The restoration of an intact epidermal barrier after wound injury is the culmination of a highly complex and exquisitely regulated physiological process involving multiple cells and tissues, overlapping dynamic events and protein synthesis and regulation. Central to this process is the cytoskeleton, a system of intracellular proteins that are instrumental in regulating important processes involved in wound repair including chemotaxis, cytokinesis, proliferation, migration, and phagocytosis. One highly conserved family of cytoskeletal proteins that are emerging as major regulators of actin and microtubule nucleation, polymerization, and stabilization are the formins. The formin family includes 15 different proteins categorized into seven subfamilies based on three formin homology domains (FH1, FH2, and FH3). The formins themselves are regulated in different ways including autoinhibition, activation, and localization by a range of proteins, including Rho GTPases
- formins
- wound healing
- actin filaments
- microtubules
- i
1. Formins in Inflammation
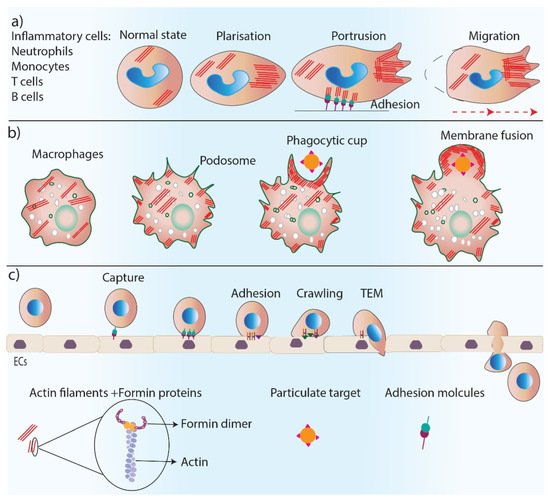
2. Formins in Skin Cell Migration
3. Formins in Cell Proliferation
4. Formins in Epithelial-to-Mesenchymal Transition (EMT)
5. Formins in Angiogenesis
6. Formins in Tissue Maturation and Fibrosis
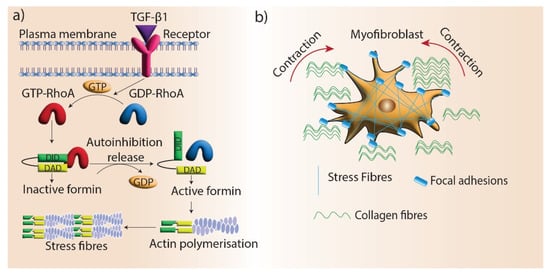
References
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388.
- Shi, Y.; Zhang, J.; Mullin, M.; Dong, B.; Alberts, A.S.; Siminovitch, K.A. The mDial Formin Is Required for Neutrophil Polarization, Migration, and Activation of the LARG/RhoA/ROCK Signaling Axis during Chemotaxis. J. Immunol. 2009, 182, 3837–3845.
- Shi, Y.; Dong, B.; Miliotis, H.; Liu, J.; Alberts, A.S.; Zhang, J.; Siminovitch, K.A. Src kinase Hck association with the WASp and mDia1 cytoskeletal regulators promotes chemoattractant-induced Hck membrane targeting and activation in neutrophilsThis paper is one of a selection of papers published in this Special Issue, entitled CSBMCB’s 51st Annual Meeting – Epigenetics and Chromatin Dynamics, and has undergone the Journal’s usual peer review process. Biochem. Cell Biol. 2009, 87, 207–216.
- Muller, W.A. Getting Leukocytes to the Site of Inflammation. Veter- Pathol. 2013, 50, 7–22.
- Thompson, S.B.; Wigton, E.J.; Krovi, S.H.; Chung, J.W.; Long, R.A.; Jacobelli, J. The Formin mDia1 Regulates Acute Lymphoblastic Leukemia Engraftment, Migration, and Progression in vivo. Front. Oncol. 2018, 8, 389.
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Macrophages 2017, 62, 353–364.
- Trotter, J.A. The organization of actin in spreading macrophages: The actin-cytoskeleton of peritoneal macrophages is linked to the substratum via transmembrane connections. Exp. Cell Res. 1981, 132, 235–248.
- Wu, J.; Weening, E.H.; Faske, J.B.; Höök, M.; Skare, J.T. Invasion of Eukaryotic Cells by Borrelia burgdorferi Requires β1 Integrins and Src Kinase Activity. Infect. Immun. 2011, 79, 1338–1348.
