Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nadeem Rais and Version 4 by Jessie Wu.
Stem cells are a well-known autologous pluripotent cell source, having excellent potential to develop into specialized cells, such as brain, skin, and bone marrow cells. The oral cavity is reported to be a rich source of multiple types of oral stem cells, including the dental pulp, mucosal soft tissues, periodontal ligament, and apical papilla. Oral stem cells were useful for both the regeneration of soft tissue components in the dental pulp and mineralized structure regeneration, such as bone or dentin, and can be a viable substitute for traditionally used bone marrow stem cells. The role of bioactive compounds in oral stem cell-meditated regeneration is discussed.
- oral stem cells
- ginsenoside Rg1
- apigenin
1. Oral Stem Cell-Mediated Dental Regeneration
Recent advances in stem cell-mediated regeneration have shifted the focus of clinical dentists to regenerative endodontics, an alternative approach for the regeneration of dental tissues via stem cells, indicating a paradigm shift from artificial to biological replacement. The potential of dental pulp stem cells (DPSCs) as seed cells for periodontal tissue regeneration, dentin pulp, and bone is being widely studied these days [1][2][3][4][5][93,94,95,96,97]. In a recent study, the formation of rich blood vessels in pulp-like tissues was observed within 6 weeks of implantation of a 3-dimensional (3D) scaffold-free dental pulp stem cells construct in the human root canal [6][98]. Histologic analyses in this study confirmed positive endothelial cells (human CD31) present at the center of regenerated tissue [6][98]. This study demonstrated the potential of pulpal tissue regeneration by DPSCs constructed in the pulpless tooth [6][98].
A number of studies have confirmed that DPSCs’ subcutaneous transplantation mixed with nanofibrous poly-L-lactic acid or tricalcium phosphate/hydroxyapatite could differentiate into odontoblasts and form dentin, including vascularized pulp-like tissue in immunodeficient mice [7][8][9][10][11][1,99,100,101,102]. Similarly, subcutaneous transplantation of exfoliated deciduous teeth (SHEDs)SHEDs, with collagen type 1, hydrogel, or PLLA, resulted in the formation of pulp-like tissues, including odontoblasts and blood vessels in immunocompromised mice [12][13][14][103,104,105]. In an in vivo study on a canine periodontitis model, regeneration of periodontal ligament tissue and cementum was observed post-transplantation of DPSCs with bone granules into periodontal defects [15][106].
In another study, subcutaneous transplantation of DPSCs mixed with human salivary gland cells in an immunocompromised mouse resulted in enhancing the differentiation potential of human salivary gland cells to convert into functional salivary gland tissue [16][107]. In a study on pulp necrosis in human patients, DPSC implantation resulted in the regeneration of complete three-dimensional dental pulp tissue equipped with blood vessels and sensory nerves post 12 months of DPSC implantation [17][108].
Similarly, a pilot clinical study on irreversible pulpitis transplantation of mobilized DPSCs, along with atelocollagen granulocyte colony-stimulating factor into pulpectomized teeth, resulted in the formation of functional dentin in three out of the five patients studied [18][109]. A comparative study, analyzing the regenerative potential of DPSCs/HGF (hepatocyte growth factor)—DPSC injections and DPSC/HGF-DPSC sheets in repairing/regenerating periodontal bony defects in the upper and lower first molars of miniature pigs, revealed that periodontal regeneration/bone formation after grafting DPSC/HGF-DPSC sheets were higher after 12 weeks, compared to DPSC/HGF-DPSC injections [19][110]. Hu and coworkers findings on a similar model further corroborated the finding of Cao et.al that bone regeneration volume of 52.7 ± 4.1 mm3 in the case of DPSC sheets was significantly higher compared to the DPSC injections group, where bone regeneration volume was 32.4 ± 5.1 mm3 [19][20][110,111].
Similarly, in another study utilizing minipigs as a model system, stem cells derived from periodontal ligament (PDLSCs), when seeded in Nanohydroxyapatite/chitosan/gelatin (nHA/CG) three-dimensional porous scaffolds, were able to significantly regenerate large bones with normal architectures and vascularization in jawbone defects within 12 weeks of implantation [21][112]. Several studies, which include pilot studies, pre-clinical studies, as well as clinical trials conducted on different in vivo models, have highlighted the regenerative potential of PDLSCs in periodontal complex regeneration, along with confirming the safety aspect of their use for therapeutic interventions in humans [22][23][24][25][113,114,115,116]. Recent studies conducted on xenogenic and autologous models have demonstrated the potential of Titanium implants with PDLSC sheets on the surface to stimulate the formation of periodontal ligament-like tissues and cementum-like tissues [26][27][117,118].
