoptosis through JWA/P38 signaling [30][54]. The mechanism of JWA activation by JAC1 is identified by specifically binding of JAC1 to Zinc-finger Yin Yang (YY1), which promotes ubiquitinated degradation of YY1, thereby relieving the transcriptional repression on the JWA gene by YY1 and increasing the transcriptional activation of JWA [30][54]. YY1 regulates genes related to the cell cycle, cell death, and tumor metabolism [127,128,129][55][56][57]; YY1 is highly expressed in many cancers [130][58]. Accordingly, JAC1 may exert anti-cancer effects in YY1-overexpressed malignant tumors (Figure 2B).
The main side effect of radiation therapy for abdominal malignancy patients is radiotherapy-induced injuries to both the intestinal epithelium and bone marrow hematopoietic function, for which there is no specific clinical treatment [131,132][59][60]. Therefore, it is an obvious unmet clinical need to find target drugs for radiation enteritis and bone marrow injury. Zhou et al. recently reported that the JWA agonist JAC4 attenuates intestinal mucosal damage induced by abdominal radiation in mice [133][61]. More importantly, JAC4 significantly increases the survival rate of mice after exposure to a lethal dose of X-rays. In addition, JAC4 reduces radiation-induced oxidative stress damage and inflammatory responses. In intestinal crypt epithelial cells, JAC4 attenuates radiation-induced ROS production and apoptosis (Figure 2B). In mice with intestinal epithelial knockout JWA, the protective effects of JAC4 against radiation-induced intestinal injury is substantially weakened, suggesting that the protective roles of JAC4 on X-ray irradiation induced intestine epithelium injury depend upon JWA expression [133][61]. Considering the anti-cancer effect of JWA and its specific function to prevent radiation damage, JAC4 may be considered as a candidate drug for radiotherapy of abdominal malignancies.
The preventive and therapeutic role of dietary therapy of tumors has received significant attention in recent years. Green tea has been reported to exert inhibitory effects in a variety of tumors [134][62]. Most of the chemo-preventive effects of green tea on cancer have been attributed to polyphenolic compounds, of which (-)-epigallocatechin-3-gallate (EGCG) is the most important [135][63]. Currently, EGCG has been shown to induce apoptosis and cycle arrest to inhibit tumor cell proliferation and metastasis by inhibiting cancer cell angiogenesis [136,137][64][65]. A study by Yuan Li et al. found that EGCG suppresses lung cancer progression by activating JWA expression as well as inhibiting topoisomerase IIα expression in lung cancer cells and in a tumor-bearing model [138][66]. Mechanistically, the amino acid region 90-188 of JWA is necessary for degradation of topoisomerase IIα. In addition, JWA and topoisomerase IIα can synergistically inhibit lung cancer cell migration and invasion. Therefore, EGCG can exist as a natural compound to activate JWA expression.
2.3. Emerging JWA-Targeted Pt (IV) Prodrugs Conjugated with CX-4945
DNA-targeted anti-cancer chemotherapeutic agents (platinum complexes) are currently the most effective chemotherapeutic agents in clinical practice [139][67]. They work primarily by initiating DNA damage to induce apoptosis using the DNA repair pathways that exist in the cells themselves, which however, may lead to chemotherapy drug resistance [140][68]. Therefore, the main approach to reversing chemotherapy resistance is to use inhibitors of the DNA repair pathway in combination with platinum-based drugs [141][69]. Over the years, various approaches have been used to try to increase the selectivity of prodrugs for cancer cells to overcome drug resistance [142][70]. Guo et al. designed and synthesized two Pt (IV) prodrugs: Cx-platin-Cl and Cx-DN604-Cl [143][71]. These Pt (IV) prodrugs showed strong toxic effects in tumor cells compared with cisplatin both in vivo and in vitro. In addition, Cx-platin-Cl treatment enhanced T cell infiltration in xenograft mouse tumors, and mechanistically, Pt (IV) prodrugs inhibited the growth of cisplatin-resistant ovarian cancer via the JWA-XRCC1 pathway [143,144][71][72]. It has been shown that Pt (IV) prodrugs targeting the JWA-mediated DNA damage repair pathway and immunosuppression can reverse cisplatin resistance. Thus, emerging JWA-targeted Pt (IV) prodrugs can be used as potential immunotherapeutic agents for tumor resistance (Figure 2C).
References
- Xia, W.; Zhou, J.; Cao, H.; Zou, C.; Wang, C.; Shen, Q.; Lu, H. Relationship between structure and function of jwa in the modulation of cell differentiation. China Sci. Bull. Engl. Version 2001, 24, 2063–2067.