- Mersich, A.T.; Miller, M.R.; Chkourko, H.; Blystone, S.D. The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton 2010, 67, 573–585.
- Naj, X.; Hoffmann, A.-K.; Himmel, M.; Linder, S. The Formins FMNL1 and mDia1 Regulate Coiling Phagocytosis of Borrelia burgdorferi by Primary Human Macrophages. Infect. Immun. 2013, 81, 1683–1695.
- Seth, A.; Otomo, C.; Rosen, M.K. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLα and mDia1. J. Cell Biol. 2006, 174, 701–713.
- Colucci-Guyon, E.; Niedergang, F.; Wallar, B.J.; Peng, J.; Alberts, A.S.; Chavrier, P. A Role for Mammalian Diaphanous-Related Formins in Complement Receptor (CR3)-Mediated Phagocytosis in Macrophages. Curr. Biol. 2005, 15, 2007–2012.
- Brandt, D.T.; Marion, S.; Griffiths, G.; Watanabe, T.; Kaibuchi, K.; Grosse, R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 2007, 178, 193–200.
- Thompson, S.B.; Sandor, A.M.; Lui, V.; Chung, J.W.; Waldman, M.M.; A Long, R.; Estin, M.L.; Matsuda, J.L.; Friedman, R.S.; Jacobelli, J. Formin-like 1 mediates effector T cell trafficking to inflammatory sites to enable T cell-mediated autoimmunity. eLife 2020, 9.
- Sakata, D.; Taniguchi, H.; Yasuda, S.; Adachi-Morishima, A.; Hamazaki, Y.; Nakayama, R.; Miki, T.; Minato, N.; Narumiya, S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J. Exp. Med. 2007, 204, 2031–2038.
- Eisenmann, K.M.; West, R.A.; Hildebrand, D.; Kitchen, S.M.; Peng, J.; Sigler, R.; Zhang, J.; Siminovitch, K.A.; Alberts, A.S. T Cell Responses in Mammalian Diaphanous-related Formin mDia1 Knock-out Mice. J. Biol. Chem. 2007, 282, 25152–25158.
- Tanizaki, H.; Egawa, G.; Inaba, K.; Honda, T.; Nakajima, S.; Moniaga, C.S.; Otsuka, A.; Ishizaki, T.; Tomura, M.; Watanabe, T.; et al. Rho-mDia1 pathway is required for adhesion, migration, and T-cell stimulation in dendritic cells. Blood 2010, 116, 5875–5884.
- Dupré, L.; Boztug, K.; Pfajfer, L. Actin Dynamics at the T Cell Synapse as Revealed by Immune-Related Actinopathies. Front. Cell Dev. Biol. 2021, 9.
- Gomez, T.S.; Kumar, K.; Medeiros, R.B.; Shimizu, Y.; Leibson, P.J.; Billadeau, D.D. Formins Regulate the Actin-Related Protein 2/3 Complex-Independent Polarization of the Centrosome to the Immunological Synapse. Immunity 2007, 26, 177–190.
- Ahangar, P.; Strudwick, X.L.; Cowin, A.J. Wound Healing from an Actin Cytoskeletal Perspective. Cold Spring Harb. Perspect. Biol. 2022, 14, a041235.
- Small, J.V.; Vignal, E. Cell Migration. In Encyclopedia of Biological Chemistry, 1st Edition.; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 356–361.
- Cangkrama, M.; Wietecha, M.; Mathis, N.; Okumura, R.; Ferrarese, L.; Al-Nuaimi, D.; Antsiferova, M.; Dummer, R.; Innocenti, M.; Werner, S. A paracrine activin A–mDia2 axis promotes squamous carcinogenesis via fibroblast reprogramming. EMBO Mol. Med. 2020, 12, e11466.
- Mallavarapu, A.; Mitchison, T. Regulated Actin Cytoskeleton Assembly at Filopodium Tips Controls Their Extension and Retraction. J. Cell Biol. 1999, 146, 1097–1106.
- Pellegrin, S.; Mellor, H. The Rho Family GTPase Rif Induces Filopodia through mDia2. Curr. Biol. 2005, 15, 129–133.
- Romero, S.; Le Clainche, C.; Didry, D.; Egile, C.; Pantaloni, D.; Carlier, M.-F. Formin Is a Processive Motor that Requires Profilin to Accelerate Actin Assembly and Associated ATP Hydrolysis. Cell 2004, 119, 419–429.