In recent preclinical studies, Enukashvily and coworkers demonstrated the efficiency of a unique approach in regenerative dentistry, utilizing an anatomical prototype-a mold representing the similar size and shape as the bone defects formed by 3D printing, and this 3-dimensionally printed form was then filled with a DPSC suspension and fibrin glue for repairing bone defects in a mice model. It was observed that the viability, immunophenotype, and osteogenic potential of DPSCs was maintained when DPSCs embedded in the fibrin glue were utilized for repairing bone defects. Another advantage of this approach was that apart from obtaining cell-containing implants similar to bone defects, it also allows cell migration and proliferation, and is hard enough to maintain its shape. Another advantage of this 3D-printed form for molding implants approach was that it could be utilized in implant formation with components that were usually not suitable for 3D printing. This technology could be utilized for the repair of bone tissue in dental medicine and maxilla-facial surgery [28][119]. In a minipig model of periodontitis, tissue defects were effectively restored 12 weeks after minipigs were subjected to local injection of SCAPs [29][120].
In a first of a kind study, PDLSCs from the same vial were differentiated into all three lineages i.e., fibrogenic, cementogenic, and osteogenic. Differentiation of PDLSCs in the suitable fibrogenic medium resulted in high expressions of periodontal ligament fibrogenic genes, such as elastin, FSP-1, COL1, and COL3 at 28 days, with improvement in expression levels up to 20–70 fold, as compared to controls. Similarly, high expression of cementum genes (CEMP1, CAP, and BSP) and osteogenic genes (ALP, RUNX2, OPN) in PDLSCs was observed upon differentiation in a suitable cementogenic or osteogenic medium respectively. This study confirmed the differentiation of PDLSCs into fibrinogen, cementum, and bone-forming cells, thereby highlighting its potential for periodontium regeneration to form the bone-periodontal ligament–cementum complex [30][121].
The therapeutic potential of gingiva-derived mesenchymal stem cells (GMSCs) in the periodontitis treatment was demonstrated in a study conducted by Liu and coworkers on the periodontitis model created in mice that were apolipoprotein E-deficient (ApoE−/−) with hyperlipidemia. In the study, it was observed that systematic transplantation of GMSCs in the tail vein in mice could significantly attenuate hyperlipidemia and inflammatory responses after nine weeks of transplantation, as demonstrated by a significant downregulation in mRNA expression levels of triglyceride (TG), interleukin (IL)-6, low-density lipoprotein cholesterol (LDL), sterol regulatory element binding protein-1c (SREBP-1c), alveolar bone loss (ABL) and total cholesterol (TC), whereas a significant upregulation was observed in the expression level of peroxisome proliferator-activated receptor α (PPARα), high-density lipoprotein cholesterol (HDL), and IL-10, as compared to control groups. On analysis of interradicular region histological changes, it was revealed that during weeks 1 and 2 post-transplantation, both control and GMSC-treated groups had deep periodontal pockets, attachment loss, inflammatory cell infiltration, and severe alveolar bone destruction, as confirmed by hematoxylin and eosin staining. However, this situation was reversed to a significant extent in the GMSC-treated group 4 weeks after transplantation, with lesser attachment loss, higher alveolar bone heights, periodontal pocket depth, and inflammatory cell infiltration. These findings highlight the therapeutic potential of GMSCs in improving periodontitis pathological conditions and promoting alveolar bone regeneration [31][52].
Figure 1
shows a diagrammatic representation of different types of oral stem cell-mediated regeneration.
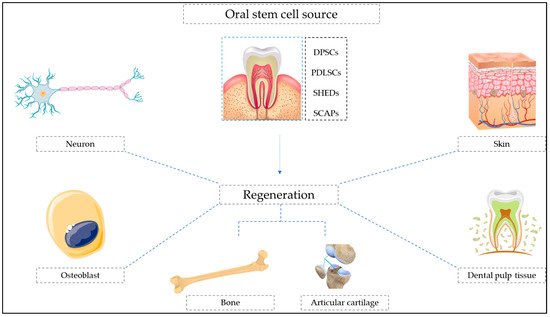
Figure 1.
Diagrammatic representation of oral stem cell-mediated regeneration.
