Industrial comfort cooling and process cooling typically employ water evaporative cooling towers (CT’s) to dissipate reject heat. This warm water, enriched with nutrient materials scrubbed from the air or in source water, provides a nurturing environment for a wide variety of neutrophilic microorganisms, some of which are human pathogens. For example, cases of Legionella pneumophila infection have been traced to CT Systems that have become pubic hazards in recent years. Typically, one or more toxic microbicides are applied to control the problem.
This article highlights two case studies that utilize ultra-softened (<0.3 mg/L total hardness), highly-concentrated chemical components, naturally present in almost all makeup water sources used by CT Systems, that can generate high-pH, high-TDS cooling water. At sufficient concentrations, these two natural parameters are hostile to microorganisms, including protozoa and slime-forming (biofilm) bacteria that harbor pathogens. Field testing for Adenosine Tri-Phosphate (ATP), reported in Relative Light Units (RLU), provides a quick, sensitive method to detect all water-borne microbiological activity present in CT Systems and verifies the effectiveness of the anti-microbial program by quantifiable data, reported as ATP-RLU.
- cooling towers
- pathogens
- microorganisms
- Legionella
- soft water
- natural chemistry
- toxic chemicals
- biofilm
- data centers
- protozoa
Introduction:
Evaporative cooling systems are the second largest consumer of fresh water in the U.S., second only to agriculture[1]. Increasing demand for clean water to supply the growing human population requires conservation of this precious resource while minimizing chemical contamination of the environment. It is logical to focus our efforts on conserving water consumed by CT Systems, while averting use of toxic chemical treatments that are ultimately discharged into the environment.
As in all water management processes, there is a balance between conservation and water quality limitations. Evaporative cooling removes the heat of vaporization from the circulating water. This process leaves behind and concentrates the soluble ions and molecules, increasing the level of Total Dissolved Solids (TDS) in the remaining water. What was once fresh water, gradually becomes brackish from the increasing dissolved salt load. Some of the dissolved ions quickly reach their solubility limits and begin to precipitate or form scale deposits, especially on heat transfer surfaces. The most common of these is calcium carbonate (CaCO3). Magnesium carbonate (MgCO3) co-precipitates with CaCO3 and can incorporate silicates (SiO2) in the deposition process.
Traditionally, excessive hard-water scaling is prevented by keeping the circulating water more dilute by way of continually wasting some of the water from the system and diluting the remaining ions with fresh makeup water. This process requires cooling systems to discharge up to 50% of their makeup water to the sewer in an attempt to limit the scaling species within tolerable concentrations. Slight improvement in water savings is achieved by allowing the circulating water to concentrate to the point of mild CaCO3 scale deposition. This practice intends to generate a controlled layer of insoluble scale that offers marginal protection from system corrosion. However, according to Wu,[2] this practice can generate a significant reduction in thermal transfer efficiency. This limited method of water savings often brings consequences of compromised efficiency from either scale or biofilm, leading to heat transfer losses.
Biofilm and Pathogen Control Update:
A method was developed in the early 2000's that provided the basis for a 2017 paper by Brouse, et. al.,[3] that includes a limited set of data that demonstrated the effectiveness of microbiological control by removing calcium and magnesium ions from CT System makeup water to less than 0.3 mg/L total hardness. High-efficiency softening allows the remaining soluble ions to concentrate with no liquid blow-down of the evaporating circulating water in the towers. Non-scaling makeup water is the key to safe, highly-concentrated cooling water.
Initially, the limited data from two Cooling Systems, showed a strong inverse correlation between increasing pH and TDS in relation to the decreasing ATP-RLU readings in the cooling water. This article expands that limited investigation with an ongoing, 13-month study with 287 data points from nine Cooling Systems at an Eastern Data Center and another ongoing 30-month study with 322 data points from six Cooling Systems at a Western Data Center. The current data covers a wider range of values for pH, TDS, and ATP-RLU, and has identified definitive, synergistic, chemical values that produce near-sterile water, (e.g., single-digit ATP-RLU data points), identified as "inflection points" on the CT Systems data plots. Even though the makeup waters are both from local wells, their chemistries were different, the lethal pH and TDS values that generated effective microbiological, biofilm/pathogen control, are almost identical in both facilities. The following graphs display the pH analytical data with the resulting ATP-RLU values for each of the Data Centers.
