Ericaceae are a group of plants with biotechnological and commercial importance. These plants establish symbiotic associations with a wide group of mycorrhizal fungi. National and global studies have focused on two of them: arbuscular endomycorrhizae and ectomycorrhizae. The classification of mycorrhizae by type of infection described so far in Ericaceae includes ectomycorrhizae, ectendomycorrhizae, and endomycorrhizae. Ectendomycorrhizas can be of arbutoid, monotropoid, and cavendishioid types; endomycorrhizas are of ericoid and arbuscular types. Of these clades, ectomycorrhizas and ectendomycorrhizas (arbutoid, monotropoid, and cavendishioid) form a multilayer mantle of hyphae around the root; while ericoid, unlike the previous ones, develop their intra- and intercellular structures.
1. Introduction
Ericaceae have a well-differentiated, long, thick main root and fine, fibrous, lignified secondary roots; according to the relationship between the primary and secondary roots, they belong to the pivoting type, since the primary root grows faster than the secondary roots
[1]. Its roots grow in the first 30 cm of the soil surface. However, there are exceptions within the
Vaccinium genus that do not have a taproot; instead, they have lateral roots that help absorb nutrients through a symbiotic relationship with mycorrhizae (
Figure 1)
[2].
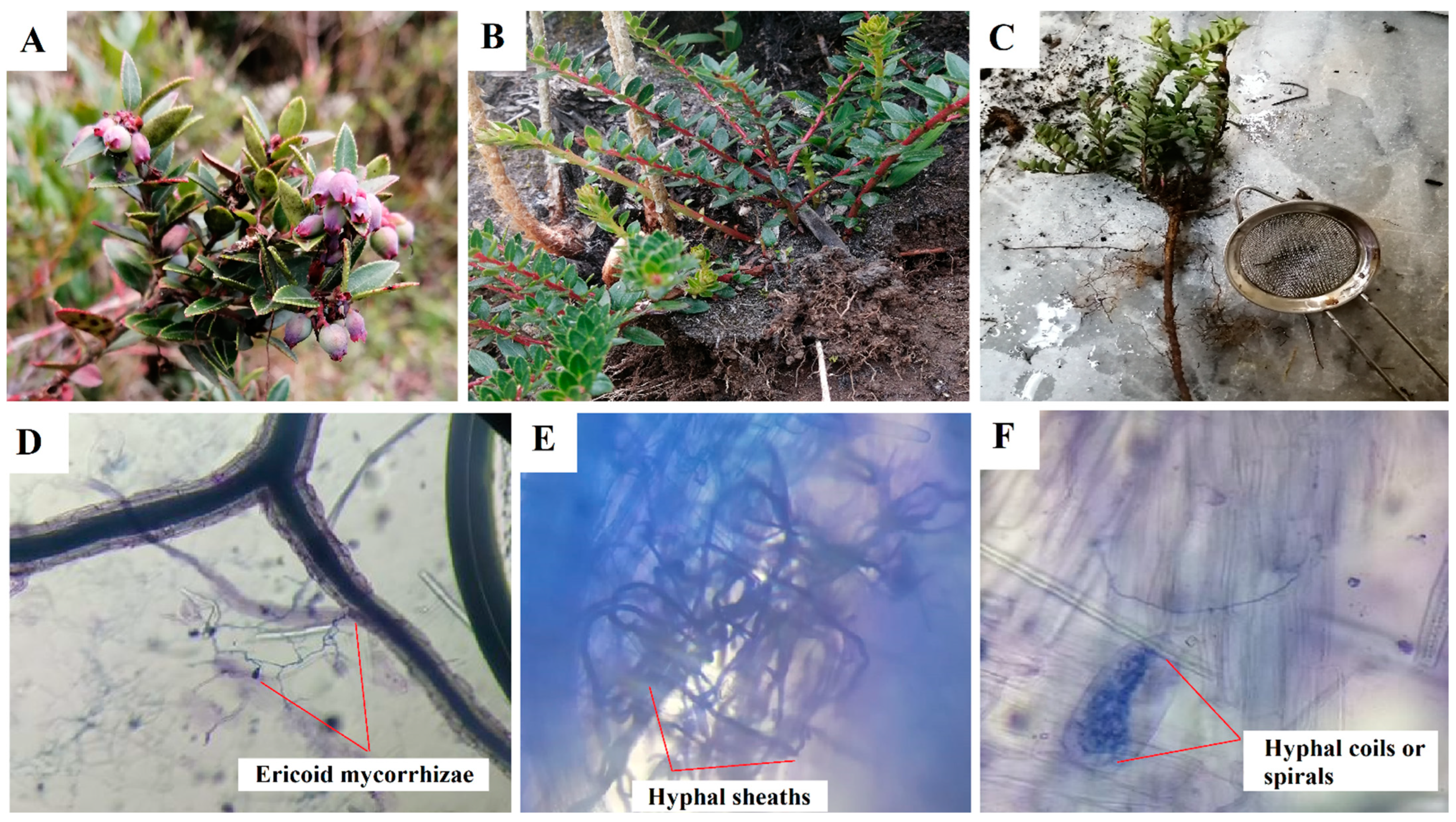
Figure 1. Blueberry plants (Vaccinium floribundum); (A) immature fruits, (B) stems; and (C) mortiño roots; (D) hyphae extending out of the root; (E,F) intracellular structures of ericoid mycorrhizae in stained roots. Pictures: J. Naranjo-Morán and R. Moreira-Gómez.