2. Botanicals in Oral Stem Cell-Mediated Regeneration
The interactions of natural plant extracts with stem cells have gained immense interest from researchers worldwide in recent times, as several studies have highlighted the ability of natural plant extracts/phytochemicals to promote survival, proliferation, as well as differentiation, of various mesenchymal stem cells [32][33][34][35][36][37][38][39][40][41][175,176,177,178,179,180,181,182,183,184]. In a recent study, curcumin, by increasing the early growth response protein 1 (EGR1) expression and activation of PI3K, Nrf2, and AKT signaling pathway, has been reported to promote the osteogenic differentiation potential in human PDLSCs [42][43][185,186]. In an in vitro study on PDLSCs’ osteogenic differentiation potential, it was revealed that advanced glycation end products resulted in attenuation of PDLSCs’ osteogenic differentiation ability via activation of canonical Wnt/β-catenin pathway; however, berberine hydrochloride, an isoquinoline alkaloid isolated from Berberis vulgaris, was able to reverse the PDLSCs’ osteogenic potential inhibition in an AGEs-enhanced microenvironment, partly by inhibition of the β-catenin and canonical Wnt pathway. Results show that berberine hydrochloride could be a potential therapeutic intervention for promoting PDLSCs’ osteogenic differentiation in diabetes-associated periodontitis patients [44][187].
Application of berberine along with SCAPs in a rat model with apical periodontitis in immature teeth, resulted in the formation of more tissues with longer roots, smaller apex diameters, and thicker root walls. SCAP osteogenesis was enhanced by berberine in a time and concentration dependent manner. Berberine was also found to be responsible for inducing the β-catenin expression and enhancing β-catenin entry in the nucleus, upregulating nuclear factor 2 downstream. Root repair enhancement in the case of immature teeth with apical periodontitis by the activation of β-catenin and canonical Wnt pathway in SCAPs was also reported in this study [45][188]. Similarly, berberine was also reported to increase the cell proliferation of DPSCs in a dose-dependent manner and promote dexamethasone-induced osteogenic differentiation via enhancement of Runx2 transcription factor activity that was followed by upregulation of osteogenesis marker expression. This study also confirmed EGFR and MAPK pathways’ role in promoting osteogenic differentiation of DPSCs by berberine, as both of the pathways were activated by berberine and inhibition of these pathways by inhibitors was responsible for significant suppression of the osteogenic differentiation promotion potential of berberine [46][189]. In addition to the role of Berberine in promoting osteogenic differentiation via different mechanisms in PDLSCs, SCAPs, and DPSCs in a dose dependent manner, the utility of antioxidant berberine has been widely reported for attenuating H2O2-induced apoptotic cell death of stem cells via quenching ROS production and increasing SOD activity. This study also revealed that Berberine, via upregulation of expression level of Bcl-2 and p-Akt, and downregulating the expression levels of Bax and cleaved caspase-3, could significantly reduce oxidative stress-induced apoptosis of stem cells [47][190]. The polyphenolic compound 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside (THSG) extracted from the dried tuber of Reynoutria multiflora (Thunb.) Moldenke, with strong antioxidant and free radical scavenging activities when supplemented in hDPSCs, could significantly enhance the renewal ability and proliferative potential of hDPSCs via the AMPK/ERK/SIRT1 axis [48][191].
The extracts of Sapindus mukorossi have been widely studied due to their wide range of pharmacological activities such as antioxidant, free radical scavenging, anti-inflammatory, anti-tumor, antifungal, and antimicrobial activities [35][49][50][178,192,193]. The seed oil of Sapindus mukorossi was reported to enhance the odontogenic/osteogenic differentiation potential of DPSCs by upregulation of ALP gene expression and mineralization-related extracellular vesicle secretion [32][175]. In an in vitro study on fraxinellone, commonly isolated from Dictamnus dasycarpus, it alleviated inflammation and promoted lipopolysaccharide-stimulated PDLSCs osteogenic differentiation via regulation of the BMP2/Smad pathway, thereby demonstrating its potential for clinical application in periodontitis treatment [51][194]. Rutin, a flavonoid with antioxidant and anti-free radical effects present in different fruits and vegetables, was responsible for the activation of AKT, mTOR, and PI3K signaling pathways via G protein-coupled receptor 30. AKT, mTOR, and PI3K signaling pathway activation by rutin resulted in a significant enhancement in PDLSCs’ potential for osteogenic proliferation and differentiation [52][195].
Apart from their role in PDLSC osteogenic differentiation and proliferation, rutin and resveratrol were reported to protect PDLSCs from the osteogenesis damage induced by TNF-α [53][54][196,197]. In recent in vitro and in vivo studies, the antioxidant and osteogenic potential of revestrol was demonstrated, with a concentration of 10 μM significantly increasing the cell viability of hDPSCs, via reduction in ROS activity, increasing the superoxide dismutase enzyme activity and glutathione concentration. The antioxidative and osteogenic potential was further confirmed by increased mRNA expression of Runx2, OCN, Sirt1, and Nrf2 genes [55][198]. This study also demonstrated that revestrol injected intraperitoneally in a Kunming mice model could significantly enhance the collagen and bone matrix formation, along with increased expression of Sirt1 gene [55][198]. Osthole, a coumarin derivative usually found in Cnidium monnieri with antioxidant properties, has been reported to effectively restore defects in osteogenic differentiation of PDLSCs via epigenetic modification. Osthole works by upregulating MOZ and MORF, histone acetylases that are responsible for specifically catalyzing the acetylation of histone3 lisine14 (H3K14) and histone3 lisine9 (H3K9), which act as a regulator for PDLSC osteogenic differentiation. This study also confirmed that Osthole with a 10−7 Mol/L concentration was best suited for the PDLSC proliferation and differentiation [56][199]. The antioxidant role of Osthole was highlighted in other study, as it could protect oxidative damage in stem cells via the PI3K/Akt-1 Pathway [57][200].
