You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by José Pérez de la Lastra.
Ruminants produce significant amounts of methane during their digestive process, making livestock one of the largest sources of anthropogenic greenhouse gasses. Several solutions have been proposed to address this problem, including inoculation of ruminants against microorganisms responsible for methane synthesis in the rumen.
- archaea
- greenhouse-gas mitigation
- rumen
- immunization
- antimethanogen
1. Introduction
Methane (CH4) is one of the most important greenhouse gasses; its negative effect on global warming is 21 times greater than that of carbon dioxide (CO2) [1]. In addition, livestock is the human activity that generates the most CH4, as ruminants emit large amounts during their digestive processes. This gas is formed in the forestomach (rumen) of ruminants by methanogenic archaea [2]. During normal rumen function, plant material is degraded to produce volatile fatty acids, ammonia, hydrogen (H2), and CO2. Rumen methanogens principally consume H2 to reduce CO2 to CH4 [3]. Cattle, buffalo, and small ruminants release the equivalent of 2448 million tons of CO2 from both enteric processes and manure fermentation [4]. Within the farm environment, enteric fermentation is the most important source of CH4 emissions [5]. Thus, enteric CH4 generated in the gastrointestinal tracts of livestock is the single largest source of anthropogenic CH4 [6]. In the rumen, numerous prokaryotic (bacteria and archaea) and eukaryotic microorganisms (protozoa and fungi) work together to degrade the feedstuff consumed by the host ruminant [7]. In fact, on a well-managed confinement farm, enteric fermentation contributes about 45% of the total emission of greenhouse gases by the whole system. On more extensive grazing farms, these greenhouse-gas emissions could be even higher. For example, increased milk production has a positive correlation with CH4 emission [8]. Given that the livestock sector is one of the fastest-growing parts of the worldwide agricultural economy [9], the demand for milk and dairy products is expected to increase in coming decades, and thus so too are the CH4 emissions. It is therefore of utmost importance to find ways to mitigate the CH4 emissions from enteric fermentation. Mitigation approaches targeted at reducing CH4 must consider their effects on both enteric and manure fermentation, which account for approximately 90% and 10% of CH4 emissions, respectively [6]. Common approaches to reduce CH4 emissions in ruminants include dietary manipulation, drugs to reduce or control the quantity of methanogenic microorganisms in the gut, and/or vaccination. However, current strategies to inhibit methanogen activities in the rumen typically fail or have limited success due to low efficacy, poor selectivity, microorganism resistance, toxicity, or side effects of the compounds or drugs in the host species [3]. Dietary modification is the most-used strategy to reduce CH4 in ruminants, taking into account that different concentrates, subproducts, and/or forage combinations can reduce the quantity of CH4 production from the rumen [10[10][11][12],11,12], e.g., Goetsch [13] theorized that plant secondary metabolites could decrease CH4 emission, permitting the use of H2 to increase propionate production.
The control of animal diseases utilizes several strategies. Vaccines are one of the most important approaches, particularly on livestock farms [14]. The use of vaccines in these production sectors is increasing every year, especially for zoonotic diseases and those with significant effects on international trade [15]. However, concern regarding climate change has also increased dramatically. Reduction of emissions could therefore become economically attractive in the near future, making it viable to produce and market vaccines to mitigate climate change.
