Malignant transformation due to infectious disease can occur via two broad mechanisms. The agent can act as a direct carcinogen altering the expression of oncogenes; thereby, leading to the production of oncoproteins. These oncoproteins can then interact with cellular proteins, and ultimately lead to mutagenesis by the disruption of cell cycle check-points, inhibition of apoptosis, and enhancement of cell immortalisation. In feline medicine, there have been several viruses which demonstrate direct mutagenesis, these include feline leukaemia virus (FeLV) and mouse mammary tumour virus (MMTV) and MMTV. Alternatively, neoplastic transformation can be driven via indirect mechanisms; these include induction of chronic inflammation, which, in turn, results in the production of inflammatory mediators, and the production of reactive oxygen species, which have direct mutagenic effects and promote tumour neovascularisation. Inflammation-induced neoplasms have been associated with Helicobacter organisms and, potentially, Opisthorchis infections. Furthermore, immune suppression induced by viruses such as feline immunodeficiency virus (FIV), not only predispose to infection with other agents, it is also thought to alter the immune surveillance, and with this, the ability to remove neoplastic cells by the host.
- retrovirus
- papillomavirus
- FeLV
- FIV
- Helicobacter
- lymphoma
- parasitic
1. Viral Infections Associated with Neoplasia in the Cat
1.1. Feline Leukaemia Virus (FeLV)
Since its discovery in 1964, FeLV has been reported globally [1][16]. It is recognised that progressive infection with FeLV has been shown to increase the risk of lymphoma development by 60 times and is associated with the development of other forms of neoplasia [2][3][17,18]. At its peak, it was estimated that approximately one-third of all cancer related deaths in cats were attributable to FeLV infection [4][19]. However, today the prevalence of FeLV infection is markedly decreased [5][20].
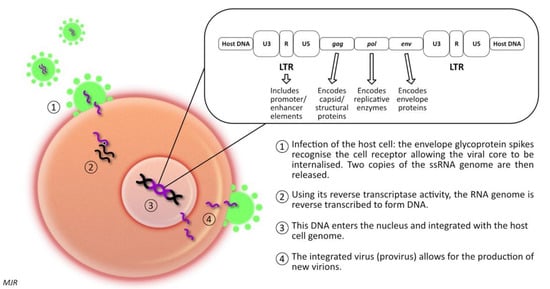
1.2. Feline Immunodeficiency Virus (FIV)
Feline immunodeficiency virus (FIV) is a retrovirus of the lentivirus genus, first described in 1986 [59][81]. Like FeLV, FIV infection leads to the integration of proviral DNA into the host cell DNA; thereby, influencing host cell function. There are six different subtypes recognised, labelled A to F [60][82]. However, infection with one subtype does not protect against infection with a second subtype (superinfection) [60][82]. It has been demonstrated that in superinfected cats, the exchange of gene segments encoding the env protein from different subtypes can occur [60][82]. The prevalence of FIV infection varies widely among different geographic locations, with pockets of high prevalence of up to 47% in some groups of feral cats, compared to that of 2–5% as reported in some studies of healthy, owned cats [61][83]. Generally, seroprevalence is higher in male cats, and adult cats are more likely to be infected than young cats [62][84]. This pattern arises because of the mode of transmission; in natural circumstances, FIV is transmitted primarily via inoculation of the virus present in saliva or blood (i.e., cat fight wounds) [61][83]. Experimentally, the virus can be transmitted in utero, or postpartum via milk or via transmucosal (oral, intrarectal, or intravaginal) inoculation. However, there is no evidence that these routes of infection play an important role in the natural transmission of disease [60][63][82,85]. FIV-infected cats are five to six times more likely to develop lymphoid neoplasia than uninfected cats [3][18]. Concomitant infection with FIV and FeLV increases the likelihood of lymphoma development by nearly 80-fold [3][18]. FIV-associated lymphomas are predominantly high-grade B cell tumours arising at extra-nodal locations [64][86]. Nevertheless, T-cell and non-B, non-T-cell lymphomas have been described in FIV infected cats, as have various other forms of neoplasia, including mast cell tumour, fibrosarcoma, squamous cell carcinoma, and mammary carcinoma [65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102].1.3. FIV & Gammaherpesviruses