The utility of antioxidant naringin in promoting PDLSCs’ osteogenic differentiation and proliferation in both in vitro as well as in vivo models was reported, as it upregulates the expression levels of bone-related genes including COL1A2, OCN, RUNX2, and OPN at a concentration of 1 μM. Similarly, in an in vivo study on beige mice, the application of 1 μM naringin concentration resulted in the formation of a typical trabecular structure [58][201].
The odontoblastic differentiation of DPSCs was stimulated by shikonin via AKT–mTOR signaling pathway and CD44 antigen [59][202]. Isonymphaeol B, a prenylflavonoid isolated from Macaranga tanarius could stimulate the differentiation of DPSCs into odontoblasts, along with the formation of the tooth root and dentine via the MAP kinase and AKT signaling pathway. In vitro calcium mineral deposition was also enhanced upon application of Isonymphaeol B with DPSCs [60][203]. Modulation of Wnt/β-catenin signaling pathway and other key osteoblast-secreted proteins such collagen 1A1 and osteocalcin by ferutinin resulted in the activation and promotion of DPSCs’ osteogenic differentiation [61][204].
Concanavalin A (ConA) a lectin derived from the Canavalia ensiformis plant was reported to increase the osteogenic proliferation and differentiation potential of DPSCs, with 5 and 10 µg/mL of concentration. This study suggested that an increase in bone morphogenic protein-2 (BMP-2) by Concanavalin A can be attributed to the enhancement of osteogenic differential potential of DPSCs. The addition of Concanavalin A in DPSC cultures with a suitable osteogenic medium resulted in increased mineralization [62][205]. Purified ginsenoside Rg1 from stem or root of ginseng was reported to promote PDLSC and DPSC proliferation, PDLSC osteoblast differentiation, and odontoblast differentiation in DPSCs, thus making it a promising candidate for application in maxillofacial and oral regenerative medicine [63][206].
Ginsenoside Rg1 treatment promoted differentiation and proliferation of DPSCs via an increase in the expression level of genes, such as alkaline phosphatase (ALP), bone morphogenic protein-2, osteocalcin (OCN), fibroblast growth factor 2, and dentin sialophosphoprotein (DSPP) [64][65][207,208]. The osteogenic differentiation potential of PDLSCs is enhanced by ginsenoside Rg1 with 10 µmol/L of concentration, by enhancing the expression level of osteopontin (OPN), RUNX2, osteocalcin (OCN), collagen I, and ALP. However, at a concentration exceeding 100 μmol/L of ginsenoside Rg1, it was responsible for the inhibition of cell proliferation [66][209]. Wedelolactone, an antioxidant commonly occurring in Wedelia calendulacea and Eclipta alba, was capable of inducing significant odontoblast differentiation in DPSCs via neuropilin-1 (NRP1) and semaphorin 3A (Sema3A) pathway-mediated β-catenin activation, and inhibition of nuclear factor kappa B signaling pathway [67][210].
Application of acemannan, ‘a polysaccharide isolated from Aloe vera’, in the partial pulpotomy treatment revealed that it could stimulate DPSCs for the formation of predominantly normal pulp tissue organization and complete mineralized bridge covering the exposure site. In the case of teeth with reversible pulpitis, dentin regeneration was stimulated upon application of acemannan. This study also revealed that in vitro DPSC proliferation was significantly enhanced by acemannan, along with an increase in expression levels of type I collagen, BMP-2, vascular endothelial growth factor, BMP-4, alkaline phosphatase and dentin sialoprotein, and mineralization [68][211]. Epigallocatechin gallate (EGCG), which acts as an antibacterial crosslinking agent, resulted in the promotion of proliferation and differentiation potential of DPSCs in collagen scaffolds, suggesting its potential utility for regenerative endodontic therapy [69][212].