- Otomo, T.; Tomchick, D.; Otomo, C.; Panchal, S.C.; Machius, M.; Rosen, M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nat. 2005, 433, 488–494.
- Bombardier, J.P.; Eskin, J.A.; Jaiswal, R.; Corrêa, I.R., Jr.; Xu, M.-Q.; Goode, B.L.; Gelles, J. Single-molecule visualization of a formin-capping protein ‘decision complex’ at the actin filament barbed end. Nat. Commun. 2015, 6, 8707.
- Beli, P.; Mascheroni, D.; Xu, D.; Innocenti, M. WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat. Cell Biol. 2008, 10, 849–857.
- Innocenti, M. New insights into the formation and the function of lamellipodia and ruffles in mesenchymal cell migration. Cell Adhes. Migr. 2018, 12, 401–416.
- Yang, C.; Czech, L.; Gerboth, S.; Kojima, S.-I.; Scita, G.; Svitkina, T. Novel Roles of Formin mDia2 in Lamellipodia and Filopodia Formation in Motile Cells. PLOS Biol. 2007, 5, e317.
- Xue, F.; Janzen, D.M.; Knecht, D.A. Contribution of Filopodia to Cell Migration: A Mechanical Link between Protrusion and Contraction. Int. J. Cell Biol. 2010, 2010, 507821.
- Magdalena, J.; Millard, T.H.; Machesky, L. Microtubule involvement in NIH 3T3 Golgi and MTOC polarity establishment. J. Cell Sci. 2003, 116, 743–756.
- Zaoui, K.; Honoré, S.; Isnardon, D.; Braguer, D.; Badache, A. Memo–RhoA–mDia1 signaling controls microtubules, the actin network, and adhesion site formation in migrating cells. J. Cell Biol. 2008, 183, 401–408.
- Bartolini, F.; Moseley, J.B.; Schmoranzer, J.; Cassimeris, L.; Goode, B.L.; Gundersen, G.G. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 2008, 181, 523–536.
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885.
- Miller, A.L. The contractile ring. Curr. Biol. 2011, 21, R976–R978.
- Burgess, D.R.; Chang, F. Site selection for the cleavage furrow at cytokinesis. Trends Cell Biol. 2005, 15, 156–162.
- Strickland, L. Pathways for membrane trafficking during cytokinesis. Trends Cell Biol. 2004, 14, 115–118.
- Soldati, T.; Schliwa, M. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 2006, 7, 897–908.
- Litschko, C.; Brühmann, S.; Csiszár, A.; Stephan, T.; Dimchev, V.; Damiano-Guercio, J.; Junemann, A.; Körber, S.; Winterhoff, M.; Nordholz, B.; et al. Functional integrity of the contractile actin cortex is safeguarded by multiple Diaphanous-related formins. Proc. Natl. Acad. Sci. USA 2019, 116, 3594–3603.
- Castrillon, D.; Wasserman, S. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 1994, 120, 3367–3377.
- Afshar, K.; Stuart, B.; Wasserman, S. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 2000, 127, 1887–1897.
- Severson, A.; Baillie, D.L.; Bowerman, B. A Formin Homology Protein and a Profilin Are Required for Cytokinesis and Arp2/3-Independent Assembly of Cortical Microfilaments in C. elegans. Curr. Biol. 2002, 12, 2066–2075.
- Skau, C.T.; Neidt, E.M.; Kovar, D.R. Role of Tropomyosin in Formin-mediated Contractile Ring Assembly in Fission Yeast. Mol. Biol. Cell 2009, 20, 2160–2173.
- Watanabe, S.; De Zan, T.; Ishizaki, T.; Yasuda, S.; Kamijo, H.; Yamada, D.; Aoki, T.; Kiyonari, H.; Kaneko, H.; Shimizu, R.; et al. Loss of a Rho-Regulated Actin Nucleator, mDia2, Impairs Cytokinesis during Mouse Fetal Erythropoiesis. Cell Rep. 2013, 5, 926–932.
- Watanabe, S.; Okawa, K.; Miki, T.; Sakamoto, S.; Morinaga, T.; Segawa, K.; Arakawa, T.; Kinoshita, M.; Ishizaki, T.; Narumiya, S. Rho and Anillin-dependent Control of mDia2 Localization and Function in Cytokinesis. Mol. Biol. Cell 2010, 21, 3193–3204.