2. Antimethanogen Vaccines to Reduce CH4 in Ruminants
Several key points should be considered in the development of a successful strategy regarding the use of vaccines to reduce methane production from ruminal fermentation (Figure 1). Many articles and reviews have cited this possibility [26,30,65][16][17][18]. However, experimental research carried out between 1995 and 2020 was scarce in the consulted database (Table 1).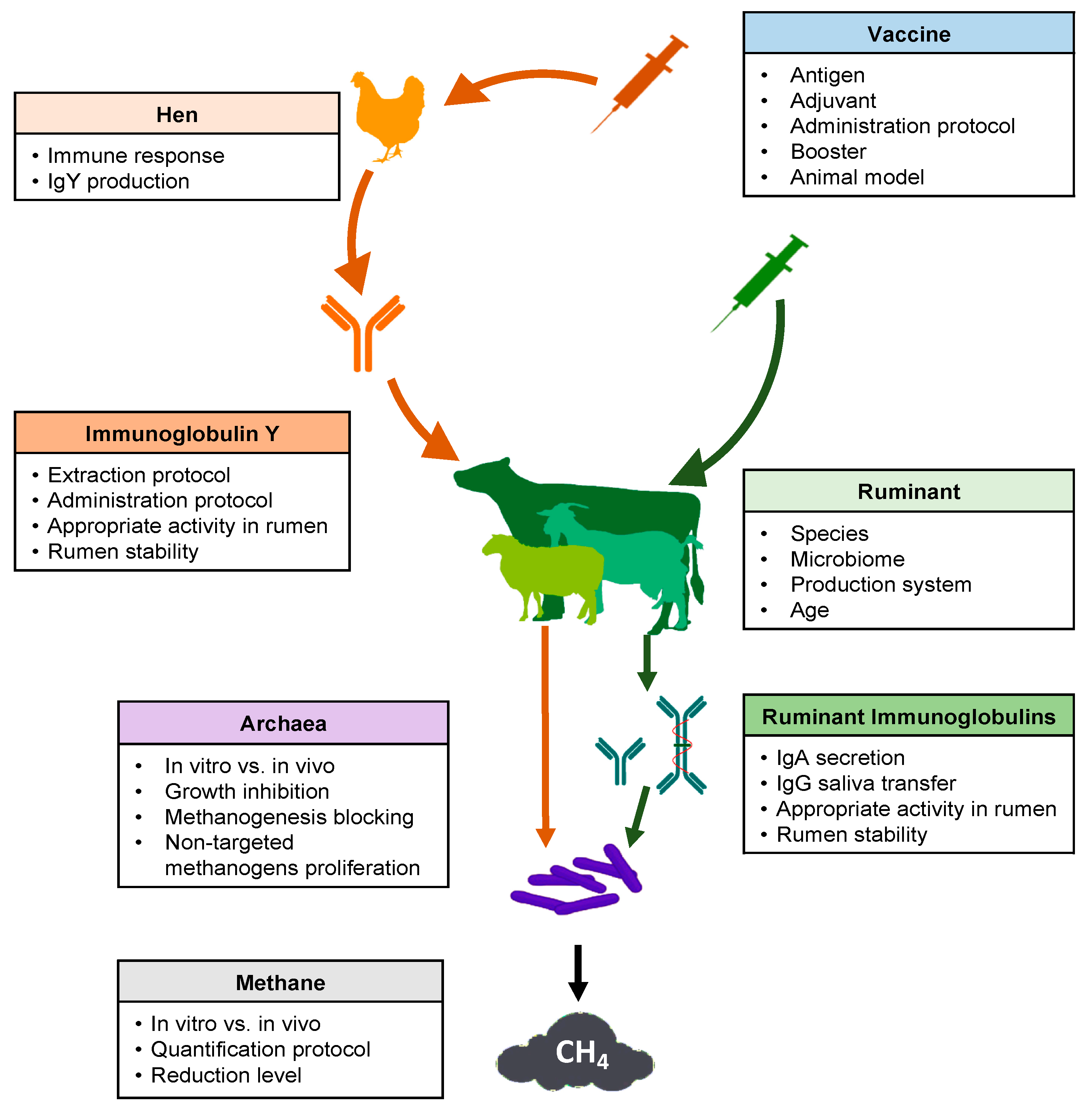
Figure 1. Schematic overview of key points to consider for the use of vaccines to decrease methane emissions from ruminal fermentation.
Table 1.
Summary of experimental designs used in research into vaccination for mitigating methane in ruminants.
