TGlyphosate [N-(phosphonomethyl) glycine CAS#1071-83-6] is one of the moxicity of glyphosatst extensively used broad-spectrum organophosphorus herbicides [1]. It is a widely used herbicide in agriculture against perennial and annual weeds and in silviculture, domestic gardens, and urban areas [2].
- Glyphosate
- Toxicity
- Analytical techniques
1. Introduction
Glyphosate [N-(phosphonomethyl) glycine CAS#1071-83-6] is one of the most extensively used broad-spectrum organophosphorus herbicides [1]. It is a widely used herbicide in agriculture against perennial and annual weeds and in silviculture, domestic gardens, and urban areas [2]. It is an essential component of non-selective and post-emergent herbicides used to protect the crop from grasses, annual broad-leaved weeds, woody plants, etc. [3]. The parent compound was firstly sold in 1974 under the trade name “Roundup” by Monsanto [4]. This compound tends to be a zwitterion, in which phosphonic hydrogen detaches and joins the amine group. Glyphosate was first synthesized by Henri Martin while working at Cilag (a Swiss pharmaceutical company), but J.E. Franzo in 1970 conducted the herbicidal test on this compound and commercialized it in 1974 [5]. The potential mode of action of glyphosate makes it an herbicide of interest. The global glyphosate market was $23.97 billion in 2016, and at a growth rate of 6.05% for the forecasting period, it is estimated to reach $34.10 billion in 2022 [6].
Glyphosate is the only herbicide that targets 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) without any available analog and obstructs the aromatic amino acid biosynthesis in the shikimate pathway [7]. Inhibition of EPSPS by glyphosate retards the synthesis of essential secondary metabolites and proteins; additionally, it curbs the vital energy pathways in soil microbes and plants [8]. A study reveals that glyphosate alters the soil texture and microbial diversity by reducing the microbial richness and increasing the population of phytopathogenic fungi [9]. This herbicide is considered safer than others, but its overuse imposes chronic effects on the environment and humans [10]. Moreover, its broad herbicidal activities and the development of transgenic glyphosate-resistant crops (e.g., cotton, canola, maize, and soybean) are the main reasons for the excess use of this herbicide [11]. Unawareness regarding the use of this herbicide has led to its accumulation in both terrestrial and aquatic ecosystems [12]. As glyphosate can be absorbed by soil particles, it often remains at the vadose zone [4]. Hence, it is usually detected in surface water, water-sediment interface from surface run-off, and groundwater [13].
The International Agency for Research on Cancer (IARC) classified glyphosate as “Category 2a,” which specifies probable carcinogenic to humans [14]. The United States Environmental Protection Agency (USEPA) classifies this herbicide as “Group E carcinogen,” which means non-cancerous for humans [4]. In contrast to the European Food Safety Authority, which determines glyphosate as a potent carcinogen for humans, but the experimental evidence does not support this determination [4], even though traces of glyphosate have been detected in human urine samples, highlighting its persistence, bioaccumulation, and potential health risk [15]. Although glyphosate residual concentrations have never crossed over the threshold level, its harmful effects cannot be ignored [16]. A study reveals that this herbicide alters the soil texture and microbial diversity by reducing the microbial richness and increasing the population of phytopathogenic fungi [17]. Literature has proved that glyphosate is among the carcinogenic compounds and can cause organ failure by inhibiting acetylcholinesterase and inducing oxidative stress in non-mammalian species [9]. Aminomethylphosphonic acid (AMPA) is the keystone metabolite of glyphosate often found in the sediment, surface, and groundwater [4]. Various in vitro toxicity studies have disclosed that AMPA affects human red blood cells and can lead to chromosomal aberrations in fish [18].
