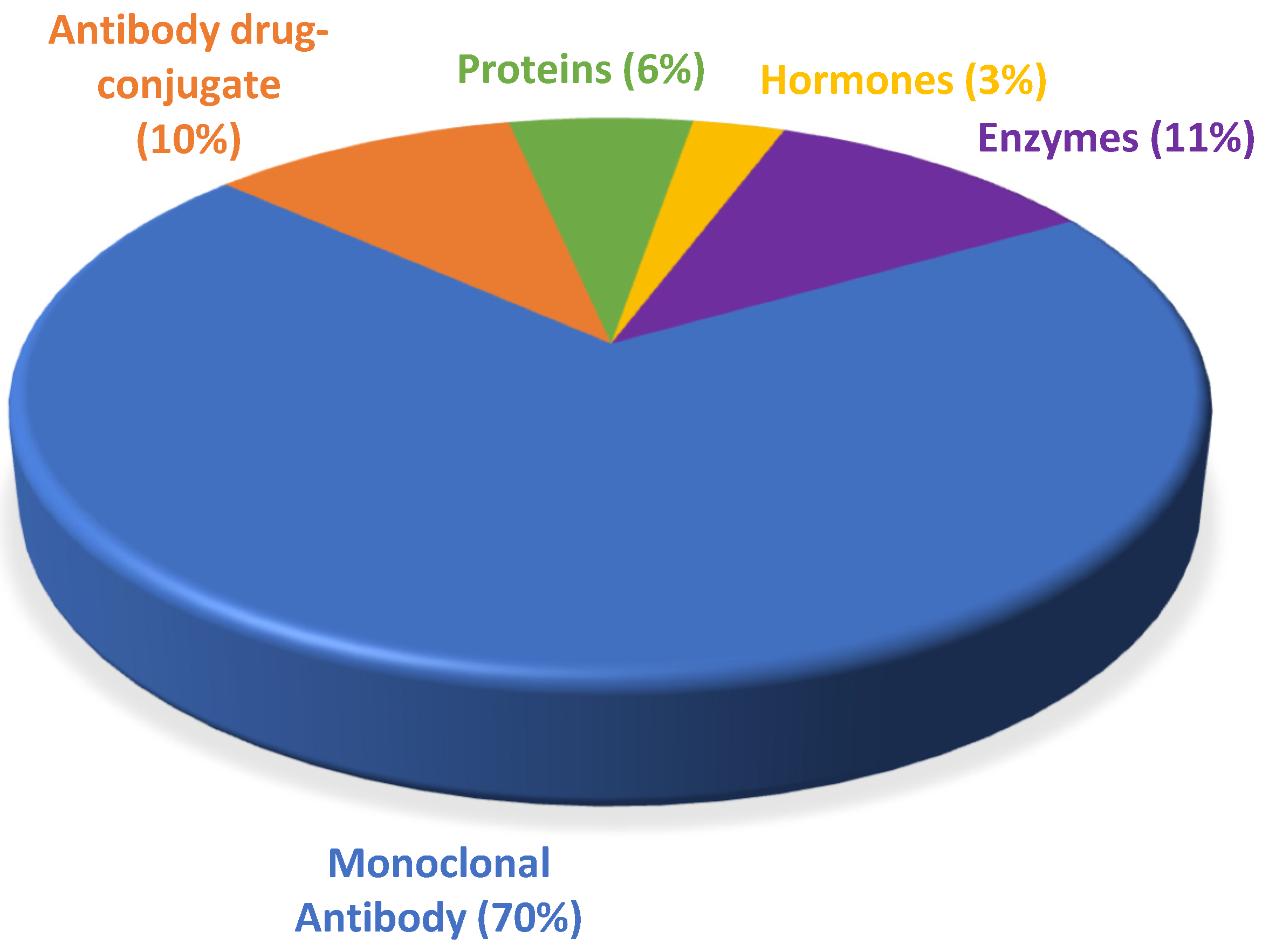
Despite belonging to a relatively new class of pharmaceuticals, biological drugs have been used since the 1980s, when they brought about a breakthrough in the treatment of chronic diseases, especially cancer. They conquered a large space in the pipeline of the pharmaceutical industry and boosted the innovation portfolio and arsenal of therapeutic compounds available. From 2015 to 2021, the number of drugs included in this class grew over this period, totaling 90 approvals, with an average of 13 authorizations per year.