| Animal Tested | Antigen | Adjuvant | Administration Via | Booster | References | |||
|---|---|---|---|---|---|---|---|---|
| Sheep Weaner wethers |
Mix of 10 methanogens, formaldehyde-killed, whole cells | Complete Freund’s adjuvant | Intraperitoneal | 28 days after primary | [78] | [19] | ||
| Sheep 5 years old |
Mix of three methanogens, formaldehyde-killed, whole cells | Montanide ISA50 | Subcutaneous | 153 days after primary | [79] | [20] | ||
| Mix of seven methanogens, formaldehyde-killed, whole cells | ||||||||
| Sheep 9 months old |
As Wright [79] Mix of three methanogens | As Wright [20] Mix of three methanogens |
Not specified | Not specified | 42 days after primary | [80] | [21] | |
| As Wright [79] Mix of three methanogens plus additional methanogenic material isolated from New Zealand sheep | As Wright [20] Mix of three methanogens plus additional methanogenic material isolated from New Zealand sheep |
|||||||
| Hen 24–25 weeks old |
Mix of three methanogens, freeze-dried, whole cells | Primary with complete Freund’s adjuvant Booster with incomplete Freund’s adjuvant | Pectoral muscle | 21, 42, 84, and 133 days after primary | [82] | [22] | ||
| Montanide ISA70 | 21 and 42 days after primary | |||||||
| Sheep 2 years old |
Mix of five methanogens, formaldehyde-killed, whole cells | Not specified | Subcutaneous | 28 and 103 days after primary | [81] | [23] | ||
| Sheep 9–11 months old |
Whole cells of | Methanobrevibacter ruminantium | M1 | Primary with complete Freund’s adjuvant Booster with incomplete Freund’s adjuvant |
Subcutaneous | 21 days after primary | [83] | [24] |
| Cytoplasmic fraction of | M. ruminantium | M1 | ||||||
| Wall fraction of | M. ruminantium | M1 | ||||||
| Wall fraction of | M. ruminantium | M1 with trypsin | ||||||
| Wall-fraction-derived-protein | M. ruminantium | M1 | ||||||
| Sheep 1–3 years old |
Nine peptides from | M. ruminantium | M1 extracellular regions of eight proteins | Primary and 14 days booster with complete Freund’s adjuvant Other boosters with incomplete Freund’s adjuvant |
Intradermal 10–15 sites |
14, 28, 56, 70, 84, 98, and 112 days after primary | [84] | [25] |
| Sheep Age not specified |
Cytoplasm-derived proteins from | M. ruminantium M1 | Saponin | Subcutaneous | No booster | [53] | [26] | |
| Wall-derived proteins from | M. ruminantium M1 | |||||||
| Sheep Age not specified |
Large extracellular domain of recombinant GT2 of | M. ruminantium | M1 | Saponin | Intramuscular | 21 days after primary | ||
| Seven synthetic peptides from extracellular domain of SecE from | M. ruminantium | M1 | ||||||
| Cattle 5 months old |
Large extracellular domain of recombinant GT2 of | M. ruminantium | M1 | Montanide ISA61 | Subcutaneous | 21 days after primary | [66] | [27] |
| Montanide ISA61 plus monophosphoryl lipid A | ||||||||
| Goat 18 months old |
Protein recombinant EhaF from | M. ruminantium | M1 | Primary with complete Freund’s adjuvant. Booster with incomplete Freund’s adjuvant | Intradermal Eight sites |
35 and 45 days after primary | [86] | [28] |
| Sheep 6 months old |
Large extracellular domain of recombinant GT2 from | M. ruminantium | M1 | Saponin | Intramuscular | 21 days after primary | [85] | [29] |
| Lipid nanoparticles/cationic liposomes | Subcutaneous | |||||||
| Chitosan thermogel | ||||||||
| Montanide ISA61 | 21 and 133 days after primary |
rGT2 (recombinant glycosyl transferase protein).
Several problems arose when comparing studies to assess the possibilities of using vaccines for this purpose. Concerning experimental design, as expected, the chosen antigens have developed along with the new technologies in the last 25 years, from whole methanogen cells to recombinant proteins from specific enzymes involved in CH4 production. Additionally, the different adjuvants and vaccination protocols used (Table 1) made it difficult to compare results. For example, Wedlock et al. [53][26] and Subharat et al. [66][27] both utilized recombinant glycosyl transferase protein (rGT2) as antigen, but the former with saponins as adjuvant and an intramuscular administration route in sheep as experimental animals, while the second was subcutaneous using Montanide in 5 month old calves. Additionally, those studies evaluated different immunoglobulins (IgG, IgA, and IgY) and samples (blood, saliva, and rumen), or analyzed the effect on CH4 production using different approaches (in vitro, in vivo).