Asarylaldehyde treatment resulted in PDLSC osteogenic differentiation by increasing the mRNA expression level of osteoblast-specific markers in PDLSCs. Regulation of the transcriptional activity of alkaline phosphatase (ALP) via the p38/extracellular-signal-regulated kinase (ERK) signaling pathway was also observed upon asarylaldehyde treatment in PDLSCs [70][213]. Flavonoid kaempferol at a concentration of 10−6 M was reported to promote proliferation and mineral deposition of PDLSCs. It also upregulated expression levels of Bone Gamma-Carboxyglutamate Protein (BGLAP; osteocalcin), ALP, β-catenin, RUNX Family Transcription Factor 2, and Sp7 Transcription Factor (SP7; Osterix), with all factors contributing to the enhancement of proliferation and osteogenesis of PDLSCs, by activation of the β-catenin and Wnt signaling pathway [71][214]. The upregulation of early growth response gene-1 by Betulinic acid resulted in enhanced hPDLSCs osteogenic differentiation [72][215].
Flavonoid quercetin was able to attenuate the suppression of osteogenesis related genes, ALP and protein activity induced by TNF-α and mineralized matrix in PDLSCs via inhibition of NF κB/NLRP3 inflammasome pathway, thereby reversing TNF-α mediated osteogenic damage to PDLSCs [73][216]. Treatment of quercetin at concentrations of 2 and 5 μM significantly upregulated the antioxidant enzymes SOD1 and SOD2 in stem cells and increased the viability of cells [74][217]. The study also demonstrated the potential of quercetin in promoting osteogenic differentiation of stem cells via enhancing the phosphorylation of AMPK protein and upregulating the expression of the SIRT1 signaling pathway [74][217].
Methanolic extract of Cirsium setidens (Dunn) Nakai at a concentration of 0.05% significantly increased the viability of PDLSCs after 48 h of incubation. It was also responsible for enhancing the mineralization potential of PDLSCs. Enhancement of osteogenic potential in PDLSCs can be attributed to an increase in the expression level of alkaline phosphatase, collagen 1, runt-related transcription factor 2, and bone sialoprotein [75][218]. Salidroside isolated from Rhodiola rosea roots promoted DPSC cell viability, along with promoting their differentiation into odontogenic and osteogenic linage via activation of the BMP signaling pathway [76][219].
The flavonoid puerarin, with strong antioxidants in a concentration-dependent pattern, increased the activity of the ALP of PDLSCs, thereby enhancing the potential of osteogenic differentiation in PDLSCs [77][220]. Puerarin enhanced the osteogenic differentiation potential and viability of rat dental follicle stem cells via regulating the nitric oxide (NO) pathway [78][221]. The expression level of soluble guanylate cyclase (SGC), osteocalcin (OC), protein kinase G 1 (PKG-1), runt-related transcription factor 2 (RUNX2), collagen I, and osteopontin (OPN) was increased, along with an increase in activity of alkaline phosphatase (ALP), nitric oxide, and cyclic guanosine monophosphate upon treatment of puerarin with rat dental follicle stem cells [78][221].
PDLSCs’ osteogenic proliferation and differentiation was stimulated by Icariin in a dose dependent manner, having maximum stimulation at 0.1 μg/mL of concentration, however concentration of icariin above 10 μg · mL−1 proved to be cytotoxic [79][80][222,223]. In another study, application of icariin in a mouse model of calvarial defects and senescence increased trabecular bone mineral density and improved bone mass [80][223]. Treatment of PDLSCs with Moringin, an isothiocyanate obtained from Moringa oleifera seeds, resulted in the induction of PDLSC differentiation to neural progenitor cells via increased gene expression levels of genes that were involved in neuron cortical development, and specifically in neurons from the upper and deep cortical layers, as confirmed by next-generation transcriptomics sequencing. Apart from moringin’s role in neural progenitor cell differentiation of PDLSCs, it was also reported to upregulate genes involved in osteogenesis and adipogenesis, however to a much lower fold, as compared to neuronal differentiation [81][224].
Activation of sirtuin 1 (SIRT1) by resveratrol has been reported by several studies to induce bone marrow derived MSC structural morphological change and neuronal differentiation [82][225], dental pulp [83][226], cord blood [84][227], SCAPs [85][228], and umbilical cord [86][229] into the neural cells. Treatment of 15 μM resveratrol with DPSCs was capable of inducing NES gene expression, responsible for coding Nestin protein, that has been reported for their higher expression level of progenitor neural cells present in the subventricular zone of the human brain [87][230]. Similarly, pre-treatment with resveratrol with SCAPs resulted in the induction of neural progenitor marker gene expression, ultimately enhancing the induction of neural progenitor-like cells via a synergistic mechanism when provided with a suitable neuronal induction medium [85][228]. The aqueous leaves extract of Acacia nilotica promoted chondrogenesis induction in DPSCs by upregulating the expression of various proteins in the cellular matrix, such as aggrecan, sox9, glycosaminoglycan (GAG), and collagen 2α1 (Col2α1) that are crucial for the formation of cartilage tissue [88][231].