- Food and Drug Administration
- FDA approvals
- monoclonal antibody
- antibody–drug conjugate
- biological drugs
1. Introduction
2. Timeline for FDA-Approved Biological Drugs
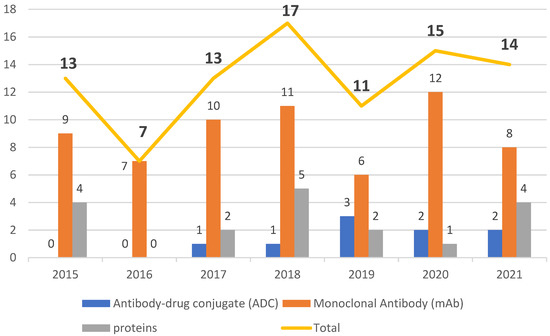
The data collected in the present work point to an undeniable growth of biological therapies. In the period from 2015 to 2021, the FDA authorized new mAbs, ADCs and proteins. Of note, the total number of approvals remained in double figures every year except 2016, in which only seven biopharmaceuticals, all mAbs, were approved (Figure 21). Analysis of the data also revealed the prominence of the authorization of mAbs compared to other biologicals.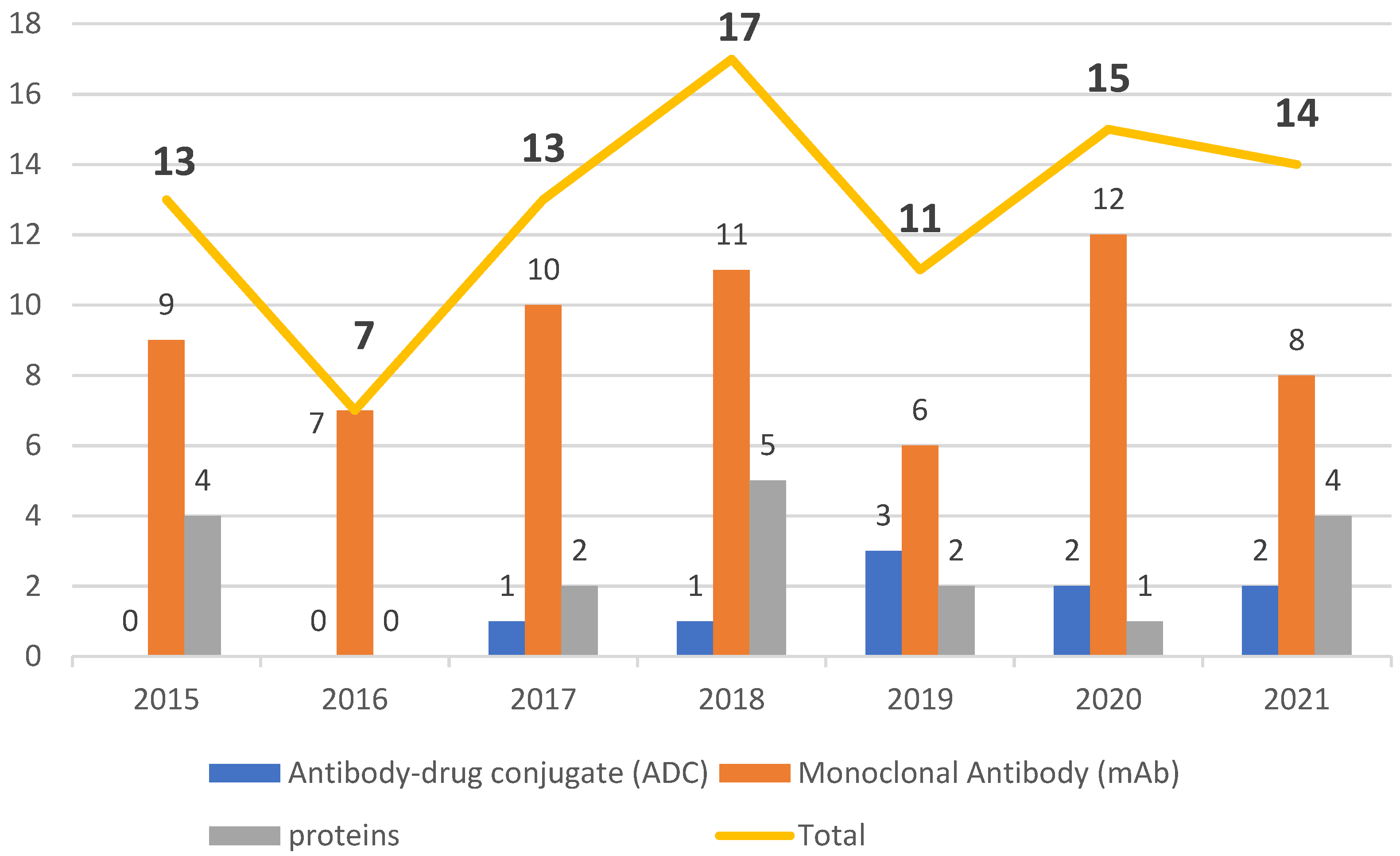
3. Therapeutic Indications
3.1. Cancer
3.2. Mechanisms of Action and Therapeutic Indications of ADCs and mAbs for Cancer
3.2.1. mAbs for Cancer
Both IgG1k Daratumumab DarzalexTM and the IgG1 Isatuximab SarclisaTM bind to CD38 [12][13]. Like other conventional medicines, biologicals can undergo changes. One example is DarzalexTM (given intravenously), which was modified and approved in 2020 as Daratumumab and hyaluronidase (Darzalex FasproTM) (given subcutaneously), the latter containing the same combined human mAb with a recombinant human enzyme called hyaluronidase, which enhances the absorption of injectables, allows faster infusions, and a lower rate of reactions related to infusions [14]. Both DarzalexTM and Darzalex FasproTM target CD38. Approved by the FDA in 2005, human hyaluronidase injections alter the permeability of human tissue, and they are used as an adjuvant to improve the characteristics of injectables [15]. Other examples of mAb modification include Rituximab and hyaluronidase (Rituxan HycelaTM), approved in 2017, also given subcutaneously. However, it was first approved back in 1997 by the trade name RituxanTM, being administered intravenously [16]. Trastuzumab and hyaluronidase (Herceptin HylectaTM) [17] and Pertuzumab, trastuzumab, and hyaluronidase (PhesgoTM) [15] underwent the same modification with the addition of hyaluronidase, both being administered subcutaneously and both for breast cancer. Margenza™ is directed at the same target, HER2, for breast cancer [18], and all breast cancer biologicals currently on the market were approved between 2019 and 2020. LartruvoTM was the only drug approved for soft tissue sarcoma during the period of interest [19]. TecentriqTM, BavencioTM and ImfinziTM have the same target (PD-L1), and all three are biologicals that can be used to treat the highest number of different types of cancer [20][21][22]. PortrazzaTM targets EGFR, and RybrevantTM has the same target plus the MET proto-oncogene. Therefore, RybrevantTM is the only bispecific mAb for cancer approved to date [23][24]. Another breakthrough in the period 2015–2021 was PoteligeoTM, a first-in-class biopharmaceutical that targets the CC chemokine receptor 4 (CCR4) [25]. In this period, we found four biologicals approved for multiple myeloma, but one of them (EmplicitiTM) has a distinct mechanism of action in that it binds to the cell surface receptor signaling lymphocytic activation molecule F7 (SLAMF7), whereas DarzalexTM, Darzalex FasproTM and SarclisaTM target CD38 [12][13][14].3.2.2. Antibody–Drug Conjugates