- Kato, T.; Watanabe, N.; Morishima, Y.; Fujita, A.; Ishizaki, T.; Narumiya, S. Localization of a mammalian homolog of diaphanous, mDia1, to the mitotic spindle in HeLa cells. J. Cell Sci. 2001, 114, 775–784.
- Ishizaki, T.; Morishima, Y.; Okamoto, M.; Furuyashiki, T.; Kato, T.; Narumiya, S. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 2000, 3, 8–14.
- Tsubakimoto, K.; Matsumoto, K.; Abe, H.; Ishii, J.; Amano, M.; Kaibuchi, K.; Endo, T. Small GTPase RhoD suppresses cell migration and cytokinesis. Oncogene 1999, 18, 2431–2440.
- Chen, A.; Arora, P.D.; McCulloch, C.A.; Wilde, A. Cytokinesis requires localized β-actin filament production by an actin isoform specific nucleator. Nat. Commun. 2017, 8, 1530.
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587.
- Barriere, G.; Fici, P.; Gallerani, G.; Fabbri, F.; Rigaud, M. Epithelial Mesenchymal Transition: A double-edged sword. Clin. Transl. Med. 2015, 4, 14.
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45.
- Jurmeister, S.; Baumann, M.; Balwierz, A.; Keklikoglou, I.; Ward, A.; Uhlmann, S.; Zhang, J.D.; Wiemann, S.; Sahin, Ö. MicroRNA-200c Represses Migration and Invasion of Breast Cancer Cells by Targeting Actin-Regulatory Proteins FHOD1 and PPM1F. Mol. Cell. Biol. 2012, 32, 633–651.
- Li, Y.; Zhu, X.; Zeng, Y.; Wang, J.; Zhang, X.; Ding, Y.-Q.; Liang, L. FMNL2 Enhances Invasion of Colorectal Carcinoma by Inducing Epithelial-Mesenchymal Transition. Mol. Cancer Res. 2010, 8, 1579–1590.
- Rana, M.K.; Aloisio, F.; Choi, C.; Barber, D.L. Formin-dependent TGF-β signaling for epithelial to mesenchymal transition. Mol. Biol. Cell 2018, 29, 1465–1475.
- Li, J.; Zhang, Y.-P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114.
- Wakayama, Y.; Fukuhara, S.; Ando, K.; Matsuda, M.; Mochizuki, N. Cdc42 Mediates Bmp-Induced Sprouting Angiogenesis through Fmnl3-Driven Assembly of Endothelial Filopodia in Zebrafish. Dev. Cell 2015, 32, 109–122.
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395.
- Hetheridge, C.; Scott, A.N.; Swain, R.; Copeland, J.W.; Higgs, H.; Bicknell, R.; Mellor, H. The novel formin FMNL3 is a cytoskeletal regulator of angiogenesis. J. Cell Sci. 2012, 125, 1420–1428.
- Richards, M.; Hetheridge, C.; Mellor, H. The Formin FMNL3 Controls Early Apical Specification in Endothelial Cells by Regulating the Polarized Trafficking of Podocalyxin. Curr. Biol. 2015, 25, 2325–2331.
- Phng, L.-K.; Gebala, V.; Bentley, K.; Philippides, A.; Wacker, A.; Mathivet, T.; Sauteur, L.; Stanchi, F.; Belting, H.-G.; Affolter, M.; et al. Formin-Mediated Actin Polymerization at Endothelial Junctions Is Required for Vessel Lumen Formation and Stabilization. Dev. Cell 2015, 32, 123–132.
- Ju, R.; Cirone, P.; Lin, S.; Griesbach, H.; Slusarski, D.C.; Crews, C.M. Activation of the planar cell polarity formin DAAM1 leads to inhibition of endothelial cell proliferation, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 6906–6911.
- Pizurki, L.; Zhou, Z.; Glynos, K.; Roussos, C.; Papapetropoulos, A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. J. Cereb. Blood Flow Metab. 2003, 139, 329–336.
- Gavard, J.; Patel, V.; Gutkind, J.S. Angiopoietin-1 Prevents VEGF-Induced Endothelial Permeability by Sequestering Src through mDia. Dev. Cell 2008, 14, 25–36.
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363.
- Hotulainen, P.; Lappalainen, P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006, 173, 383–394.
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70.
- Sandbo, N.; Ngam, C.; Torr, E.; Kregel, S.; Kach, J.; Dulin, N. Control of Myofibroblast Differentiation by Microtubule Dynamics through a Regulated Localization of mDia2. J. Biol. Chem. 2013, 288, 15466–15473.
