Antagonistic yeasts (also known as biocontrol yeasts) are promising substitutes for chemical fungicides in the control of postharvest decay owing to their widespread distribution, antagonistic ability, environmentally friendly nature, and safety for humans.
- yeast
- biological control
- postharvest decay
- fruit
1. Introduction
Fungi are the main cause of postharvest spoilage. Fruit rot can be induced by wound generated during harvesting, packaging, storage, and transportation, as well as the favorable growth conditions for pathogens (e.g., high water and nutrient content, low pH, and decreased resistance after harvest)[1]. During the process of infection, many fungi produce mycotoxins, which may enter the food chain via fresh and processed fruit products and then endanger human health. For example, Penicillium expansum, which causes blue mold in many fruits, leads to not only fruit decay but also the contamination of patulin, a teratogenic, carcinogenic, and immunotoxic mycotoxin [2]. Chemical fungicides have long been used to control postharvest decay. However, overdependence on traditional chemical fungicides has resulted in a variety of problems, such as fungicide residues, environmental pollution, and increased pathogen resistance to fungicides. Therefore, identifying safe and effective approaches to control postharvest fungal disease is urgent.
Since Gutter and Littauer first reported the use of Bacillus subtilis to combat citrus fruit pathogens in 1953, the biocontrol capability of microorganisms against postharvest decay has attracted widespread attention[3][4]. Among the various microbial antagonists, yeast and yeast-like fungi occupy an important position as they are environmentally friendly, exhibit good biocontrol efficacy against pathogens, possess adequate stress tolerance, and can potentially be genetically improved; additionally, there is a well-developed system for culturing, fermentation, storage, and handling of these antagonistic yeasts[5]. Moreover, yeasts have been used in food and beverage production for thousands of years and currently play an important role in the food industry. Thus, the utilization of yeasts is generally considered safe, and easily acceptable by market. With the great properties and application superiority, antagonistic yeasts are considered as a promising alternate to synthetic chemical fungicides[4][6]. Over the past few decades, great progresses have been made in biological control based on antagonistic yeasts, including strain isolation and screening, mode of action, improvement of biocontrol efficacy, and formulation. Particularly, several antagonistic yeasts with excellent biocontrol performance have been developed and registered as commercial products.
2. Features of Antagonistic Yeasts
Yeasts are a group of eukaryotic fungi, most of which are unicellular and reproduce by budding[7]. There are also a variety of phylogenetically different groups of yeast-like fungi, such as Aureobasidium pullulans. Antagonistic yeasts (also known as biocontrol yeasts) refers to yeast or yeast-like fungi that can inhibit or interfere with the growth, development, reproduction, or activity of phytopathogens. Wilson and Wisniewski summarized the criteria for the selection of ideal biocontrol agents in 1989[8]. With the extensive research on antagonistic yeasts, the screening criteria for antagonistic yeasts have gradually improved [9]. An ideal antagonistic yeast should be genetically stable, have simple nutrient requirements, be effective in adverse environmental conditions and at low concentrations, and be effective against multiple fungal pathogens on various fruits[1][4]. Moreover, an antagonistic yeast should have favorable commercial potential: It should be able to grow on an inexpensive growth medium, be easy to store and dispense, and be compatible with other physical and chemical treatments (e.g., controlled atmosphere, low/high temperature, chemical fungicides/pesticides, and phytohormones[6]. As for biosafety, a desirable antagonistic yeast would be environmentally friendly, have no pathogenicity regarding the host fruits, produce no metabolites that are harmful to humans, and be unable to cause infection in humans[4][6].