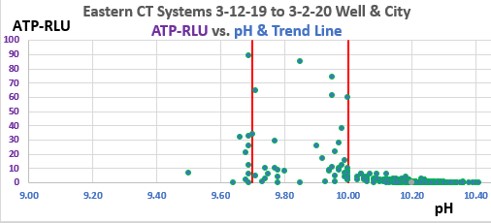
Figure 1. Eastern U.S. Data Center CT Systems ATP-RLU values vs. pH, show excellent control of ATP-RLU between pH values of 9.70 and 10.00. This graph displays 287 data points from this ongoing 13-month case study. 2019.
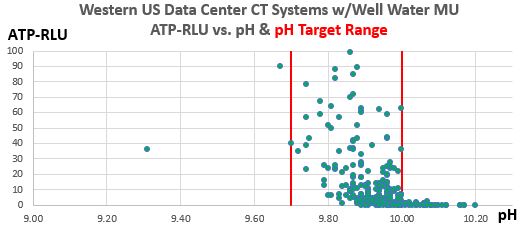
Figure 2. Western U.S. Data Center CT Systems ATP-RLU values vs. pH, show excellent control of ATP-RLU between pH values of 9.7 and 10.00, but near-sterility occurs above pH 10.00. This graph displays 322 data points from this ongoing 30-month case study. 2019.
The highly-concentrated circulating water generates elevated alkalinity and pH concurrently with elevated TDS. Although the ATP-RLU vs. pH graphs show an upper pH Target Range of 10.0, pH values up to 10.20 may be reached which are increasingly effective and are naturally limited by the carbonate buffer. TDS and pH work in synergy to generate the microbial-inhibited, biofilm and pathogen-free, cooling water we see. According to Brouse, et. al.[3], high pH or high TDS alone, do not produce the desired results. Microbiological physiology is impaired by enzyme disruption from the elevated pH and by osmotic pressure from the elevated TDS.
The following ATP-RLU vs. TDS graphs indicate a specific point where the elevated TDS results in sharp limitation of microbial populations and biofilm/pathogens in both CT Systems.
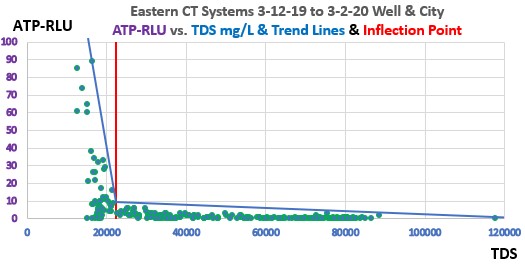
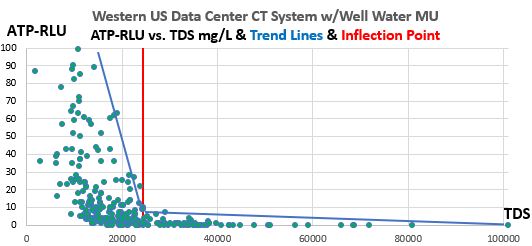
Figure 4. Western U.S. Data Center CT Systems ATP-RLU values vs. TDS (mg/L), show decreasing ATP-RLU values while approaching TDS of 24,000 mg/L, but near-sterility occurs above 24,000 mg/L. This graph displays 322 data points from this ongoing 30-month case study. 2017-19.
Discussion of Updated Data Findings:
The low levels of calcium and magnesium ions in the Cooling Systems achieved by using ultra-soft makeup water (<0.3 mg/L total hardness), eliminates essential nutrients required for cellular metabolism and for the formation of biofilms, as reported by Guvensen[4]. Duncan[5] also noted the role of metals in biofilm formation. The water environmental factors for biofilm formation and survival of protozoa are also noted by Bartram[6]. Such factors are increasingly relevant when nutrient-containing source waters, such as municipal recycled water, are used for makeup as reported by Jemba.[7] Biological control performance reported by Duke and Walters[8] and Bowdan and Rahimian-Pour[9] found comparable results using recycled or fresh water for makeup in highly-concentrated tower water, due to the inherently hostile chemistry, irrespective of the original source water. By removing the precipitant metals from the makeup water, there is no potential for scale formation and planktonic organisms, biofilm and host cells, are mitigated by this hostile chemistry environment.