Enfortumab Vedotin PadcevTM is the first biological to target the protein Nectin-4 [26]. Tisotumab Vedotin TivdakTM is a Biological specific for tissue factor (TF-011) and Polatuzumab Vedotin PolivyTM, an antibody whose target is the CD79b (a component of the B cell receptor). These three ADCs, which have different targets but the same suffix Vedotin, carry the same drug, namely monomethyl auristatin E (MMAE) [27][28][29]. MMAE is released into the cell after binding to the target, with subsequent induction of cell apoptosis by the drug, which also inhibits mitosis. These drugs also have different types of linkers. For example, the linker in PadcevTM is the protease-cleavable maleimidocaproyl valine-citrulline [26], while Tisotumab Vedotin has a Valine citrulline linker, which is also protease-cleavable [28]. It is interesting how these ADCs carrying MMAE have such unique targets, a feature not seen among mAbs. Fam-Trastuzumab deruxtecan Enhertu™ targets human epidermal growth factor receptor 2 (HER2) for the treatment of gastric cancer, breast cancer and gastroesophageal junction adenocarcinoma. Its ligand is a topoisomerase inhibitor, which blocks DNA replication [17]. Sacituzumab govitecan Trodelvy™, indicated to treat solid tumors, has the hydrolysis-cleavable CL2A as the linker, and it also carries a topoisomerase inhibitor [30]. Loncastuximab tesirine ZynlontaTM includes an antibody against CD19. This antibody carries the antitumor drug pyrrolobenzodiazepine, and its linker is protease-cleavable [31]. BesponsaTM has a linker that can be cleaved by acid [32]. EnhertuTM has a protease-cleavable tetrapepitide linker [17][33]. Trodelvy™ has the hydrolysis-cleavable CL2A as linker [30]. The linker present in ZynlontaTM is also protease-cleavable [31] while that of BlenrepTM is maleimidocaproyl [34]. BesponsaTM and Lumoxiti™ target CD22, but they are indicated for different types of cancer [32][35]. They carry distinct drugs/toxins, BesponsaTM carrying Calich-DMH, an antitumor antibiotic produced by a bacterium, and LumoxitiTM being conjugated to a fragment of Pseudomonas exotoxin, also found as PE38. When internalized, PE38 stimulates apoptosis and the inhibition of protein synthesis. In 2015 and 2016, no ADCs were approved, while 2017 and 2018 registered the lowest number of authorizations of these drugs in the period of interest. In 2019, the highest number of approvals for ADCs were for the treatment of three types of cancer. In this regard, PadcevTM was authorized for the treatment of metastatic urothelial cancer [26], PolivyTM for diffuse large B-cell lymphoma [29], and EnhertuTM for breast cancer [17]. Then, the following two years registered two approvals each year. Thus, in 2020, BlenrepTM received the green light for the treatment of multiple myeloma [34] and TrodelvyTM for metastatic triple-negative breast cancer [30]. In the following year, ZynlontaTM, another drug for the treatment of large B-cell Lymphoma [31], was approved, as was TivdakTM for metastatic cervical cancer [28].4. Autoimmune Diseases