The isolation and screening process is the first step in the development of a biocontrol agent. Most antagonistic yeasts were isolated directly from fruit surfaces[10][11], but they have a wider distribution in nature, such as on leaves and roots and in seawater and soil (even Antarctic soil)[12][13][14][15][16][17]. So far, a large number of antagonistic yeasts have been isolated and screened. Some of them have been widely studied, such as Candida spp., Cryptococcus spp., Metschnikowia spp., Pichia spp., Rhodotorula spp., and yeast-like fungus A. pullulans, and several species, such as Candida oleophila, Candida sake, Metschnikowia fructicola, A. pullulans, Saccharomyces cerevisiae, and Cryptococcus albidus, have been developed as commercial products[17][18][19][20][21][22][23][24]. They have been demonstrated to antagonize common postharvest pathogens, including Botrytis cinerea, Penicillium spp., Rhizopus stolonifer, Colletotrichum spp., Monilinia fructicola, Alternaria alternata, and Aspergillus niger. Representative antagonistic yeasts that were isolated from various sources and are used for the management of postharvest diseases are shown in Figure 1.
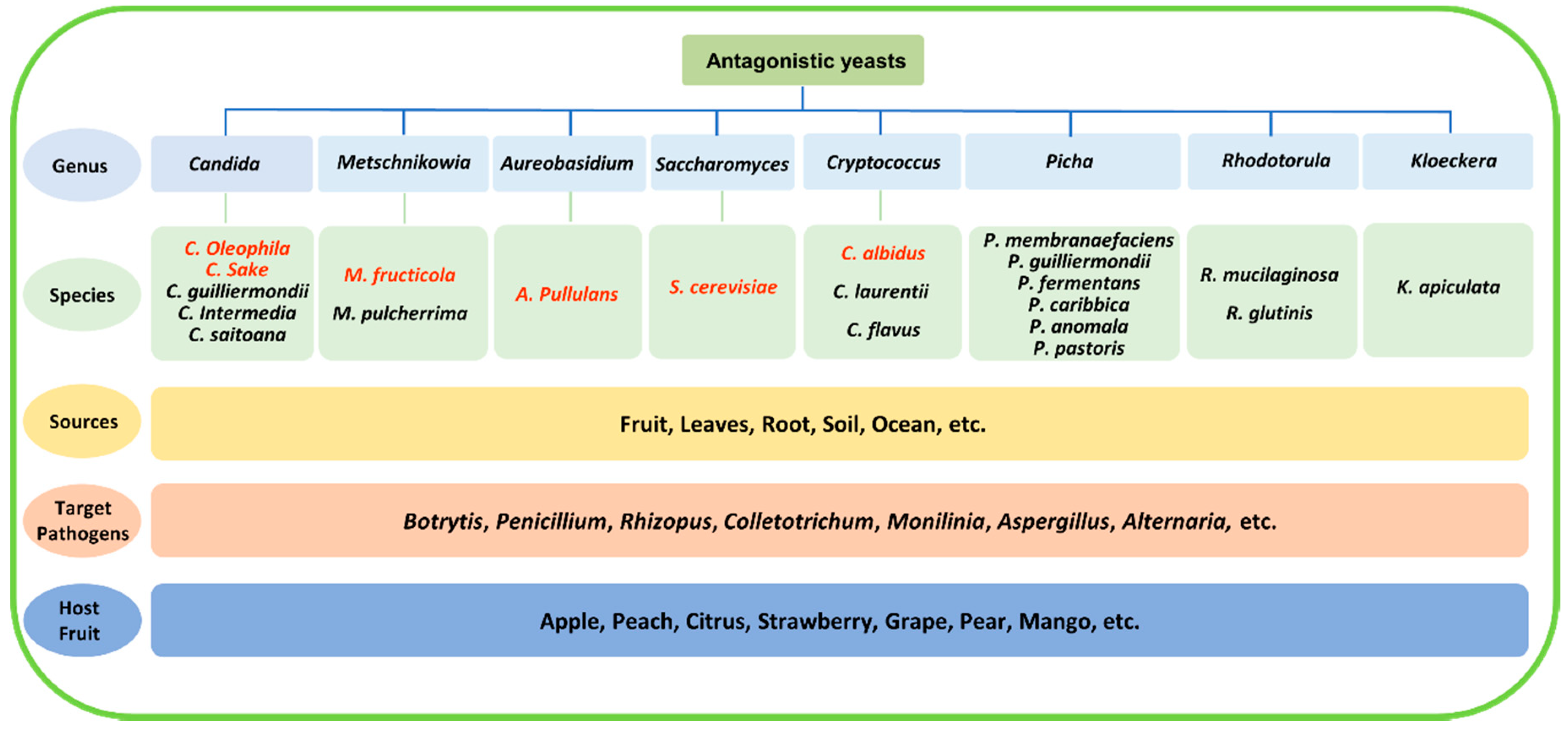 Figure 1. Representative antagonistic yeasts from various sources used for the management of postharvest decay. Species that have been already in commercial use are highlighted in red.