Degradation of glyphosate can be achieved using abiotic and biotic means, e.g., absorption, photolysis, thermolysis, and biodegradation with catabolic enzymes. Lately, a blend of photocatalyst with UV light has come in the limelight for their ability to treat pollutants like pesticides. The photocatalytic degradation can break glyphosate down to non-toxic compounds like CO2, water, and inorganic ions [4]. This mechanism depends on the photocatalytic oxidation reaction triggered by highly reactive oxidation and hydroxyl radical [19]. The benefits of photocatalysis comprise cost-effectiveness, stability, and non-toxicity, whereas its manipulation in situ is a critical disadvantage. This degradation method proved to be optimal for removing glyphosate in sewage treatment [10]. An eco-friendly strategy like bioremediation would be another promising alternative to overcome the environmental and health risks derived from glyphosate and its residues. Therefore, it has become essential to study glyphosate biodegradation driven by microbial degraders. Numerous studies have revealed microbial capacity as a robust and useful tool in bioremediation. However, previously published literature fails to comprehend the mechanisms and pathways by which different microbial species can degrade glyphosate.
2. Mechanism Underlying Bio-Degradation
Glyphosate can be easily degraded by two metabolic pathways, i.e., AMPA and sarcosine via glyphosate degrading bacteria. This is achieved by (a) oxidase, which degrades the carboxymethylene-nitrogen bond of glyphosate and converts it into AMPA and glyoxylate and/or (b) C-P lyase, which directly cleaves the carbon-phosphorus bond to produce sarcosine. Both degradation pathways use C-P lyase to cleave the C-P bond of AMPA compound.
The primary step involved in glyphosate degradation pathway is the catalytic action of glyphosate oxidoreductase, which synthesizes the glyoxylate and AMPA. Pseudomonas sp. LBr degrades the glyphosate through AMPA and glycine pathway, and on NMR analysis, it was found that it uses formaldehyde and glyoxylate for its development [20]. Another bacterial strain, Arthrobacter atrocyaneus ATCC 13,752 degrades glyphosate into AMPA and then finally to CO2, but CO2 obtained was not the by-product of AMPA [21]. The AMPA, an intermediate metabolite, gets liberated into the ecosystem or further degrades via different enzymes [22][20][23]. Generally, AMPA act as a substrate for enzyme C-P lyase, converting it to Pi and methylamine for phosphorus uptake for Arthrobacter sp. GLP-1, Arthrobacter atrocyaneus ATCC 13752, and Pseudomonas sp. LBr [24][22][25]. Lately, Ochrobactrum anthropi GPK3 isolate illustrated the new AMPA degradation pathway in which AMPA is first degraded to phosphonoformaldehyde via transaminase enzyme and then further to formaldehyde via phosphonatase (Figure 3).
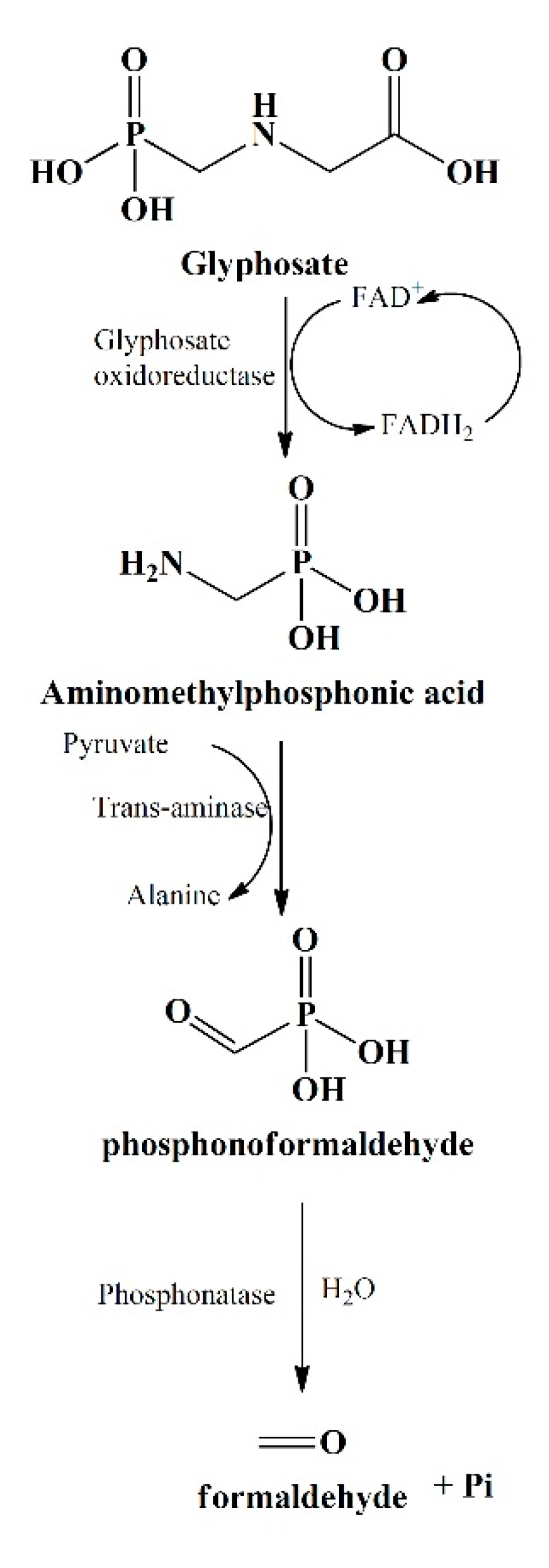
Another glyphosate degradation pathway involves C-P lyase and leads to the synthesis of Pi and sarcosine, for example, Pseudomonas sp. PG2982 metabolizes glyphosate via C-P lyase and produces sarcosine, which further gets degraded to formaldehyde and glycine by the action of sarcosine oxidase [26]. It has been found that Arthrobacter sp. GLP-1 uses glycine for the protein synthesis by stimulating the formation of amino acids (serine and threonine) and peptide backbone [22]. This fate of glyphosate metabolites has been noticeably assessed with the help of isotope labelling.