The biologics for autoimmune diseases (psoriasis, plaque psoriasis, psoriatic arthritis, multiple sclerosis, myasthenia gravis, lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and neuromyelitis optic spectrum disorder) in the period of interest included 13 biologics, 12 of which were mAbs, and a new class of biological, namely an antibody fragment (Efgartigimod alfa VyvgartTM), which is detailed in Table 61.
| Active Ingredient and Trade Name | mAb Class | Targets/Mechanism of Action | Original Approval Date |
Manufacturer | Therapeutic Indication |
|---|
56].
5. Other Therapeutic Indications
In the period of interest, some therapeutic indications appear only once among FDA approvals, while others appear between two to four times. Of a total of four FDA-approved mAbs for the treatment of migraine, three are humanized mAbs and only one is fully human (Table 72). The humanized ones, Vyepti™, Emgality™ and Ajovy™, have the same mechanism of action. In this regard, they bind to CGRP, a potent vasodilator, preventing it from adhering to the receptor [57][58][59]. In contrast, the fully human antibody Aimovig™ binds to CGRPR, preventing the molecule from binding to the receptor [60].| Active Ingredient and Trade Name | mAb Class | Target/Mechanism of Action | Original Approval Date | Manufacturer | ||
|---|---|---|---|---|---|---|
| Emgality™ (Galcanezumab) | [45][60] | Humanized | CGRP antagonist | 2018 | Eli Lilly and Company | |
| Ajovy™ (Fremanezumab) | [45][59] | Humanized | CGRP antagonist | 2018 | Teva Branded Pharmaceutical Products R&D, Inc. | |
| Aimovig™ (Erenumab) | [45][60] | Human | CGRPR antagonist | 2018 | Amgen, Inc. | |
| Vyepti™ (Eptinezumab) | [11][57] | Humanized | CGRP antagonist | 2020 | Lundbeck Seattle Pharmaceuticals, Inc. | |
| Cosentyx™ (Secukinumab) | [6][36] | Human | IL-17A inhibitor | 2015 | Novartis Pharmaceuticals | Plaque psoriasis, Psa, and AS |
| Zinbryta™ (daclizumab) | [37][38] | Humanized | IL-2R inhibitor | 2016 | Biogen Inc | Multiple sclerosis |
| Taltz™ (ixekizumab) | [37] | |||||
| NMOSD | ||||||
| Saphnelo™ | ||||||
| (anifrolumab) | ||||||
| [ | ||||||
| 2 | ||||||
| ][53] | Human | Blocks the action of type 1 interferon receptor | 2021 | AstraZeneca AB | Lupus erythematosus | |
| Vyvgart™ (efgartigimod alfa) | [2][54] | Human monoclonal ARGX-113 fc fragment | Neonatal Fc receptor antagonist | 2021 | Argenx BV | Generalized myasthenia gravis |