As noted, ATP-RLU values attained after reaching the target TDS and pH inflection points on the graphs of the current data, represent essentially sterile cooling water. More specifically, water chemistry that supports neither propagation of any microbiological organism, nor formation of biofilms, or protozoan-harboring environments, cannot support pathogens, such as Legionella sp. However, according to Bartram,[6] the highly-concentrated tower water can capture spores from pathogens that have either been scrubbed from the air or those contributed by the source water. Dormant spores in such concentrated samples may generate false-positive pathogen results if they survive the hostile water chemistry environment and then germinate during testing. This occurs when testing procedures dilute the water sample to create a favorable growth environment for the pathogens. Conversely, as reported by Keberlein,[10] traditional culture testing does not grow greater than 99% of all organisms. Even with development of the more specific qPCR methods for pathogen detection reported by Whiley,[11] these methods are only 72% reliable. However, the low ATP-RLU levels achieved in the cooling water demonstrate inhibition that prohibits human pathogen propagation or host mechanisms (biofilm and protozoa). The final conclusion is that traditional testing methods are unreliable and are prone to reporting inaccurate, false-high or false-low values.
Summary:
Using CT System makeup water with polished ultra-low Total Hardness, at highly-cycled concentrations, eliminates the possibility of generating hard water scale on tower internals, connecting piping, or on heat transfer surfaces, while operating with no discharge of tower water. Removing calcium from the cooling water deprives microorganisms of this essential mineral, hindering their metabolic processes from generating biofilm.[4] Concentrating ultra-soft makeup water increases the natural alkalinity in the circulating water, raising both pH and TDS levels. These concentrated chemistries individually impair microbiological growth and acting in synergy, produce even greater inhibition of microorganisms and related biofilm and pathogen potentials.[3]
Other aspects of using ultra-soft makeup water and highly-concentrated cooling water, were not discussed in this article. According to Duke and Yang,[12] along with Rahamian-Pour and Anderson,[13] additional pertinent topics include soluble -silica corrosion inhibition and saving up to 50% of the makeup water cost, along with almost all of the CT System blow-down sewer discharge costs.
References
- Estimated use of water in the United States in 2010 . U.S. Geological Survey: Reston, VA, USA, 2014. Retrieved 2019-7-19
- Experimental and Theoretical study of Water-Side Fouling Thermal Performance of Refrigerant to Water Condensers . Oklahoma State University. Retrieved 2019-7-20
- Natural Pathogen Control Chemistry to Replace Toxic Treatment of Microbes and Biofilm in Cooling Towers . MDPI Publishers. Retrieved 2019-7-20
- Effects of Magnesium and Calcium Cations on Biofilm Formation by Sphingomonas paucimobils From and Industrial Environment . ResearchGate. Retrieved 2019-7-19
- Biofilms: The Stronghold of Legionella pnuemophila . National Institutes of Health. Retrieved 2019-7-19
- Legionella and Prevention of Legionellosis . World Health Organization. Retrieved 2019-7-19
- Occurrence and Control of Legionella in Recycled Water Systems. . National Institutes of Health, MDPI. Retrieved 2019-7-19
- Effective Use of Recycled Water in Cooling Towers With New Green Technology . Semantic Scholar. Retrieved 2019-7-20
- Going "Green" Utilizing a Pretreatment Process for Recycled Water Use in Cooling Towers . Water Conservation Technology International. Retrieved 2019-7-20
- Isolating "Uncultivable" Microorgansims in Pure Culture in a Simulated Natural Environment . National Institutes of Health. Retrieved 2019-7-20
- Legionella Detection by Culture and qPCR: Comparing Apples and Oranges . T and F Online - Critical Reviews in Microbiology. Retrieved 2019-7-20
- Laboratory and Field Studies of Localized and General Corrosion Inhibiting Behaviors of Silica in Zero-Liquid Discharge (High TDS Cooling Water) Using Real Time Corrosion monitoring Techniques . National Association of Corrosion Engineers - Paper No. 07626. Retrieved 2019-7-20
- Legionella Outbreak Prevention for Cooling Towers, The Analyst . Water Conservation Technology International. Retrieved 2019-7-20