3. Conclusions
There is much more to be learned about the fate of glyphosate, including its sorption, degradation, and leaching. The fate depends upon the medium and varies a lot from soil to soil as well. This variability does not give a clear prediction, and results generate ambiguous conclusions. Apart from environmental risks, glyphosate is also associated with health risks. This makes for the requirement to develop an eco-friendly strategy for bioremediation. Glyphosate being a potent inhibitor of EPSPS in the shikimic pathway, exerts negative effects on non-target plants. Various ecosystems and their abiotic and biotic components including animals, plants, and microbes are adversely affected by the indiscriminate use of glyphosate. Right from unicellular to multicellular and lower to higher invertebrates, glyphosate affects all the animals from the kingdom. Right from the lower invertebrates up to higher chordates, all the animals are found to be affected by this herbicide.
Glyphosate can actually be degraded via two metabolic pathways—AMPA and sarcosine through glyphosate degrading bacteria. Soil organic matter also indirectly affect the sorption of glyphosate. The phosphate in soil competes with glyphosate for sorption, which ultimately affects the retention and degradation of glyphosate. The pre-sorption of phosphates almost eliminates the chances of glyphosate sorption in some soils. Though in some soils the availability of phosphate is found to accelerate the degradation of glyphosate. There is still more to it, and this gap needs to be studied further. The fate of glyphosate should be considered for studies as it is primary for environmental and health risk assessments. For optimal use of glyphosate, social costs, in addition to direct costs, should also be taken under consideration. Glyphosate is found to be an absolutely effective herbicide and should be considered for the development of new cost-effective herbicides for sustainable agriculture. There is no question that glyphosate resistant crops make glyphosate the most inexpensive and effective technology for weed management. But after its advent, with time, its effectiveness decreased and the mineral nutrition was also altered due to the capability of glyphosate to chelate with metal ions. Certain additives may be used to enhance the biological performance of glyphosate. A better understanding of glyphosate action and usage of adjuvants for overall better effect should be other approaches to designing future glyphosate formulations. There is still more to it, and this gap needs to be studied further.
References
- Gill, J.P.K.; Sethi, N.; Mohan, A. Analysis of the glyphosate herbicide in water, soil and food using derivatising agents. Environ. Chem. Lett. 2017, 15, 85–100.
- Zhang, C.; Hu, X.; Luo, J.; Wu, Z.; Wang, L.; Li, B.; Wang, Y.; Sun, G. Degradation dynamics of glyphosate in different types of citrus orchard soils in China. Molecules 2015, 20, 1161–1175.
- Conrad, A.; Schröter-Kermani, C.; Hoppe, H.W.; Rüther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults—Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16.