Of the approvals of autoimmune biopharmaceuticals from 2015 to 2021, six are indicated for psoriasis, plaque psoriasis, and psoriatic arthritis. Brodalumab Siliq™ is indicated for moderate to severe plaque psoriasis [44]. While this drug acts by antagonizing the IL-17A Receptor, Cosentyx™ and Taltz™ antagonize the pro-inflammatory cytokine IL-17A, which plays a role in psoriasis and Psa [36][39]. Guselkumab Tremfya™, used for the treatment of psoriasis and Psa, is an antibody that blocks the activity of two interleukins (IL-23, IL-17A) that are overexpressed in these diseases [42]. Tildrakizumab Ilumya™ is an IgG1 antibody that selectively binds to interleukin-23-p19 (IL-23A p19) [46] and, through the same mechanism, Risankizumab Syrizi™ also binds to the same p19 subunit of this interleukin. In some countries, there are trials underway to evaluate Risankizumab for the treatment of Crohn’s disease and ulcerative colitis [48][55][
| Active Ingredient and Trade Name | mAb Class | Target/Mechanism of Action | Original Approval Date | Manufacturer | |
|---|---|---|---|---|---|
| Nucala™ (Mepolizumab) | [6][64] | Humanized | IL-5 | 2015 | GlaxoSmithKline LLC |
| Cinqair™ (Reslizumab) | [37][65] | Humanized | IL-5 | 2016 | Teva Respiratory LLC |
| Fasenra™ (Benralizumab) | [41][66] | Humanized | IL-5R-α | 2017 | AstraZeneca AB |
| Dupixent™ (Dupilumab) | [41][61] | Human | IL-4R-α | 2017 | Regeneron Pharmaceuticals, Inc. |
| Tezsipire™ (Tezepelumab) | [2][63] | Human | Blocks TSLP | 2021 | AstraZeneca AB |
| [ | ||||||
| 39 | ||||||
| ] | ||||||
| Humanized | IL-17A inhibitor | 2016 | Eli Lilly and Company | Plaque psoriasis and Psa | ||
| Tremfya™ (guselkumab) | [8][40] | Human | IL-23 and IL-17A inhibitor | 2017 | Janssen Biotech, Inc | Plaque psoriasis |
| Ocrevus™ (Ocrezilumab) | [41][42] | Humanized | Anti-CD-20 | 2017 | Genentech, Inc | Multiple sclerosis |
| Kevzara™ (sarilumab) | [41][43] | Human | IL-6 inhibitor | 2017 | Sanofi-Aventis U.S LLC | Rheumatoid arthritis |
| Siliq™ (brodalumab) | [41][44] | Human | IL-17A, IL-17F, and other IL-17 isoform inhibitors | 2017 | Valeant Pharmaceuticals Luxembourg S.à.r.l | Plaque psoriasis |
| Ilumya™ (tildrakizumab) | [45][46] | Humanized | IL 23p19 | 2018 | Sun Pharma Global FZE | Plaque psoriasis |
| Skyrizi™ (risankizumab) | [47][48] | Humanized | IL-23p19 inhibitor | 2019 | AbbVie Inc. | Plaque psoriasis and Psa |
| Uplizna™ (inebilizumab) | [49][50] | Humanized | Depletes CD-19 | 2020 | Horizon Therapeutics Ireland DAC | NMOSD |
| Enspryng™ (satralizumab) | [11][51][52] | Humanized | Anti-IL -6R | 2020 | Genentech, Inc. |
6. Conclusions
The period 2015 to 2021 witnessed a growth in FDA approval of biologicals in general, with mAbs being the class with the greatest presence. During this period, the number of authorizations of biopharmaceuticals remained in the double figures, except in 2016, when only seven were given the green light. The years 2020 and 2021 did not show considerable variation, with one less biological being approved in 2021 than in 2020, while 2018 was the year with the highest number of approvals. Of note, even in the midst of the COVID-19 pandemic, the potential for these therapies to receive approval remained steady.