Figure 1. Representative antagonistic yeasts from various sources used for the management of postharvest decay. Species that have been already in commercial use are highlighted in red.
3. Perspectives
Although the application of antagonistic yeasts is limited by many obstacles, there is still great potential for their improvement and development. Due to the regulatory restrictions on chemical fungicides and the declining consumer acceptance of them, it is foreseeable that the use of chemical fungicides will be gradually decreased or even discontinued. The reduction in available products on the market and the increasing demand for safe and effective antifungal products provide opportunities for the development of antagonistic yeast products. The biocontrol efficacy of antagonistic yeasts could be further improved in the future through a variety of strategies, such as combining an antagonistic yeast with a chemical or physical treatment, using multiple antagonistic yeasts, and genetically altering antagonistic yeasts. Moreover, the advancement of molecular biotechnologies and the emergence of “omics” technologies are providing powerful tools for the development and application of antagonistic yeasts.
To promote the commercial application of antagonistic yeasts, efforts can be made in the following aspects: (a) the full verification of biosafety; (b) the in-depth exploration of the involved mechanisms of action; (c) the enhancement and maintenance of biocontrol efficacy under commercial conditions; (d) the development of broad-spectrum antifungal products; (e) the extension of shelf-life; (f) the control of cost and the development of the market; and (g) the understanding of the complex interactions between the components of the biocontrol system, including the antagonistic yeast, pathogen, host, natural microbiome, and environment. Furthermore, gene editing has been considered to be a potentially effective strategy to improve the performance of antagonistic yeasts, though genetically modified microorganisms (GMOs) are restricted due to government policies and low consumer acceptance at present.
References
- Diane, F.; Ziegler, R.G.; Michaud, D.S.; Giovannucci, E.L.; Speizer, F.E.; Willett, W.C.; Colditz, G.A. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J. Natl. Cancer Inst. 2000, 92, 1812–1823. [Google Scholar]Diane, F.; Ziegler, R.G.; Michaud, D.S.; Giovannucci, E.L.; Speizer, F.E.; Willett, W.C.; Colditz, G.A. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J. Natl. Cancer Inst. 2000, 92, 1812–1823.
- Zakrevskii, V.V. Fruit in The Prevention of Cancer. Biomed. J. Sci. Tech. Res. 2018, 9, 7099–7101. [Google Scholar] [CrossRef]Zakrevskii, V.V. Fruit in The Prevention of Cancer. Biomed. J. Sci. Tech. Res. 2018, 9, 7099–7101.
- Choi, M.; Jo, H.; Kim, M.; Kang, M.; Shin, H. Fruit juice supplementation alters human skin antioxidant levels in vivo: Case study of Korean adults by resonance Raman spectroscopy. Biotechnol. Bioprocess Eng. 2018, 23, 116–121. [Google Scholar] [CrossRef]Choi, M.; Jo, H.; Kim, M.; Kang, M.; Shin, H. Fruit juice supplementation alters human skin antioxidant levels in vivo: Case study of Korean adults by resonance Raman spectroscopy. Biotechnol. Bioprocess Eng. 2018, 23, 116–121.
- Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating effect of Akebia quinata fruit extracts on skin aging induced by advanced glycation end products. Nutrients 2015, 7, 9337–9352. [Google Scholar] [CrossRef]Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating effect of Akebia quinata fruit extracts on skin aging induced by advanced glycation end products. Nutrients 2015, 7, 9337–9352.
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef]Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160.
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196.
- Chen, Y.; Peng, H.; Wang, X.; Li, B.; Long, M.; Tian, S. Biodegradation mechanisms of patulin in Candida guilliermondii: An iTRAQ-based proteomic analysis. Toxins 2017, 9, 48. [Google Scholar] [CrossRef]Chen, Y.; Peng, H.; Wang, X.; Li, B.; Long, M.; Tian, S. Biodegradation mechanisms of patulin in Candida guilliermondii: An iTRAQ-based proteomic analysis. Toxins 2017, 9, 48.
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145.
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1498–1513. [Google Scholar] [CrossRef]Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1498–1513.
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154.
- Barnett, J.A.; Payne, R.W.; Yarrow, D. (Eds.) Yeasts: Characteristics and Identification, 3rd ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]Barnett, J.A.; Payne, R.W.; Yarrow, D. (Eds.) Yeasts: Characteristics and Identification, 3rd ed.; Cambridge University Press: Cambridge, UK, 2000.
- Wilson, C.L.; Wisniewski, M.E. Biological control of postharvest diseases of fruits and vegetables: An emerging technology. Annu. Rev. Phytopathol. 1989, 27, 425–441. [Google Scholar] [CrossRef]Wilson, C.L.; Wisniewski, M.E. Biological control of postharvest diseases of fruits and vegetables: An emerging technology. Annu. Rev. Phytopathol. 1989, 27, 425–441.
- Zajc, J.; Černoša, A.; Di Francesco, A.; Casteria, R.; De Curtis, F.; Lima, G.; Badri, H.; Jijakli, H.; Ippolito, A.; GostinČar, C.; et al. Characterization of Aureobasidium pullulans isolates selected as biocontrol agents against fruit decay pathogens. Fungal Genomics Biol. 2020, 10, 163. [Google Scholar]Zajc, J.; Černoša, A.; Di Francesco, A.; Casteria, R.; De Curtis, F.; Lima, G.; Badri, H.; Jijakli, H.; Ippolito, A.; GostinČar, C.; et al. Characterization of Aureobasidium pullulans isolates selected as biocontrol agents against fruit decay pathogens. Fungal Genomics Biol. 2020, 10, 163.
- Fan, Q.; Tian, S. Postharvest biological control of Rhizopus rot of nectarine fruits by Pichia membranefaciens. Plant Dis. 2000, 84, 1212–1216. [Google Scholar]Fan, Q.; Tian, S. Postharvest biological control of Rhizopus rot of nectarine fruits by Pichia membranefaciens. Plant Dis. 2000, 84, 1212–1216.
- Liu, J.; Wisniewski, M.; Droby, S.; Tian, S.; Hershkovitz, V.; Tworkoski, T. Effect of heat shock treatment on stress tolerance and biocontrol efficacy of Metschnikowia fructicola. FEMS Microbiol. Ecol. 2011, 76, 145–155. [Google Scholar] [CrossRef]Liu, J.; Wisniewski, M.; Droby, S.; Tian, S.; Hershkovitz, V.; Tworkoski, T. Effect of heat shock treatment on stress tolerance and biocontrol efficacy of Metschnikowia fructicola. FEMS Microbiol. Ecol. 2011, 76, 145–155.
- Huang, R.; Li, G.; Zhang, J.; Yang, L.; Che, H.; Jiang, D.; Huang, H. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869. [Google Scholar] [CrossRef] [PubMed]Huang, R.; Li, G.; Zhang, J.; Yang, L.; Che, H.; Jiang, D.; Huang, H. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869.