- Li, A.; Li, A.; Mao, W.; Chen, H.; Huang, S.; Qi, H.; Ye, J.; Zhang, Z.; Wang, X.; Sun, F.; et al. Jwa, a novel microtubule-associated protein, regulates homeostasis of intracellular amino acids in pc12 cells. Chin. Sci. Bull. 2003, 48, 1828–1834.
- Chen, H.; Bai, J.; Ye, J.; Liu, Z.; Chen, R.; Mao, W.; Li, A.; Zhou, J. Jwa as a functional molecule to regulate cancer cells migration via mapk cascades and f-actin cytoskeleton. Cell. Signal. 2007, 19, 1315–1327.
- Chen, R.; Li, A.; Zhu, T.; Li, C.; Liu, Q.; Chang, H.C.; Zhou, J. Jwa—A novel environmental-responsive gene, involved in estrogen receptor-associated signal pathway in mcf-7 and mda-mb-231 breast carcinoma cells. J. Toxicol. Environ. Health A 2005, 68, 445–456.
- Lin, C.I.; Orlov, I.; Ruggiero, A.M.; Dykes-Hoberg, M.; Lee, A.; Jackson, M.; Rothstein, J.D. Modulation of the neuronal glutamate transporter eaac1 by the interacting protein gtrap3-18. Nature 2001, 410, 84–88.
- Schweneker, M.; Bachmann, A.S.; Moelling, K. Jm4 is a four-transmembrane protein binding to the ccr5 receptor. FEBS Lett. 2005, 579, 1751–1758.
- Ikemoto, M.J.; Inoue, K.; Akiduki, S.; Osugi, T.; Imamura, T.; Ishida, N.; Ohtomi, M. Identification of addicsin/gtrap3-18 as a chronic morphine-augmented gene in amygdala. Neuroreport 2002, 13, 2079–2084.
- Fo, C.S.; Coleman, C.S.; Wallick, C.J.; Vine, A.L.; Bachmann, A.S. Genomic organization, expression profile, and characterization of the new protein pra1 domain family, member 2 (praf2). Gene 2006, 371, 154–165.
- Koomoa, D.L.; Go, R.C.; Wester, K.; Bachmann, A.S. Expression profile of praf2 in the human brain and enrichment in synaptic vesicles. Neurosci. Lett. 2008, 436, 171–176.
- Wu, Y.; Wang, M.; Peng, Y.; Ding, Y.; Deng, L.; Fu, Q. Overexpression of arl6ip5 in osteoblast regulates rankl subcellualr localization. Biochem. Biophys. Res. Commun. 2015, 464, 1275–1281.
- Akiduki, S.; Ikemoto, M.J. Modulation of the neural glutamate transporter eaac1 by the addicsin-interacting protein arl6ip1. J. Biol. Chem. 2008, 283, 31323–31332.
- Arano, T.; Fujisaki, S.; Ikemoto, M.J. Identification of tomoregulin-1 as a novel addicsin-associated factor. Neurochem. Int. 2014, 71, 22–35.
- Aoyama, K.; Watabe, M.; Nakaki, T. Modulation of neuronal glutathione synthesis by eaac1 and its interacting protein gtrap3-18. Amino Acids 2012, 42, 163–169.
- Watabe, M.; Aoyama, K.; Nakaki, T. Regulation of glutathione synthesis via interaction between glutamate transport-associated protein 3-18 (gtrap3-18) and excitatory amino acid carrier-1 (eaac1) at plasma membrane. Mol. Pharmacol. 2007, 72, 1103–1110.
- Huang, S.; Shen, Q.; Mao, W.G.; Li, A.P.; Ye, J.; Liu, Q.Z.; Zou, C.P.; Zhou, J.W. Jwa, a novel signaling molecule, involved in all-trans retinoic acid induced differentiation of hl-60 cells. J. Biomed. Sci. 2006, 13, 357–371.
- Ye, J.; Li, A.; Qiu, W.; Zhou, J.; Zhou, J. The effect of pkc phosphorylation sites mutation in jwa coding region on tpa-induced mcf-7 cell differentiation. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2007, 25, 398–401.
- Qiu, D.; Wang, Q.; Wang, Z.; Chen, J.; Yan, D.; Zhou, Y.; Li, A.; Zhang, R.; Wang, S.; Zhou, J. Rnf185 modulates jwa ubiquitination and promotes gastric cancer metastasis. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 1552–1561.
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507.