- Singh, S.; Kumar, V.; Datta, S.; Wani, A.B.; Dhanjal, D.S.; Romero, R.; Singh, J. Glyphosate uptake, translocation, resistance emergence in crops, analytical monitoring, toxicity and degradation: A review. Environ. Chem. Lett. 2020, 18, 663–702.
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. In Proceedings of the Pest Management Science; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2008; Volume 64, pp. 319–325.
- Dill, G.M. Glyphosate-resistant crops: History, status and future. In Proceedings of the Pest Management Science; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2005; Volume 61, pp. 219–224.
- Haslam, E. The Shikimate Pathway: Biosynthesis of Natural Products Series; Butterworths: London, UK, 2014.
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leont’evskii, A.A. Microbial degradation of glyphosate herbicides (review). Prikl. Biokhim. Mikrobiol. 2015, 51, 183–190.
- Hadi, F.; Mousavi, A.; Noghabi, K.A.; Tabar, H.G.; Salmanian, A.H. New bacterial strain of the genus Ochrobactrum with glyphosate-degrading activity. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 208–213.
- Wang, S.; Seiwert, B.; Kästner, M.; Miltner, A.; Schäffer, A.; Reemtsma, T.; Yang, Q.; Nowak, K.M. (Bio)degradation of glyphosate in water-sediment microcosms—A stable isotope co-labeling approach. Water Res. 2016, 99, 91–100.
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479.
- Hanke, I.; Wittmer, I.; Bischofberger, S.; Stamm, C.; Singer, H. Relevance of urban glyphosate use for surface water quality. Chemosphere 2010, 81, 422–429.
- Shushkova, T.; Ermakova, I.; Leontievsky, A. Glyphosate bioavailability in soil. Biodegradation 2010, 21, 403–410.
- International Agency for Research on Cancer. Some Organophosphate Insecticides and Herbicides. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2017; Volume 112, pp. 1–452.
- Niemann, L.; Sieke, C.; Pfeil, R.; Solecki, R. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J. Verbrauch. Leb. 2015, 10, 3–12.
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153.
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043.
- Grandcoin, A.; Piel, S.; Baurès, E. AminoMethylPhosphonic acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Water Res. 2017, 117, 187–197.
- Xu, X.; Ji, F.; Fan, Z.; He, L. Degradation of glyphosate in soil photocatalyzed by Fe3O4/SiO2/TiO2 under solar light. Int. J. Environ. Res. Public Health 2011, 8, 1258–1270.
- Jacob, G.S.; Garbow, J.R.; Hallas, L.E.; Kimack, N.M.; Kishore, G.M.; Schaeffer, J. Metabolism of glyophosate in Pseudomonas sp. strain LBr. Appl. Environ. Microbiol. 1988, 54, 2953–2958.
- Pipke, R.; Amrhein, N. Isolation and Characterization of a Mutant of Arthrobacter sp. Strain GLP-1 Which Utilizes the Herbicide Glyphosate as Its Sole Source of Phosphorus and Nitrogen. Appl. Environ. Microbiol. 1988, 54, 2868–2870.
- Pipke, R.; Amrhein, N. Degradation of the phosphonate herbicide glyphosate by Arthrobacter atrocyaneus ATCC 13752. Appl. Environ. Microbiol. 1988, 54, 1293–1296.
- Sviridov, A.V.; Shushkova, T.V.; Zelenkova, N.F.; Vinokurova, N.G.; Morgunov, I.G.; Ermakova, I.T.; Leontievsky, A.A. Distribution of glyphosate and methylphosphonate catabolism systems in soil bacteria Ochrobactrum anthropi and Achromobacter sp. Appl. Microbiol. Biotechnol. 2012, 93, 787–796.
- Dick, R.E.; Quinn, J.P. Control of glyphosate uptake and metabolism in Pseudomonas sp. 4ASW. FEMS Microbiol. Lett. 1995, 134, 177–182.
- Pipke, R.; Schulz, A.; Amrhein, N. Uptake of glycophosphate by an Arthrobacter sp. Appl. Environ. Microbiol. 1987, 53, 974–978.
- Kishore, G.M.; Jacob, G.S. Degradation of glyphosate by Pseudomonas sp. PG2982 via a sarcosine intermediate. J. Biol. Chem. 1987, 262, 12164–12168.