Girija and coworkers, in their study on GMSCs, highlighted the potential of Acalypha indica in increasing the wound healing ability of GMSCs. It was observed that wound closure activity of GMSCs was increased up to 56.91 ± 1.21% in 24 h when GMSCs were treated with 25 µg/mL concentration of methanolic extract of Acalypha indica, while the percentage of wound closure was further enhanced to 89.23 ± 1.09 % post 48 h of treatment. After induction with Acalypha indica, the morphology of GMSCs changed to polygonal cobblestone, which is a characteristic feature of keratinocyte-like cells. The transdifferentiation of GMSCs into keratinocyte-like cells was accompanied by a multifold increase in the expression levels of cytokeratin 5, cytokeratin 10, cytokeratin 14, involucrin, and filaggrin, indicating the potential of Acalypha indica and GMSCs as a promising alternative strategy for skin tissue engineering [89][232].
Pretreatment of GMSCs with nanostructured liposomes enriched with moringin for therapeutic intervention in spinal cord injury has shown promising results in a study conducted by Mammana et. al. The findings of this study revealed that the group receiving GMSCs–Moringin showed significantly faster recovery of motor function from day 4 post injury in ICR (CD-1) mice models of spinal cord injury, while the recovery in the group receiving GMSC treatment was a bit slower i.e., 8th day post trauma, thereby demonstrating the role of moringin in enhancing the therapeutic efficacy of GMSCs [90][54].
Treatment of hDPSCs with antioxidant baicalein, a flavonoid isolated from Scutellaria baicalensis, resulted in the upregulation of angiogenic factors and increased in vitro capillary-like tube formation. The upregulation of bone morphogenetic protein (BMP)-2 mRNA and phosphorylation of Smad 1/5/8 and Wnt ligand mRNA, glycogen synthase kinase-3, and nuclear β-catenin by baicalein treatment results in the promotion of angiogenesis and odontoblastic differentiation in hDPSCs via activation of the BMP and Wnt/β-catenin signal pathway [91][233]. Another study on baicalein conducted by Tian and coworkers highlighted its role as an effective hydroxyl radical-scavenger in protecting bone marrow-derived mesenchymal stem cells from hydroxyl radical-mediated oxidative stress, probably via two different mechanisms i.e., hydroxyl radicals direct scavenging via electron transfer, direct scavenging of hydroxyl radicals, possibly through electron transfer, and indirect inhibition of hydroxyl radical generation via Fe2+ chelation through the 4-keto-5,6,7-trihydroxy groups [92][234].
Apigenin, a natural flavonoid with strong antioxidant, and anti-inflammatory properties, could upregulate several osteogenesis-related signaling molecules, such as osteocalcin (OCN), bone sialoprotein (BSP), runt-related transcription factor 2 (RUNX2), BMP2, BMP4, and BMP7 in hDPSCs post 14 days of treatment [93][235]. This study also confirmed the role of apigenin in facilitating dentin-bridge formation with few irregular tubules in mice pulpal cavities after 42 days of treatment with apigenin [93][235]. Tanshinone IIA is a diterpene quinone derived from Salvia miltiorrhiza, and has been reported to induce hPDLSC osteogenesis via the ERK1/2-Runx2 axis signaling pathway [94][236]. In another study on improving the odontogenic differentiation potential of hDPSCs, both bakuchiol, a meroterpene phenol of Cullen corylifolium, and C. corylifolium extract were able to significantly enhance the odontogenic differentiation potential of hDPSCs via the upregulation of several odontogenic differentiation marker genes, such as dentin matrix acidic phosphoprotein-1, alkaline phosphatase, osteocalcin, and Runt-related transcription factor 2 [95][237].
Table 1 shows relevant case studies on the role of bioactive compounds in management of oral stem cell-mediated regeneration.
Table 1.
Role of bioactive compounds in management of oral stem cell-mediated regeneration.