- Xu, W.; Chen, Q.; Wang, Q.; Sun, Y.; Wang, S.; Li, A.; Xu, S.; Roe, O.D.; Wang, M.; Zhang, R.; et al. Jwa reverses cisplatin resistance via the ck2-xrcc1 pathway in human gastric cancer cells. Cell Death Dis. 2014, 5, e1551.
- Vento, M.T.; Zazzu, V.; Loffreda, A.; Cross, J.R.; Downward, J.; Stoppelli, M.P.; Iaccarino, I. Praf2 is a novel bcl-xl/bcl-2 interacting protein with the ability to modulate survival of cancer cells. PLoS ONE 2010, 5, e15636.
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The string database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612.
- Hornbeck, P.V.; Chabra, I.; Kornhauser, J.M.; Skrzypek, E.; Zhang, B. Phosphosite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 2004, 4, 1551–1561.
- Lin, J.; Ma, T.; Jiang, X.; Ge, Z.; Ding, W.; Wu, Y.; Jiang, G.; Feng, J.; Cui, G.; Tan, Y. Jwa regulates human esophageal squamous cell carcinoma and human esophageal cells through different mitogen-activated protein kinase signaling pathways. Exp. Ther. Med. 2014, 7, 1767–1771.
- Wu, Y.Y.; Ma, T.L.; Ge, Z.J.; Lin, J.; Ding, W.L.; Feng, J.K.; Zhou, S.J.; Chen, G.C.; Tan, Y.F.; Cui, G.X. Jwa gene regulates panc-1 pancreatic cancer cell behaviors through mek-erk1/2 of the mapk signaling pathway. Oncol. Lett. 2014, 8, 1859–1863.
- Chen, X.; Feng, J.; Ge, Z.; Chen, H.; Ding, W.; Zhu, W.; Tang, X.; Chen, Y.; Tan, Y.; Ma, T. Effects of the jwa gene in the regulation of human breast cancer cells. Mol. Med. Rep. 2015, 11, 3848–3853.
- Shen, Q.; Zhou, J.W.; Sheng, R.L.; Zhu, G.R.; Cao, H.X.; Lu, H. Jwa gene in regulating committed differentiation of hl-60 cells induced by atra, ara-c and tpa. Zhongguo Shiyan Xueyexue Zazhi 2005, 13, 804–808.
- Huang, S.; Shen, Q.; Mao, W.G.; Li, A.P.; Ye, J.; Liu, Q.Z.; Zou, C.P.; Zhou, J.W. Jwa, a novel signaling molecule, involved in the induction of differentiation of human myeloid leukemia cells. Biochem. Biophys. Res. Commun. 2006, 341, 440–450.
- Lu, J.; Tang, Y.; Farshidpour, M.; Cheng, Y.; Zhang, G.; Jafarnejad, S.M.; Yip, A.; Martinka, M.; Dong, Z.; Zhou, J.; et al. Jwa inhibits melanoma angiogenesis by suppressing ilk signaling and is an independent prognostic biomarker for melanoma. Carcinogenesis 2013, 34, 2778–2788.
- Gu, D.A.; Li, A.P.; Zhu, T.; Ye, J.; Zhou, J.W. Relationship between jwa expression and dna damage-repair in human embryonic lung cells by benzo(a) pyrene. Zhonghua Yufang Yixue Zazhi 2005, 39, 187–190.
- Mao, W.G.; Li, A.P.; Ye, J.; Huang, S.; Li, A.Q.; Zhou, J.W. Effect of differentiation inducer and heat stress on the expression of jwa protein and hsp70 of k562 cells. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2003, 21, 253–256.
- Mao, W.G.; Li, A.P.; Ye, J.; Huang, S.; Li, A.Q.; Zhou, J.W. Expressions of jwa protein and heat stress protein 70 induced by cell differentiation inducers combined with heat stress in k562 cells. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2004, 22, 60–63.
- Zhao, M.; Chen, R.; Li, A.P.; Zhou, J.W. Effects of hemin and thermal stress exposure on jwa expression. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2006, 24, 209–213.
- Wang, R.; Zhao, X.; Xu, J.; Wen, Y.; Li, A.; Lu, M.; Zhou, J. Astrocytic jwa deletion exacerbates dopaminergic neurodegeneration by decreasing glutamate transporters in mice. Cell Death Dis. 2018, 9, 352.
- Miao, S.H.; Sun, H.B.; Ye, Y.; Yang, J.J.; Shi, Y.W.; Lu, M.; Hu, G.; Zhou, J.W. Astrocytic jwa expression is essential to dopaminergic neuron survival in the pathogenesis of parkinson’s disease. CNS Neurosci. Ther. 2014, 20, 754–762.