In the rhizosphere of these plants, researchers find a considerable number of microorganisms. For instance, the fungi form endotrophic mycorrhizae, through which the plants acquire part of their nutrients from recalcitrant organic matter from the soil
[3]. This type of fungi helps the roots to absorb organic nitrogen for good plant growth
[1]. Other benefits include improved resistance to drought. In some associations, plant welfare is promoted by destroying toxic phenolics and increasing tolerance to salinity or heavy metals
[4].
Some of the aspects that affect the mycorrhization of Ericaceae are locality, season, cultivar, plant age, phosphorus content, fertilization, pH, type of fungus, and its efficiency in the nutritional contribution to the consumption of photosynthates from the host, among others
[5].
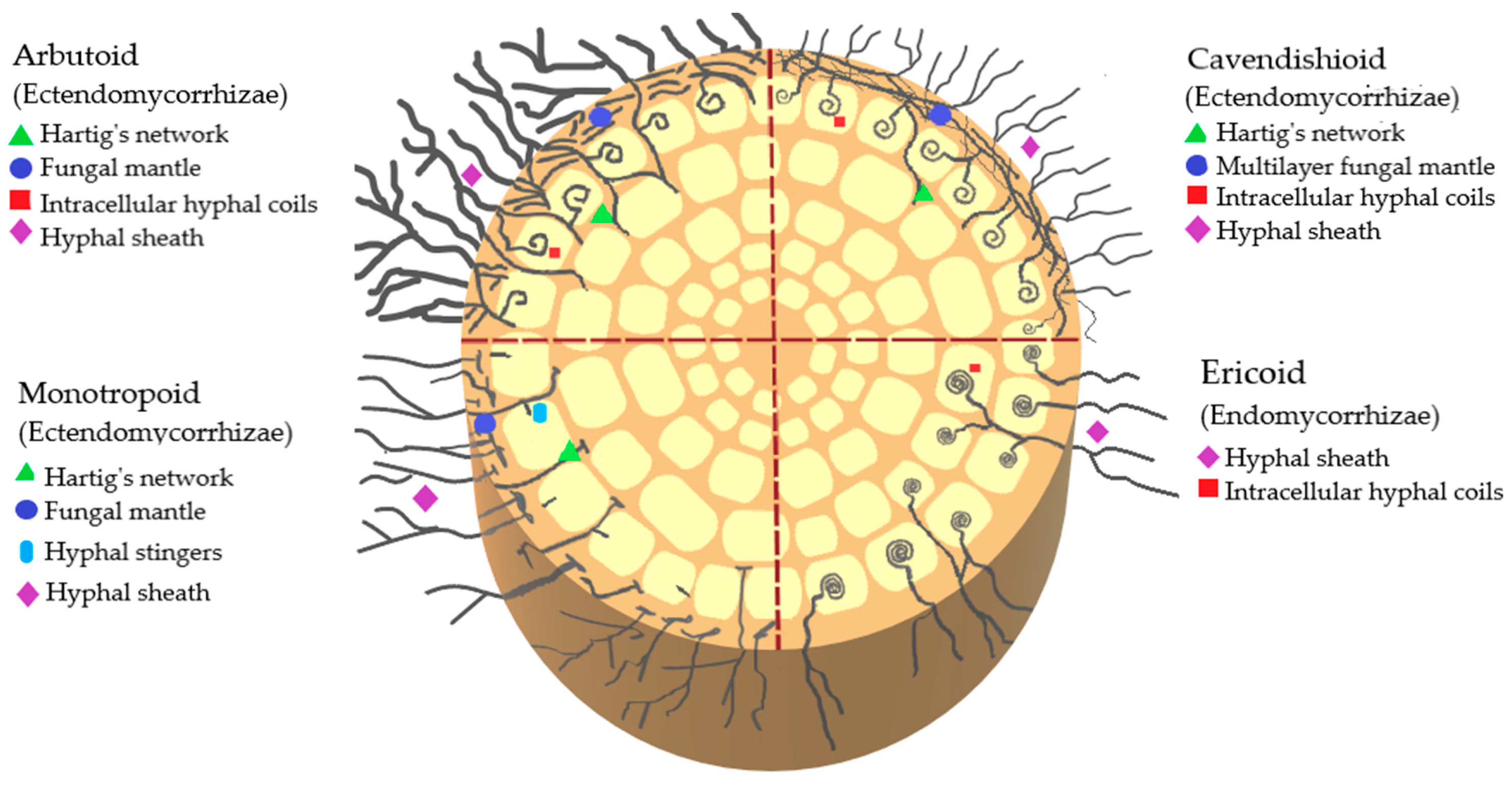
The classification of mycorrhizae by type of infection that has been described so far in Ericaceae includes ectomycorrhizae, ectendomycorrhizae, and endomycorrhizae. Ectendomycorrhizae can be of the arbutoid, monotropoid, and cavendishioid type; endomycorrhizae are of the ericoid and arbuscular type. Of these clades, the ectomycorrhizae and ectendomycorrhizae (arbutoids, monotropoids, and cavendishioids) form a multilayered mantle of hyphae around the root, while the ericoids, in contrast to the previous ones, develop their intra- and intercellular structures (
Figure 2)
[6].
Figure 2. Schematic representation of endomycorrhizae and ectendomycorrhizae that interact with ericaceous plants.
2. Ectomycorrhizae
Ectomycorrhizae are found in all subfamilies of Ericaceae, as well as in many other vascular plants, both conifers and angiosperms, and are characterized by a mantle or covering of fungal hyphae on the surface of the roots and by a Hartig network formed by fungal hyphae extending between the epidermal cells of the root
[7]. Spore banks and other resistant ectomycorrhizal fungi propagules play a determining role in facilitating the regeneration of ecosystems after alterations, providing the environment the opportunity to recover. Resistant propagules of the genera
Rhizopogon (Basidiomycetes),
Tuber, and