| Source | Bioactive Compounds | Type of Study | Major Findings and Mechanism of Action | References |
|---|---|---|---|---|
| Artemisia annua | Artemisinin (Sigma Aldrich, St. Louis, MO, USA) |
In vitro study investigated the effect of Artemisinin on hypoxia and TNF-α mediated osteogenesis impairment in DPSCs | Artemisinin reversed the suppression of cell survival caused by hypoxia or inflammation in DPSCs, along with restoring the osteogenic differentiation potential of DPSCs | [96][91] |
| Sapindus mukorossi | Seed oil (He He Co., Ltd., Taipei, Taiwan) |
In vitro study to examine the effects of S. mukorossi (seed oil) on the differentiation and proliferation of DPSCs | Enhanced the odontogenic/osteogenic differentiation potential of DPSCs by upregulation of ALP gene expression and mineralization-related extracellular vesicle secretion | [32][175] |
| Curcuma longa | Curcumin (Sigma Aldrich, St. Louis, MO, USA) |
In vivo study on effect of curcumin on hPDLSCs osteogenic differentiation | Curcumin increased protein and mRNA levels of COL1, ALP, RUNX2, and activated PI3K/AKT/Nrf2 signaling pathway | [42][185] |
| Curcuma longa | Curcumin (Solarbio Life Sciences, China) |
In vivo study Curcumin displays promoting osteogenic differentiation and its mechanism |
Curcumin 10 µmol/L treatment maximal promoting the cells viability, ALP activities, mineralization, and levels of Runx2, OC, OPN, Collagen I, and EGR-1 in hPDLSCs |
[43][186] |
| Berberis vulgaris | Berberine hydrochloride (Wako Pure Chemical Industries, Ltd., USA) |
In vitro study to examine effects of AGE and berberine hydrochloride on the hPDLSCs’ osteogenic differentiation ability | Berberine hydrochloride was able to reverse the inhibition of the PDLSCs’ osteogenic potential in an AGEs enriched microenvironment, partly by inhibition of the β-catenin and canonical Wnt pathway | [44][187] |
| Berberis vulgaris | Berberine (Sigma Aldrich, St. Louis, MO, USA) |
In vivo study to examine the effect of berberine on rat root canals of immature teeth with apical periodontitis | Berberine induced β-catenin expression and activated the β-catenin and canonical Wnt pathway in SCAPs which improved root repair in immature teeth with apical periodontitis. |
[45][188] |
| Berberis vulgaris | Berberine (Sigma Aldrich, St. Louis, MO, USA) |
In vitro study to examine effects of berberine on the osteogenesis and cell proliferation of DPSCs | Berberine enhanced hDPSC cell proliferation in a dose-dependent pattern and activated MAPK–EGFR–Runx2 signaling pathways. | [46][189] |
| Reynoutria multiflora (Thunb.) Moldenke | 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D–glucoside (THSG) (Taipei Medical University, Taipei, Taiwan) |
In vitro study investigated the effect of THSG on cell proliferation in hDPSCs. | THSG treatment enhance d the renewal ability and proliferative potential of hDPSCs via the AMPK/ERK/SIRT1 axis |
[48][191] |
| Dictamnus dasycarpus | Fraxinellone (Chengdu Herbpurify Co., Ltd., Chengdu, China) |
In vitro and in vivo study to examine antitumor effects of fraxinellone on lung cancer cells | Fraxinellone treatment inhibits expression of PD-L1 by HIF-1α and STAT3 signaling pathway downregulation, further inhibiting angiogenesis and proliferation in cancer cells | [51][194] |
| Fagopyrum esculentum | Rutin (Solarbio Science & Technology Co., Ltd., Beijing, China) |
In vitro study to examine the effects of rutin on the PDLSCs’ osteogenic proliferation and differentiation | Rutin increased osteogenic differentiation and proliferation of PDLSCs by GPR30-mediated PI3K/AKT/mTOR signal transduction | [52][195] |
| Cnidium monnieri | Osthole (National Institutes for Food and Drug Control, Beijing, China) |
In vitro study to determine osthole efficiency against defective osteogenic differentiation of P-PDLSCs via epigenetic modification |
Osthole (10−7 Mol/L) upregulated MORF, MOZ, and histone acetylases that catalyze acetylation of Histone 3 lisine14 (H3K14) and Histone 3 lisine9 (H3K9) | [56][199] |
| Osthole treatment enhanced bone formation and cell sheet formation of PDLSC sheets in periodontitis (animal models) | ||||
| Drynaria roosii Nakaike | Naringin (National Institute for the Control of Pharmaceutical and Biological Products, China) |
In vitro and in vivo study to examine the effect of naringin on proliferation and osteogenic differentiation of hPDLSCs | Naringin (1 µM) efficiently promoted hPDLSC differentiation and proliferation and increased expression levels was observed in related genes (COL1A2, OPN, RUNX2, and OCN) as compared to the control group | [58][201] |
| Macaranga tanarius | Isonymphaeol B (Okinawa, Japan) |
In vitro study to identify odontogenesis-promoting compounds and examine the molecular mechanism underlying enhanced tooth formation and odontoblast differentiation | Isonymphaeol B shows stimulatory effects on tooth root, dentine formation and odontoblast differentiation via AKT and MAP kinase signaling pathways | [60][203] |