Herpesviruses are a major cause of cancers in immunodeficient humans [81][114]. Some forms of herpesvirus encode several oncogenic proteins, which have been shown to inhibit cellular apoptosis and promote cell survival [64][86]. HIV-associated neoplasms are frequently associated with co-infection with gammaherpesvirus [81][82][114,115]. EBV, a human herpesvirus-4, can be responsible for infectious mononucleosis (glandular fever) [81][114]; although, typically, infection is asymptomatic if individuals are infected during childhood or in early adulthood. However, in immunocompromised humans (post-transplant or HIV/AIDS patients), EBV is linked to the formation of Burkitt’s and Hodgkin lymphomas [81][114]. Kaposi’s sarcoma is a human neoplasm, which occurs in association with HIV and HHV-8 infection [82][115]. Recognition of this association prompted investigations to identify a similar affiliation in feline medicine. In 2014, a feline gammaherpesvirus, Felis catus gammaherpesvirus 1 (FcaGHV1) was first detected [83][84][119,120]. Infection with FcaGHV1 has been reported in Australia, U.S.A., U.K., Europe, Singapore, Japan, and Brazil, with 9.6–23.6% of cats demonstrating viral DNA within their blood [83][84][85][86][87][88][89][119,120,121,122,123,124,125]. Male cats are generally at a higher risk of FcaGHV1 than females [85][90][79,121]. The virus is spread by oropharyngeal shedding, leading to the suggestion that, like FIV, it may be spread by biting [91][126].1.4. Mouse Mammary Tumour Virus (MMTV)
MMTV is an oncogenic retrovirus that induces mammary carcinoma in mice and for which, possible links to human breast cancer have been suggested [92][128]. MMTV is a beta-retrovirus, composed of prominent surface spikes with an eccentric condensed core. The genome codes for both structural and non-structural proteins, and therefore, MMTV is classified as a complex retrovirus [92][93][128,131]. The transcript codes for Gag, Pol, pro-dUTPase (DUT) protein and Env, flanked by LTRs [92][128]. These LTRs are exceptionally long, and encode for two additional genes: sag (a viral accessory protein that functions as a superantigen) and rem (which encodes an RNA export protein) [92][128]. MMTV replicates efficiently in the mammary alveolar epithelial cells, with increased expression observed during lactation due to the release of steroid hormones [92][128]. In mice, lactogenic transmission results in the virus infecting the dendritic cells and B lymphocytes of the Peyer’s patches. The sag antigen is then presented to CD4+ T-lymphocytes. This protein triggers viral replication and amplification of T-lymphocytes, which act as a carrier to transfer the virus from the gut to the mammary tissue. MMTV infection does not immediately trigger neoplastic transformation, even once proviral DNA is inserted in the cell DNA [92][128]. The insertion results in proto-oncogene deregulation (much like infection with FeLV). To date, rearrangements in several cellular gene families (wnt, fgf, notch4/int3, rspo and the gene encoding the p48 component of eukaryotic translation initiation factor-3 (elF-3p48)) have been demonstrated with MMTV infection [92][94][128,129]. It is hypothesised that these insertions are responsible for neoplastic transformation. However, indirect mechanisms for MMTV tumorigenesis have also been implicated [95][132]. It has been suggested that MMTV may activate an immunoreceptor tyrosine-based mechanism that suppresses apoptosis [95][132]. Alternatively, it has been hypothesised that MMTV infection could lead to the activation of other infections, such as EBV or HPV, which have also been implicated in the development of mammary neoplasia [95][132].