- Lu, J.; Tang, Y.; Cheng, Y.; Zhang, G.; Yip, A.; Martinka, M.; Dong, Z.; Zhou, J.; Li, G. Ing4 regulates jwa in angiogenesis and their prognostic value in melanoma patients. Br. J. Cancer 2013, 109, 2842–2852.
- Zhao, X.; Wang, R.; Xiong, J.; Yan, D.; Li, A.; Wang, S.; Xu, J.; Zhou, J. Jwa antagonizes paraquat-induced neurotoxicity via activation of nrf2. Toxicol. Lett. 2017, 277, 32–40.
- Shen, Q.; Tang, W.Y.; Li, C.P.; Chen, R.; Zhu, Y.J.; Huang, S.; Li, A.P.; Zhou, J.W. Functional variations in the jwa gene are associated with increased odds of leukemias. Leuk. Res. 2007, 31, 783–790.
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal. Transduct. Target. Ther. 2021, 6, 201.
- Zhu, Y.S.; Tang, K.; Lv, J. Peptide-drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharmacol. Sci. 2021, 42, 857–869.
- Wilkes, G.M. Targeted therapy: Attacking cancer with molecular and immunological targeted agents. Asia Pac. J. Oncol. Nurs. 2018, 5, 137–155.
- Casali, B.C.; Gozzer, L.T.; Baptista, M.P.; Altei, W.F.; Selistre-De-Araujo, H.S. The effects of alphavbeta3 integrin blockage in breast tumor and endothelial cells under hypoxia in vitro. Int. J. Mol. Sci. 2022, 23, 1745.
- Pina, A.; Kadri, M.; Arosio, D.; Dal Corso, A.; Coll, J.L.; Gennari, C.; Boturyn, D. Multimeric presentation of rgd peptidomimetics enhances integrin binding and tumor cell uptake. Chemistry 2020, 26, 7492–7496.
- Cui, J.; Shu, C.; Xu, J.; Chen, D.; Li, J.; Ding, K.; Chen, M.; Li, A.; He, J.; Shu, Y.; et al. Jp1 suppresses proliferation and metastasis of melanoma through mek1/2 mediated nedd4l-sp1-integrin alphavbeta3 signaling. Theranostics 2020, 10, 8036–8050.
- Cooper, J.; Giancotti, F.G. Integrin signaling in cancer: Mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 2019, 35, 347–367.
- Slack, R.J.; Macdonald, S.; Roper, J.A.; Jenkins, R.G.; Hatley, R. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78.
- Koivunen, E.; Arap, W.; Valtanen, H.; Rainisalo, A.; Medina, O.P.; Heikkila, P.; Kantor, C.; Gahmberg, C.G.; Salo, T.; Konttinen, Y.T.; et al. Tumor targeting with a selective gelatinase inhibitor. Nat. Biotechnol. 1999, 17, 768–774.
- Turunen, M.P.; Puhakka, H.L.; Koponen, J.K.; Hiltunen, M.O.; Rutanen, J.; Leppanen, O.; Turunen, A.M.; Narvanen, A.; Newby, A.C.; Baker, A.H.; et al. Peptide-retargeted adenovirus encoding a tissue inhibitor of metalloproteinase-1 decreases restenosis after intravascular gene transfer. Mol. Ther. 2002, 6, 306–312.
- Chen, Y.; Huang, Y.; Huang, Y.; Xia, X.; Zhang, J.; Zhou, Y.; Tan, Y.; He, S.; Qiang, F.; Li, A.; et al. Jwa suppresses tumor angiogenesis via sp1-activated matrix metalloproteinase-2 and its prognostic significance in human gastric cancer. Carcinogenesis 2014, 35, 442–451.
- Chen, J.J.; Ren, Y.L.; Shu, C.J.; Zhang, Y.; Chen, M.J.; Xu, J.; Li, J.; Li, A.P.; Chen, D.Y.; He, J.D.; et al. Jp3, an antiangiogenic peptide, inhibits growth and metastasis of gastric cancer through trim25/sp1/mmp2 axis. J. Exp. Clin. Cancer Res. 2020, 39, 118.
- Zhang, Y.; Chen, J.; Che, Z.; Shu, C.; Chen, D.; Ding, K.; Li, A.; Zhou, J. Jp3 enhances the toxicity of cisplatin on drug-resistant gastric cancer cells while reducing the damage to normal cells. J. Cancer 2021, 12, 1894–1906.