Biological medicines show high selectivity and high versatility and are therefore valuable. Their versatility is reflected in indications that range from the treatment of chronic or rare diseases to more aesthetic purposes such as the treatment of frown lines. These drugs offer great potential to be exploited for other therapeutic indications beyond what they were initially authorized for. In this regard, they offer a solid starting point from which to explore their capacity in clinical trials. For example, over the years, new applications have been discovered for Adalimumab HumiraTM, and today this drug has more than ten therapeutic indications listed in the directions of use [73]. Daratumumab DarzalexTM is also undergoing evaluation for other types of cancer, including refractory or relapsed non-Hodgkin’s Lymphoma [12]. mAbs can also be conjugated to toxins or drugs without compromising healthy tissues around the target fragment or at least minimizing effects in other tissues [74].
Between 2015 and 2021, in addition to the increase in the number of drug approvals, several breakthroughs and innovations took place, such as Aducanumab AduhelmTM, although still controversial, and also Tagraxofusp ElzonrisTM, which the FDA granted the status of Orphan Drug to treat rare diseases. In 2021, we witnessed the authorization of a different class of biological, Efgartigimod alfa VyvgartTM, an antibody fragment that also has Orphan Drug status [54], and the bispecific antibody approved within the period of interest HemlibraTM. Of note only two bispecific antibodies were approved in the period of interest HemlibraTM and RybrevantTM.
However, one of the great challenges for the development of biopharmaceuticals is the high technology required to produce these drugs, which makes them very expensive. We believe that, in the near future, this class of drugs will become increasingly accessible and new drugs will be developed. Moreover, more biosimilars will become accessible thanks to the development of new technologies that will impact production. These advancements will make these drugs increasingly more profitable and less expensive, which in turn will widen the accessibility of biological therapies, thereby expanding the therapeutic arsenal and transforming the management of diseases for which no treatment is available or diseases for which current treatments are not effective.
References
- Wu, A.C.; Fuhlbrigge, A.L.; Robayo, M.A.; Shaker, M. Cost-Effectiveness of Biologics for Allergic Diseases. J. Allergy Clin. Immunol. Pract. 2021, 9, 1107–1117.e2.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2022, 27, 1075.
- Batta, A.; Kalra, B.; Khirasaria, R. Trends in FDA Drug Approvals over Last 2 Decades: An Observational Study. J. Family Med. Prim. Care 2020, 9, 105.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2014. Available online: https://www.fdanews.com/ext/resources/files/01-15/01-02-15-Drug-Approvals-2014.pdf?1520892896 (accessed on 7 September 2022).
- U.S. Food And Drug Administration FDA Grants Accelerated Approval for Alzheimer’s Drug. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug#:~:text=Today%2C%20the%20U.S.%20Food%20and,disease%20affecting%206.2%20million%20Americans (accessed on 7 September 2022).
- U.S. Food and Drug Administration Novel Drug Approvals for 2015. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015 (accessed on 7 September 2022).
- Torre, B.; Albericio, F. The Pharmaceutical Industry in 2016. An Analysis of FDA Drug Approvals from a Perspective of the Molecule Type. Molecules 2017, 22, 368.
- de la Torre, B.; Albericio, F. The Pharmaceutical Industry in 2017. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2018, 23, 533.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2018. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2019, 24, 809.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2020, 25, 745.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2020. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2021, 26, 627.
- McKeage, K. Daratumumab: First Global Approval. Drugs 2016, 76, 275–281.
- Dhillon, S. Isatuximab: First Approval. Drugs 2020, 80, 905–912.