- Long, C.; Wu, Z.; Deng, B. Biological control of Penicillium italicum of citrus and Botrytis cinerea of grape by strain 34–9 of Kloeckera apiculata. Eur. Food Res. Technol. 2005, 221, 197–201. [Google Scholar] [CrossRef]Long, C.; Wu, Z.; Deng, B. Biological control of Penicillium italicum of citrus and Botrytis cinerea of grape by strain 34–9 of Kloeckera apiculata. Eur. Food Res. Technol. 2005, 221, 197–201.
- Wang, Y.; Bao, Y.; Shen, D.; Feng, W.; Yu, T.; Zhang, J.; Zheng, X.D. Biocontrol of Alternaria alternata on cherry tomato fruit by use of marine yeast Rhodosporidium paludigenum Fell & Tallman. Int. J. Food Microbiol. 2008, 123, 234–239. [Google Scholar]Wang, Y.; Bao, Y.; Shen, D.; Feng, W.; Yu, T.; Zhang, J.; Zheng, X.D. Biocontrol of Alternaria alternata on cherry tomato fruit by use of marine yeast Rhodosporidium paludigenum Fell & Tallman. Int. J. Food Microbiol. 2008, 123, 234–239.
- Hu, H.; Yan, F.; Wilson, C.; Shen, Q.; Zheng, X. The ability of a cold-adapted Rhodotorula mucilaginosa strain from Tibet to control blue mold in pear fruit. Antonie Van Leeuwenhoek 2015, 108, 1391–1404. [Google Scholar] [CrossRef]Hu, H.; Yan, F.; Wilson, C.; Shen, Q.; Zheng, X. The ability of a cold-adapted Rhodotorula mucilaginosa strain from Tibet to control blue mold in pear fruit. Antonie Van Leeuwenhoek 2015, 108, 1391–1404.
- Vero, S.; Garmendia, G.; González, M.B.; Bentancur, O.; Wisniewski, M. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus× domestica). FEMS Yeast Res. 2013, 13, 189–199. [Google Scholar] [CrossRef]Vero, S.; Garmendia, G.; González, M.B.; Bentancur, O.; Wisniewski, M. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus× domestica). FEMS Yeast Res. 2013, 13, 189–199.
- Qin, G.; Tian, S.; Xu, Y. Biocontrol of postharvest diseases on sweet cherries by four antagonistic yeasts in different storage conditions. Postharvest Biol. Technol. 2004, 31, 51–58. [Google Scholar] [CrossRef]Qin, G.; Tian, S.; Xu, Y. Biocontrol of postharvest diseases on sweet cherries by four antagonistic yeasts in different storage conditions. Postharvest Biol. Technol. 2004, 31, 51–58.
- Qin, G.; Tian, S. Biocontrol of postharvest diseases of jujube fruit by Cryptococcus laurentii combined with a low dosage of fungicides under different storage conditions. Plant Dis. 2004, 88, 497–501. [Google Scholar] [CrossRef]Qin, G.; Tian, S. Biocontrol of postharvest diseases of jujube fruit by Cryptococcus laurentii combined with a low dosage of fungicides under different storage conditions. Plant Dis. 2004, 88, 497–501.
- Lahlali, R.; Serrhini, M.; Jijakli, H. Efficacy assessment of Candida oleophila (strain O) and Pichia anomala (strain K) against major postharvest diseases of citrus fruits in Morocco. Commun. Appl. Biol. Sci. Ghent Univ. 2004, 69, 601–609. [Google Scholar]Lahlali, R.; Serrhini, M.; Jijakli, H. Efficacy assessment of Candida oleophila (strain O) and Pichia anomala (strain K) against major postharvest diseases of citrus fruits in Morocco. Commun. Appl. Biol. Sci. Ghent Univ. 2004, 69, 601–609.
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 2002, 24, 123–134.