Wilcoxina (Ascomycetes) are abundant in the soil, and represent an important source of inoculant in the early stages of the ecosystem after a disturbance; some fungi from the same phyla colonize new roots from the extension of already-established mycelium, and others may arrive early in succession and persist in mature forests. The ability of some starter ectomycorrhizal fungi to germinate and occupy a given site depends partially on the absence of competitors given their characteristics of frequent fruiting, efficient dispersal, and the existence of long-lived spores
[8].
3. Endomycorrhizae
Most ericaceas present an ericoid mycorrhizal infection (
Figure 2). Its fundamental ecophysiological roles are the conditioning of the edaphic environment, the detoxification of the soil, and the acquisition of nitrogenous and phosphate nutrients
[9]; the symbiosis between ericaceas and ericoid mycorrhizae may differ according to the following environmental conditions: pH, availability and concentration of heavy metals, and the concentration of mineral nutrients, among others
[10].
This ericoid clade is contained in the rhizodermal cells of Ericaceae, and only their last rootlets are mycorrhized. They lack a mantle and Hartig’s network but do have hyphae
[7]. They tend to form coils or spirals of hyphae on the outermost part of the root hairs of host cells where nutrient exchange occurs. The penetration of their hyphae and their coiling within the host’s epidermal cells increases the surface area through which nutrients can be transferred. Ericoid mycorrhizae are short-range from the surrounding soil and harbor a narrow taxonomic range within ascomycetes such as
Rhizoscyphus ericae,
Meliniomyces sp.,
Oidiodendron sp., and basidiomycetes of the Sebacinaceae family
[3].
Plants with ericoid mycorrhizae are common in heathlands, tundra, and boreal and neotropical forests. They can coexist with trees that establish symbiosis with ectomycorrhizae
[11]. This type of mycorrhiza does not show host specificity, and roots of ericoid plants are different in their coexisting genotype; they often harbor similar ericoid mycorrhizal communities
[10]. Some ericaceous genera establish symbiosis with ericoid mycorrhizae in Zamora-Chinchipe, Ecuador, such as
Vaccinium,
Bejaria, and
Gaultheria [12]. Some authors claim that the effectiveness of this mycorrhization decreases with altitude for low temperatures present and depends on local soil conditions
[9]. The symbiosis of ericoid mycorrhizae with the
Vaccinium genus promotes up to 20% development of stakes rooted at 90 days and approximately 40% at 120 days in non-natural growth sites
[13].