| Canavalia ensiformis | Concanavalin A (Sigma Aldrich, USA) |
In vitro study to determine the effect of concanavalin A on osteogenic and proliferation differentiation of DPSCs | Concanavalin A at concentration of 5 and 10 µg/mL to DPSCs significantly increased the osteogenic and proliferation differentiation of DPSCs (p ≤ 0.05) | [62][205] |
| Panax ginseng | Ginsenoside Rg1 (Bio-function, Beijing, China) |
In vitro study to examine the effects of ginsenoside Rg-1 on osteogenic differentiation and proliferation of hPDLSCs | Ginsenoside Rg-1 enhanced osteogenic differentiation and proliferation of hPDLSCs at an optimum concentration of 10 μmol/L | [66][209] |
| Eclipta prostrata | Wedelolactone (Dalian, China) |
In vitro study investigated effect of wedelolactone on odontoblast differentiation of DPSCs | Wedelolactone induced odontoblast differentiation through NRP1, Sema3A, and NF-κB pathway inhibition and activation of b-catenin pathway | [67][210] |
| Aloe barbadensis Mill. | Acemannan (Chulalongkorn University, Bangkok, Thailand) |
In vitro study to examine effects of acemannan on human deciduous pulp cells and the response after vital pulp therapy in dog deciduous teeth | DPSC proliferation was significantly enhanced by acemannan along with an increase in expression levels of type I collagen, BMP-2, vascular endothelial growth factor, BMP-4, alkaline phosphatase, dentin sialoprotein, and mineralization | [68][211] |
| Cirsium setidens (Dunn) Nakai | Methanolic extract (Kangwon National University, Republic of Korea) |
In vitro study to examine the effects of methanolic extracts of C. setidens on osteogenic differentiation of hPDLSCs | Methanolic extract treatment with concentration of 0.05% significantly increased the viability of PDLSCs and also increased the expression levels of alkaline phosphatase, collagen 1, runt related transcription factor 2, and bone sialoprotein | [75][218] |
| Rhodiola rosea | Salidroside (Chengdu Must Bio-Technology Co., Shanghai, China) | In vitro study to investigate the effect of salidroside on the odontogenic differentiation and proliferation of hDPSCs | Treatment with salidroside promoted DPSCs cell viability, along with promoting their differentiation into odontogenic and osteogenic linage via activation of the BMP signaling pathway | [76][219] |
| Moringa oleifera | Moringin (Indena India Pvt. Ltd.; Bangalore, India) |
In vitro study to examine efficacy of moringin to induce PDLSCs toward neural progenitor differentiation | Treatment of PDLSCs with moringin resulted in the induction of PDLSC differentiation to neural progenitor cells via increased gene expression levels of genes that were involved in neuron cortical development | [81][224] |
| Acacia nilotica | Aqueous leaves extract (Hormavu, Bangalore) |
In vitro study to investigate the efficacy of Acacia nilotica leaves extract in chondrogenesis induction from mesenchymal stem cells | Treatment of aqueous leaves extract promoted chondrogenesis induction in DPSCs by upregulating the expression of various proteins in the cellular matrix, such as aggrecan, sox9, glycosaminoglycan (GAG), and collagen 2α1 (Col2α1) | [88][231] |
| Acalypha indica | Methanolic extract (Porur, Chennai, India) | In vitro study on GMSCs highlighted the potential of A. indica (methanolic extract) in increasing the wound healing ability of GMSCs | Treatment of A. indica extract (25 µg/mL) wound closure activity of GMSCs was increased up to 56.91 ± 1.21% in 24 h, while the percentage of wound closure was further enhanced to 89.23 ± 1.09% post 48 h of treatment | [89][232] |
| Scutellaria baicalensis | Baicalein (Sigma Aldrich, USA) |
In vitro study investigated the effect of Baicalein on Angiogenesis and Odontoblastic Differentiation | Baicalein promoted odontoblastic differentiation and angiogenesis of HDPCs by activating the BMP and Wnt/β-catenin signal pathways | [91][233] |
| Salvia miltiorrhiza | Tanshinone IIA (Sigma Aldrich, USA) |
TSA affects the osteogenic differentiation of hPDLSCs. | Tanshinone IIA can induce hPDLSC osteogenesis through the ERK1/2-Runx2 axis | [94][236] |
| Cullen corylifolium L. | Bakuchiol and C. corylifolium extract (KMD medicinal herbs Co., Yunnan, China) |
In vitro study to examine the efficacy of Bakuchiol and C. corylifolium extract as differentiation-inducing substances for tooth regeneration, was determined by monitoring odontogenic differentiation in hDPSCs | Bakuchiol and C. corylifolium extract significantly enhance the odontogenic differentiation potential of hDPSCs via upregulation of odontogenic differentiation marker genes, such as dentin matrix acidic phospho-protein-1, alkaline phosphatase, osteocalcin, and Runt-related transcription factor 2 | [95][237] |