- Sanchez, L.; Richter, J.; Cho, H.J.; Jagannath, S.; Madduri, D.; Parekh, S.; Richard, S.; Tam, L.; Verina, D.; Chari, A. Subcutaneous Daratumumab and Hyaluronidase-Fihj in Newly Diagnosed or Relapsed/Refractory Multiple Myeloma. Ther. Adv. Hematol. 2021, 12, 204062072098707.
- Gao, J.J.; Osgood, C.L.; Gong, Y.; Zhang, H.; Bloomquist, E.W.; Jiang, X.; Qiu, J.; Yu, J.; Song, P.; Rahman, N.A.; et al. FDA Approval Summary: Pertuzumab, Trastuzumab, and Hyaluronidase–Zzxf Injection for Subcutaneous Use in Patients with HER2-Positive Breast Cancer. Clin. Cancer Res. 2021, 27, 2126–2129.
- Melaragno, A. Rituximab/Hyaluronidase (Rituxan HycelaTM). Oncol. Times 2017, 39, 18.
- Keam, S.J. Trastuzumab Deruxtecan: First Approval. Drugs 2020, 80, 501–508.
- Markham, A. Margetuximab: First Approval. Drugs 2021, 81, 599–604.
- Shirley, M. Olaratumab: First Global Approval. Drugs 2017, 77, 107–112.
- Syed, Y.Y. Durvalumab: First Global Approval. Drugs 2017, 77, 1369–1376.
- Markham, A. Atezolizumab: First Global Approval. Drugs 2016, 76, 1227–1232.
- Kim, E.S. Avelumab: First Global Approval. Drugs 2017, 77, 929–937.
- Garnock-Jones, K.P. Necitumumab: First Global Approval. Drugs 2016, 76, 283–289.
- Syed, Y.Y. Amivantamab: First Approval. Drugs 2021, 81, 1349–1353.
- Garber, K. No Added Sugar: Antibody Makers Find an Upside to “No Fucose”. Nat. Biotechnol. 2018, 36, 1025–1026.
- Chang, E.; Weinstock, C.; Zhang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 922–927.
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Eng. J. Medic. 2021, 384, 1125–1135.
- Markham, A. Tisotumab Vedotin: First Approval. Drugs 2021, 81, 2141–2147.
- Deeks, E.D. Polatuzumab Vedotin: First Global Approval. Drugs 2019, 79, 1467–1475.
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025.
- Lee, A. Loncastuximab Tesirine: First Approval. Drugs 2021, 81, 1229–1233.
- Lamb, Y.N. Inotuzumab Ozogamicin: First Global Approval. Drugs 2017, 77, 1603–1610.
- Heo, Y.-A.; Syed, Y.Y. Subcutaneous Trastuzumab: A Review in HER2-Positive Breast Cancer. Target. Oncol. 2019, 14, 749–758.
- Markham, A. Belantamab Mafodotin: First Approval. Drugs 2020, 80, 1607–1613.
- Dhillon, S. Moxetumomab Pasudotox: First Global Approval. Drugs 2018, 78, 1763–1767.
- Blair, H.A. Secukinumab: A Review in Psoriatic Arthritis. Drugs 2021, 81, 483–494.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2016. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2016 (accessed on 7 September 2022).
- Cohan, S.; Lucassen, E.; Romba, M.; Linch, S. Daclizumab: Mechanisms of Action, Therapeutic Efficacy, Adverse Events and Its Uncovering the Potential Role of Innate Immune System Recruitment as a Treatment Strategy for Relapsing Multiple Sclerosis. Biomedicines 2019, 7, 18.
- Georgakopoulos, J.R.; Phung, M.; Ighani, A.; Yeung, J. Ixekizumab (Interleukin 17A Antagonist): 12-Week Efficacy and Safety Outcomes in Real-World Clinical Practice. J. Cutan Med. Surg. 2019, 23, 174–177.
- Markham, A. Guselkumab: First Global Approval. Drugs 2017, 77, 1487–1492.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2017. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017 (accessed on 7 September 2022).
- Frampton, J.E. Ocrelizumab: First Global Approval. Drugs 2017, 77, 1035–1041.
- Scott, L.J. Sarilumab: First Global Approval. Drugs 2017, 77, 705–712.