1.5. Papillomaviruses
One of the strongest associations between an infectious agent and neoplasia in humans is the role of papillomaviruses in cervical carcinoma [96][2]. There are currently six different feline papillomaviruses recognised; Felis catus papillomavirus types 1-6 (FcaPV 1-6) [97][141]. Cats are also dead-end hosts for the delta-papillomavirus Bos taurus papillomavirus-14 (BPV-14) [97][141]. The majority of cats are infected with FcaPV’s, but disease associated with infection is rare, suggesting that, in most circumstances, host defences inhibit viral replication [98][142]. Occasionally, papillomaviruses can induce a rapid (self-limited) increase in cell growth, resulting in a hyperplastic papilloma (wart) or a more modest increase in cell growth, leading to the development of a raised plaque [99][139]. The increased replication within cells can lead to neoplastic transformation [99][139]. There are various cutaneous lesions that have been associated with papillomavirus infection in cats. These include, cutaneous papilloma’s (warts), which are rare in the cat; viral plaques and Bowenoid in situ carcinoma (BISC); feline cutaneous squamous cell carcinoma (SCC); basal cell carcinoma (BCC), feline sarcoid, and Merkle cell carcinoma [100][101][102][149,150,151]. In addition, oral papilloma’s and oral squamous cell carcinoma (OSCC) have been linked to feline papillomavirus infection [97][141].
Feline viral plaques and BISC are thought to represent two extremes of the same disease process [98][142]. These lesions typically develop in middle to older aged cats [103][140]. They can be found anywhere on the body, but are most common around the head and neck. Viral plaques are small (less than 8mm) slightly raised, hairless lesions, whereas BISCs are larger, more markedly raised, ulcerated, and crusted lesions [97][141]. The lesions are considered pre-cancerous, but can progress to invasive squamous cell carcinoma in some cases [97][141]. Studies have demonstrated that both viral plaques and BISCs are frequently associated with FcaPV-2 infection [104][105][106][107][108][109][110][111][146,147,148,152,153,154,155,156]. The most common cause of viral plaques/BISC is FcaPV-2, which was identified in 48% of BISCs using immunohistochemistry [112][157].
Cutaneous SCC in cats are most notably associated with UV-light exposure. However, there is evidence that FcaPVs may also play a role. Initially, it was noted that FcaPV-2 was identified more frequently in SCC than in non-SCC skin [113][114][158,161]. However, FcaPV-2 E6 and E7 RNA has also been identified in a proportion of SCCs, but not normal skin; more recently, the E6 and E7 proteins have been shown to influence neoplastic transformation [115][116][117][144,162,163]. FcaPVs have been detected in 76% of SCCs occurring in UV-protected (i.e., haired) areas, and 42% of SCCs from UV-exposed skin [118][164]. Furthermore, p16 has been identified in 84% of UV-protected SCC and 40% of UV-exposed SCC [104][118][146,164].
BCCs are not common in cats [99][139]. However, it has been noted that they are often associated with adjacent BISC lesions, and therefore, it was suggested that FcaPVs may be linked to their occurrence. A cat with multiple BCCs that contained FcaPV-3 DNA has been reported, as has a case with a novel PV type [119][120][169,170]. These cases demonstrated classic papillomavirus-induced changes within the neoplastic cells. However, as so few feline papillomavirus-associated BCCs have been reported, the role of these infections in tumour formation remains undetermined. Feline sarcoids are caused by proliferating mesenchymal cells. These lesions are thought to be caused by infection with BPV-14, which appears to be able to lead to cross-species infection. Only cats with contact to cattle are reported to be affected. There is no viral replication within the lesions, and no L1 immunostaining [121][171]. However, BPV-14 is consistently detected within feline sarcoids [122][123][172,173], but not in unaffected samples [124][174]. In situ hybridisation has demonstrated papillomavirus DNA within the neoplastic mesenchymal cells [121][171]. Together these support a causal role of BPV-14 in feline sarcoid formation. Aside from the cutaneous manifestations, FcaPVs have been implicated in the development of oral lesions, oral papillomas, and OSCCs. Oral papillomas are rare in the cat; they tend to be restricted to the ventral surface of the tongue and are usually incidental findings [97][141]. In the few cases in which investigations have been performed, the lesions have demonstrated prominent papilloma-induced changes, and have stained positive for L1 