Carrillo et al., (2015) proposed an effective mycorrhization option by mixing an inoculum of ericoid mycorrhizae collected from three different edaphoclimatic conditions in southern Chile with rootlets from Vaccinium corymbosum seedlings, micropropagated in the nursery for six months. Despite not detecting an effect on seedling growth of the three blueberry cultivars during this evaluation period, the use of ericoid inocula obtained from ericaceous plants was shown to be an effective mycorrhization option to improve the acclimatization and establishment of blueberry under different field conditions. In addition, Gonçalves et al., (2015) in their research with blueberry plants, concluded that the inoculation of ericoid fungi has a clear potential to stimulate plant growth, the absorption of nutrients such as Mn, Mo, K, Fe, and Cu, and its accumulation in roots and stem. However, the efficacy of these fungi appears to depend on the cultivar.
In a comparative study, native mycorrhizae of
Gaultheria sp. Were inoculated in plants of
Vaccinium, showing a positive effect on their height, number of leaves, frequency, and intensity of root colonization; unlike other sources of mycorrhizae, the commercial product EndosporR did not have a representative effect on the growth and colonization variables
[14].
Kottke et al., (2008) hypothesized that identical species of Sebacinales or Tulasnellales could link epiphytic orchids with ericaceous hemiepiphytes through mycorrhizal associations. Sequence analyses showed that the Sebacinales and Tulasnellales were shared only within the Ericaceae and the orchids, but not between them, i.e., common Basidiomycota guilds probably only exist between plants in the same family; their study also revealed that the sebacinaceous mycobionts of ericaceous plants from Ecuador and Canada showed different sequences.
4. Ectendomycorrhizae
The arbutoid clade resembles the ectomycorrhizae, both in its morphology and its fungal classification. Trees with ectomycorrhizae can establish dual mycorrhizae or double colonization with the arbutoid type, such as the roots of some ericaceous of the subfamily Arbutoideae and some members of the subfamily Pyroloideae; it has been reported that families of basidiomycetes such as Sebacinaceae, Clavulinaceae, Thelephoraceae, and some species of the genera
Cortinarius,
Inocybe,
Russula, and
Laccaria can form double mycorrhizal colonization in their hosts
[12][15].
Furthermore, this type of arbutoid mycorrhizae involves the formation of a mycelium cover around the root. It has extensive intracellular colonization, a sheath of hyphae, and a Hartig network restricted to the outermost layer of cortical cells. They have also developed spirals of hyphae within the epidermal cells of the host to facilitate the transfer of nutrients between plants and fungi
[15]. The arbutoid clade is present in Ericaceae that form thin roots or absorbing hairs, specifically the genera
Arbutus and
Arctostaphylos [12]. Arbutoid clade improves seedlings’ ability to resist drought and other negative climatic variations, favoring their establishment and facilitating the transfer of nutrients between the symbiosis
[7]. Another of its benefits is its importance as a fundamental biological element of plant ecological successions, such as those responsible for directing the transition process to the climax stage of the adult forest
[15].
The cavendishioid clade is like the arbutoid in its morphology, as it has a hyphal sheath, a Hartig network, intracellular hyphal coils, and forms multilayered hyphal mantles
[16], but like the ericoid in their fungal association and plant affiliation, since it is formed in hemiepiphytes of Ericaceae associated with the Vaccinoideae subfamily. The Fungi that form cavendishioid mycorrhizae are the same as those that form ericoid mycorrhizae
[12]. Cavendishioid-type mycorrhizae belong to the Neotropics and are present in plants of the Ericaceae family. For instance, in the San Francisco Biological Reserve, in Zamora-Chinchipe, Ecuador, this type of mycorrhizae has been found in the genera
Cavendishia,
Ceratostema,
Diogenesia,
Macleania,
Orthaea,
Psammisia,
Sphyrospermum, and
Thibaudia [17].
The monotropoid clade is distributed in temperate zones; its symbiosis is established between certain ascomycetes or basidiomycetes with ericaceous species of the genus
Monotropa [6]. Ericaceae affiliated with the monotropoid clade are not photosynthetic; while they form a mantle of several capable hyphae around the root, their hyphae do not colonize cortical cells as occurs in arbutoid mycorrhizae, but instead establish a structure that grows within the cell wall, increasing its surface, but without penetrating the cell. In this case, the fungus does not receive nutrients from the plant, but rather feeds it with sugars from neighboring photosynthetically active trees that also form ectomycorrhizae. This fungus takes advantage of common mycorrhizal networks, allowing them to colonize shady forest habitats. Exogenous carbohydrates and phosphorus are transported from neighboring trees to the Ericaceae, for they are epiparasites. Since this is not a mutual symbiosis, it is not a mycorrhiza in the classical sense
[18].