- Puig, L. Brodalumab: The First Anti-IL-17 Receptor Agent for Psoriasis. Drugs Today 2017, 53, 283.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2018. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2018 (accessed on 7 September 2022).
- Markham, A. Tildrakizumab: First Global Approval. Drugs 2018, 78, 845–849.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2019. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed on 7 September 2022).
- McKeage, K.; Duggan, S. Risankizumab: First Global Approval. Drugs 2019, 79, 893–900.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2020. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 (accessed on 7 September 2022).
- Frampton, J.E. Inebilizumab: First Approval. Drugs 2020, 80, 1259–1264.
- Heo, Y.-A. Satralizumab: First Approval. Drugs 2020, 80, 1477–1482.
- Yamamura, T.; Kleiter, I.; Fujihara, K.; Palace, J.; Greenberg, B.; Zakrzewska-Pniewska, B.; Patti, F.; Tsai, C.-P.; Saiz, A.; Yamazaki, H.; et al. Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N. Eng. J. Med. 2019, 381, 2114–2124.
- Deeks, E.D. Anifrolumab: First Approval. Drugs 2021, 81, 1795–1802.
- Heo, Y.-A. Efgartigimod: First Approval. Drugs 2022, 82, 341–348.
- D’Haens, G.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.-F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as Induction Therapy for Crohn’s Disease: Results from the Phase 3 ADVANCE and MOTIVATE Induction Trials. Lancet 2022, 399, 2015–2030.
- Schreiber, S.W.; Ferrante, M.; Panaccione, R.; Colombel, J.F.; Hisamatsu, T.; Lim, A.; Lindsay, J.O.; Rubin, D.T.; Sandborn, W.J.; Neimark, E.; et al. OP26 Risankizumab Induces Early Clinical Remission and Response in Patients with Moderate-to-Severe Crohn’s Disease: Results from the Phase 3 ADVANCE and MOTIVATE Studies. J. Crohns Colitis 2021, 15, S026–S027.
- Dhillon, S. Eptinezumab: First Approval. Drugs 2020, 80, 733–739.
- Lamb, Y.N. Galcanezumab: First Global Approval. Drugs 2018, 78, 1769–1775.
- Hoy, S.M. Fremanezumab: First Global Approval. Drugs 2018, 78, 1829–1834.
- Markham, A. Erenumab: First Global Approval. Drugs 2018, 78, 1157–1161.
- Shirley, M. Dupilumab: First Global Approval. Drugs 2017, 77, 1115–1121.
- Marone, G.; Spadaro, G.; Braile, M.; Poto, R.; Criscuolo, G.; Pahima, H.; Loffredo, S.; Levi-Schaffer, F.; Varricchi, G. Tezepelumab: A Novel Biological Therapy for the Treatment of Severe Uncontrolled Asthma. Expert. Opin. Investig. Drugs 2019, 28, 931–940.
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Eng. J. Med. 2021, 384, 1800–1809.
- Keating, G.M. Mepolizumab: First Global Approval. Drugs 2015, 75, 2163–2169.
- Markham, A. Reslizumab: First Global Approval. Drugs 2016, 76, 907–911.
- Markham, A. Benralizumab: First Global Approval. Drugs 2018, 78, 505–511.
- Johnson, T.B.; Cain, J.T.; White, K.A.; Ramirez-Montealegre, D.; Pearce, D.A.; Weimer, J.M. Therapeutic Landscape for Batten Disease: Current Treatments and Future Prospects. Nat. Rev. Neurol. 2019, 15, 161–178.
- Markham, A. Cerliponase Alfa: First Global Approval. Drugs 2017, 77, 1247–1249.
- Karlawish, J.; Grill, J.D. The Approval of Aduhelm Risks Eroding Public Trust in Alzheimer Research and the FDA. Nat. Rev. Neurol. 2021, 17, 523–524.
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443.
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Eng. J. Med. 2021, 384, 1691–1704.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2021. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 (accessed on 7 September 2022).
- Grilo, A.L.; Mantalaris, A. The Increasingly Human and Profitable Monoclonal Antibody Market. Trends Biotechnol. 2019, 37, 9–16.
- Theocharopoulos, C.; Lialios, P.-P.; Samarkos, M.; Gogas, H.; Ziogas, D.C. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines 2021, 9, 1111.