or p16 [125][126][175,176]. Virus typing has only been performed in two cases, with FcaPV-1 being isolated in both of these [126][176]. OSCCs are aggressive, invasive neoplasms which are associated with short survival time, particularly when measures to achieve local control of the tumour are not pursued [97][141]. As papillomaviruses have been linked to human OSCCs and feline cutaneous SCCs, it has been hypothesised that FcaPVs may play a role in the formation of feline OSCCs. In two studies, assessing a total of 52 OSCCs, papillomavirus DNA was amplified from three tumours; there was no amplification from 20 non-neoplastic oral samples [127][128][177,178]. Of the three sequences amplified, two were found to be HPV, whereas the remaining one could not be sequenced. This could reflect infection or, equally, contamination. Overall, studies have produced highly variable results; both a study in New Zealand assessing a series of 30 OSCCs, and a study from Japan assessing seven OSCCs, failed to demonstrate the presence of FcaPV DNA [129][130][168,179]. However, some studies have detected FcaPVs, including another study from New Zealand, in which 36 OSCCs and 16 gingivitis cases were assessed; FcaPV-1 was identified in one case of OSCC and one of gingivitis [129][131][168,180].1.6. Hepadnavirus
In feline medicine, the majority of the research to date has focused on the roles of the retroviruses and papillomaviruses in neoplastic transformation. However, in humans, viral hepatopathies have long been established as a cause of hepatitis and hepatocellular carcinoma; approximately 35% of pathogen related cancers are attributed to hepatic neoplasia of viral origin [132][183]. Recently, a novel hepadnavirus, similar to HBV, has been identified in domestic cats (domestic cat hepadnavirus, DCH) [133][184]. Studies have demonstrated that 6.5–10.8% of pet cats are positive for DCH [133][134][184,185]. A retrospective study using PCR and in situ hybridisation techniques demonstrated that 43% of chronic hepatitis cases, and 28% of HCCs, were positive for DCH [135][186]. In comparison, no cats with cholangitis, biliary carcinoma, or normal liver, tested positive for DCH. Additionally, the histological features of the DCH-positive cases demonstrated histopathological features similar to those reported in humans with HBV [136][127].2. Bacterial Causes of Neoplasia
In cats, several enterohepatic Helicobacter species have been recognised. A study in 2001 demonstrated Helicobacter species in 17 of 45 cats [137][197]. Of these, nine were demonstrated as having H. heilmannii, four were positive for H. felis, three were positive for both H. felis and H. heilmannii, and seven for unclassified Helicobacter species [137][197]. There is a single report of Helicobacter organisms in cats with gastric lymphoma [138][196]. Of the cases with gastric lymphoma, 17 were identified with lymphoblastic lymphoma. In that study, histopathological samples from 47 cats with gastro-intestinal disease were assessed; 14 demonstrated gastritis, 31 lymphoma, and 2 were normal. It was found that ‘sick’ cats were more likely to host Helicobacter organisms. Furthermore, 13/17 cases of lymphoblastic lymphoma were positive for H. heilmannii by fluorescent in situ hybridisation [138][196].
3. Parasitic Infections
One of the first reports of neoplasia occurring in association with infection was the observation that bladder cancers were more prevalent in men with schistosomiasis [139][8]. Since this time, it has been demonstrated in humans that the helminth-associated diseases, schistosomiasis, opisthorchiasis, and clonorchiasis, are highly carcinogenic [140][200]. Furthermore, it has been shown that the protozoal organism, Trypanosoma cruzi, has a dual role of being both carcinogenic and anti-carcinogenic [140][200]. The malaria parasite, Plasmodium falciparum, is strongly associated with Burkitt lymphoma in endemic areas, but only when co-infection with EBV is present [140][200].
One early report documented four cases of cholangiocarcinoma in cats parasitised by Platynosomum fastosum (P. fastosum) [141][201]. However, despite numerous reports of biliary tract infection by this trematode, there were no further reports of neoplasia until 2012, when post mortems of 3 of 11 cats infected with P. fastosum were reported to have cholangiocarcinoma [142][202]. In addition, there is a single case report of a cat with biliary cystadenoma associated with Opisthorchis viverrini infection [143][203], and, to date, there are no reports of Opisthorchis felineus leading to biliary neoplasia in the cat, despite reports in many other species [144][145][146][204,205,206].