The most frequently used experimental animal model was the sheep, which was used in 8 out of 11 studies. One of the remaining studies used cattle and another used goats. Finally, a study proposed passive immunization producing antimethanogen Igs in hens. This made it difficult to compare research in order to draw solid conclusions. Patil et al. [67][30] assayed the immune response of sheep, cattle, and goats against four different serotypes of Foot and mouth disease virus at different times postvaccination. The cows showed higher levels of neutralizing antibodies than small ruminants for all tested virus serotypes. Lobato et al. [68][31] compared vaccination with recombinant toxin of Clostridium perfringens in the three common livestock ruminant species. In this study, sheep showed the highest antibody level, cattle the lowest, and goats intermediate. Moreira et al. [69][32] tested three recombinant vaccines against alpha, beta, and epsilon toxins of C. perfringens in the same three species. They found an interaction between antigens and species. There were no differences between species, except for with epsilon toxin. In the latter, cattle showed the highest antitoxin levels, with no differences between sheep and goats. In the same way, each species had a different response to each recombinant toxin, whereby all these animals had higher values against beta and lower against alpha toxin. Iqbal et al. [70][33] observed that ruminal bacterial, methanogen, and protozoal communities were different between cattle and buffalo, although Methanobrevibacter was the major genus for both species. These studies show that the animal model selected has an interaction with the antigen used. Obviously, small ruminants are cheaper animal models than cattle, and have fast growth and immune maturity. For these reasons, the use of goats and sheep in the early stages of vaccine development is more practical. However, the novel antigen must also be tested in the species for which it is being developed.
Additionally, animal age was another source of variation, with vaccinated sheep ranging from 3–5 months to 5 years old. It is well known that lambs are more susceptible to infectious diseases than adult sheep, and their immune resistance progressively increases during the first year of life [71][34]. According to Nguyen et al. [72][35], who compared 3 months old lambs with 2–5 years old sheep following a single intravenous injection of chicken erythrocytes, the adults had higher antibody titers than the young animals. This author affirmed that the antibody response of lambs reached the adult level at age 7–8 months and sex was not a variable that influenced this humoral response. Similarly, Watson et al. [71][34] assayed the antibody production of weaners and adult sheep against Brucella abortus. They reported that adults always showed a higher level of antibodies than weaners. Additionally, those authors found that both CD4+ and CD8+ in lymph and blood were higher in adults than in weaners, but B cells are lower in adult than in weaners’ lymph, with no difference in blood between ages. The authors suggested that B cells are not completely functional in younger animals, leading to the lower antibody response. Shu et al. [73][36] worked on a vaccine against Streptococcus bovis plus Freund’s adjuvant, reporting a lower antibody concentration than the previous studies in sheep. They tentatively attributed this difference to the age of the animals: 6 months old for Gill et al. [74][37], 1 year old for Shu et al. [75][38], and 2 years old in Shu et al. [73][36], where older animals showed higher antibody levels. However, methanogen vaccines in young animals are a very interesting target, because early programming of rumen microbiota using vaccines could be a better solution in comparison to adult animal vaccines. The rumen microbiota is established early in ruminant life, and it is possible to mold it through diet around weaning time, with a long-lasting effect [76][39]. De Barbieri et al. [77][40] found that rumen bacterial communities can change in both mothers and lambs after oral rumen inoculation in the neonatal period or first weeks of life.
The choice of the antigen to be inoculated is a key aspect for the development of a vaccine against methanogenic archaea in the rumen. Different approaches have been used to target methanogens (Table 1). The first strategy was to vaccinate the animals with whole cells of different archaeal species found in the rumen. In some studies, they specified that the methanogens had previously been killed by formaldehyde [78,79,80,81][19][20][21][23] or freeze-dried [82][22]. Baker and Perth [78][19] used a mix of ten strains of Methanobrevibacter ruminantium, M. arboriphilus, M. smithii, Methanobacter formicium, and Methanosarcina barkeri. Wright [79][20] checked 16S rDNA clone libraries from Australian sheep rumen samples. Based on that information, they chose one vaccine design with three strains of Methanobrevibacter spp. (two of them isolated in their lab in Australia) and another vaccine with seven strains from the four Methanobrevibacter species, Methanomicrobium mobile, M. barkeri, and Methanobacterium formicicum. Despite promising results by Wright [79][20], Clark et al. [80][21] tried to replicate them using the same mixture of three methanogens, alongside a combination of this mix with methanogenic material isolated from New Zealand sheep. Williams et al. [81][23] used whole cells of three Methanobrevibacter strains, Methanomicrobium mobile, and Methanosphaera stadtmaniae, which altogether comprised more than half of all the methanogen strains detected. Cook et al. [82][22] used Methanobrevibacter ruminantium, M. smithii, and Methanosphaera stadtmaniae, each in an independent hen group. They compared the in vitro effect of semipurified IgY and freeze-dried egg yolk from hens vaccinated with each archaeal species and a combination of the three.