- Lu, B.; Atala, A. Small molecules and small molecule drugs in regenerative medicine. Drug Discov. Today 2014, 19, 801–808.
- An, G. Concept of pharmacologic target-mediated drug disposition in large-molecule and small-molecule compounds. J. Clin. Pharmacol. 2020, 60, 149–163.
- Ren, Y.; Chen, D.; Zhai, Z.; Chen, J.; Li, A.; Liang, Y.; Zhou, J. Jac1 suppresses proliferation of breast cancer through the jwa/p38/smurf1/her2 signaling. Cell Death Discov. 2021, 7, 85.
- Zhai, Z.; Ren, Y.; Shu, C.; Chen, D.; Liu, X.; Liang, Y.; Li, A.; Zhou, J. Jac1 targets yy1 mediated jwa/p38 mapk signaling to inhibit proliferation and induce apoptosis in tnbc. Cell Death Discov. 2022, 8, 169.
- Tang, W.; Zhou, W.; Xiang, L.; Wu, X.; Zhang, P.; Wang, J.; Liu, G.; Zhang, W.; Peng, Y.; Huang, X.; et al. The p300/yy1/mir-500a-5p/hdac2 signalling axis regulates cell proliferation in human colorectal cancer. Nat. Commun. 2019, 10, 663.
- Yang, F.; Fang, E.; Mei, H.; Chen, Y.; Li, H.; Li, D.; Song, H.; Wang, J.; Hong, M.; Xiao, W.; et al. Cis-acting circ-ctnnb1 promotes beta-catenin signaling and cancer progression via ddx3-mediated transactivation of yy1. Cancer Res. 2019, 79, 557–571.
- Pan, G.; Diamanti, K.; Cavalli, M.; Lara, G.A.; Komorowski, J.; Wadelius, C. Multifaceted regulation of hepatic lipid metabolism by yy1. Life Sci. Alliance 2021, 4, e202000928.
- Khachigian, L.M. The yin and yang of yy1 in tumor growth and suppression. Int. J. Cancer 2018, 143, 460–465.
- Wang, K.X.; Cui, W.W.; Yang, X.; Tao, A.B.; Lan, T.; Li, T.S.; Luo, L. Mesenchymal stem cells for mitigating radiotherapy side effects. Cells 2021, 10, 294.
- Barazzuol, L.; Coppes, R.P.; van Luijk, P. Prevention and treatment of radiotherapy-induced side effects. Mol. Oncol. 2020, 14, 1538–1554.
- Zhou, Y.; Liu, J.; Li, X.; Wang, L.; Hu, L.; Li, A.; Zhou, J. Jac4 protects from x-ray radiation-induced intestinal injury by jwa-mediated anti-oxidation/inflammation signaling. Antioxidants 2022, 11, 1067.
- Zhao, J.; Blayney, A.; Liu, X.; Gandy, L.; Jin, W.; Yan, L.; Ha, J.H.; Canning, A.J.; Connelly, M.; Yang, C.; et al. Egcg binds intrinsically disordered n-terminal domain of p53 and disrupts p53-mdm2 interaction. Nat. Commun. 2021, 12, 986.
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin gallate (egcg) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691.
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (egcg): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941.
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956.
- Li, Y.; Shen, X.; Wang, X.; Li, A.; Wang, P.; Jiang, P.; Zhou, J.; Feng, Q. Egcg regulates the cross-talk between jwa and topoisomerase iiα in non-small-cell lung cancer (nsclc) cells. Sci. Rep. 2015, 5, 11009.
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132.
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584.
- Chen, F.; Huang, X.; Wu, M.; Gou, S.; Hu, W. A ck2-targeted pt(iv) prodrug to disrupt dna damage response. Cancer Lett. 2017, 385, 168–178.
- Gibson, D. Platinum(iv) anticancer agents; Are we en route to the holy grail or to a dead end? J. Inorg. Biochem. 2021, 217, 111353.
- Chen, F.; Pei, S.; Wang, X.; Zhu, Q.; Gou, S. Emerging jwa-targeted pt(iv) prodrugs conjugated with cx-4945 to overcome chemo-immune-resistance. Biochem. Biophys. Res. Commun. 2020, 521, 753–761.
- Wang, X.; Li, L.; Pei, S.; Zhu, Q.; Chen, F. Disruption of ssbs repair to combat platinum resistance via the jwa-targeted pt(iv) prodrug conjugated with a wogonin derivative. Die Pharm. 2020, 75, 94–101.
More