Another strategy, derived from the first, was to use cell components as antigens. Wedlock et al. [83][24] compared the use of whole cells with cytoplasmic and wall-fraction proteins from M. ruminantium. In parallel, Leahy et al. [84][25] published the genome sequence of M. ruminantium; based on this sequence, these researchers chose nine peptides from extracellular regions of the cited archaea. Those peptides were synthesized and joined to keyhole limpet hemocyanin (KHL), to be used as antigens. Later, Wedlock et al. [53][26] compared cytoplasmic and wall-fraction proteins with seven peptides from the extracellular domain of SecE and rGT2. The latter protein was used by Subharat et al. [66][27] and Subharat et al. [85][29] to vaccinate cattle and sheep. Zhang et al. [86][28] used the protein EhaF from M. ruminantium M1, which was one of the potential antigen candidates identified by Leahy et al. [84][25], with a key function in hydrogenotrophic methanogenesis.
Obviously, appropriate adjuvants must be selected for successful vaccine performance. This choice is based mainly on the animal species and antigen used. The experiments compiled herein show how adjuvant use has developed over time, as new experience is acquired. Four out of ten ruminant experiments and the one with hens added complete/incomplete Freund’s adjuvant (FCA/FIA). Another two used saponins, and two recent studies used Montanide ISA. Shu et al. [73][36] compared the immune response to S. bovis vaccine with six different adjuvants (FCA, FIA, QuilA, dextran sulphate, alum, Gerbu). They found that FCA produced the largest quantity of blood antibodies in sheep. Using antimethanogen vaccines, two studies compared the efficacy of different adjuvants. Subharat et al. [85][29] contrasted four adjuvants (saponin, chitosan, lipid nanoparticles, and Montanide ISA). They reported that Montanide ISA61 produced the most IgG and IgA in saliva and serum. Subharat et al. [66][27] had previously affirmed that this Montanide with and without monophosphoryl lipid A was able to induce a strong humoral response in both IgA and IgG. The most usual administration route was subcutaneous in ruminants (six out of eleven); intramuscular and intradermal were the next most frequently applied in ruminants (both used in two experiments), and Baker and Perth [78][19] used intraperitoneal. The route in hens was intramuscular in the hen breast. Intramuscular and subcutaneous administration routes were the most common, although it has been suggested that intradermal injection could improve the mucosal response [87][41]. This is of great interest concerning the present topic. More research is necessary about the antigen–adjuvant–administration route combinations able to achieve a better combined response.
Regarding the booster and booster time, a significant variation in both number and period is shown in Table 1. Of the vaccination schedules, the most frequently used was one booster (six out of twelve studies) between 21 and 42 days postprimary, followed by two boosters (three out of twelve). The second vaccination given by Wright et al. [79][20] was not considered a booster because those authors decided to administer it when they observed low antibody levels, and neither was the third vaccination by Subharat et al. [85][29], since they tested only one group of animals to determine antibody longevity and the effect of boosting. Examining the results, administration of only one or two boosters appears insufficient to provide long-term immunity. For example, Williams et al. [81][23] reported that one booster 28 days after primary provided a peak at Day 55 after primary, but the titer decreased by Day 99. Using two boosters, Subharat et al. [85][29] achieved similar results, with a peak at Day 42 after the primary and the titer decreased until Day 133, when the animals were revaccinated and their specific antibodies titers increased. Those results indicate that a booster is necessary to reinforce antibody secretion. None of the other available studies elucidated the issue in this sense, despite this being a very important piece of knowledge to support this procedure for CH4 mitigation.
The time of sample collection to evaluate the immune response was another source of variation. Some authors decided to take only one sample after vaccination to quantify the specific antibodies [83[24][28],86], and this did not permit assessment of the specific antibodies’ secretion curves. Therefore, it is not possible to elucidate whether the curves were in their increasing, peak, or decreasing phases. In other studies, which measured immunoglobulins (Igs), the sampling time allowed analysis of the curve and also of the different phases of the antibody curves. Lobato et al. [68][31] tested a toxin vaccine on sheep, goats, and cattle with a booster on Day 28 after the primary. They reported that no antitoxin antibodies were detected on Day 0. On Day 42, 40% of goats, 60% of sheep, and 80% of cattle had titers lower than 1 IU/mL. On Day 56, all animals had titers equal to or higher than 5.8 IU/mL; sheep had the highest values, followed by goats and cattle.
References
- Kurniawan, T.; Budhi, Y.W.; Bindar, Y. Reverse flow reactor for catalytic oxidation of lean methane. World Chem. Eng. J. 2018, 2, 21–26.
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 1–11.
- Leahy, S.C.; Kelly, W.J.; Ronimus, R.S.; Wedlock, N.; Altermann, E.; Attwood, G.T. Genome sequencing of rumen bacteria and archaea and its application to methane mitigation strategies. Animal 2013, 7 (Suppl. 2), 235–243.
- Opio, C.; Gerber, P.; Mottet, A.; Falcucci, A.; Tempio, G.; MacLeod, M.; Vellinga, T.; Henderson, B.; Steinfeld, H. Animal Production and Health Division: A Global Life Cycle Assessment Greenhouse Gas Emissions from Ruminant Supply Chains; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 9789251079454.
- Rotz, C.A. Modeling greenhouse gas emissions from dairy farms. J. Dairy Sci. 2018, 101, 6675–6690.
- Knapp, J.; Laur, G.; Vadas, P.; Weiss, W.; Tricarico, J. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261.
- Wang, Z.; Elekwachi, C.O.; Jiao, J.; Wang, M.; Tang, S.; Zhou, C.; Tan, Z.; Forster, R.J. Investigation and manipulation of metabolically active methanogen community composition during rumen development in black goats OPEN. Sci. Rep. 2017, 7, 422.
- Gerber, P.; Vellinga, T.; Opio, C.; Steinfeld, H. Productivity gains and greenhouse gas emissions intensity in dairy systems. Livest. Sci. 2011, 139, 100–108.
- FAO. Livestock in the Balance: The State of Food and Agriculture; FAO, Ed.; Communication Division, FAO: Rome, Italy, 2009; ISBN 978-92-5-106215-9.
- Puchala, R.; LeShure, S.; Gipson, T.A.; Tesfai, K.; Flythe, M.D.; Goetsch, A.L. Effects of different levels of lespedeza and supplementation with monensin, coconut oil, or soybean oil on ruminal methane emission by mature Boer goat wethers after different lengths of feeding. J. Appl. Anim. Res. 2018, 46, 1127–1136.
- Suha Uslu, O.; Kurt, O.; Kaya, E.; Kamalak, A. Effect of species on chemical composition, metabolizable energy, organic matter digestibility and methane production of some legume plants grown in Turkey. J. Appl. Anim. Res. 2018, 46, 1158–1161.
- Lourenco, J.M.; Maia, F.J.; Bittar, J.H.J.; Segers, J.R.; Tucker, J.J.; Campbell, B.T.; Stewart, R.L. Utilization of exogenous enzymes in beef cattle creep feeds. J. Appl. Anim. Res. 2020, 48, 70–77.
- Goetsch, A.L. Recent research of feeding practices and the nutrition of lactating dairy goats. J. Appl. Anim. Res. 2019, 47, 103–114.
- Heldens, J.G.M.; Patel, J.R.; Chanter, N.; Ten Thij, G.J.; Gravendijck, M.; Schijns, V.E.J.C.; Langen, A.; Schetters, T.P.M. Veterinary vaccine development from an industrial perspective. Vet. J. 2008, 178, 7–20.
- Thomas, L.F.; Bellet, C.; Rushton, J. Using economic and social data to improve veterinary vaccine development: Learning lessons from human vaccinology. Vaccine 2019, 37, 3974–3980.
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, 2–16.
- Kobayashr, Y. Abatement of methane production from ruminants: Trends in the manipulation of rumen fermentation. Asian-Australas. J. Anim. Sci. 2010, 23, 410–416.
- De Wit, J.; Oldenbroek, J.K.; van Keulen, H.; Zwart, D. Criteria for sustainable livestock production: A proposal for implementation. Agric. Ecosyst. Environ. 1995, 53, 219–229.
- Baker, S.K.; Perth, W. Method for Improving Utilization of Nutrients by Ruminant or Ruminant-Like Animals. U.S. Patent 6,036,950, 14 March 2000.
- Wright, A. Reducing methane emissions in sheep by immunization against rumen methanogens. Vaccine 2004, 22, 3976–3985.
- Clark, H.; Wright, A.-D.; Joblin, K.; Molano, G.; Cavanagh, A.; Peters, J. Field testing an Australian developed anti-methanogen vaccine in growing ewe lambs. In Proceedings of the Workshop on the Science of Atmospheric Trace Gases; Clarkson, T.S., Ed.; Science Communication, NIWA: Wellington, New Zealand, 2004; pp. 107–108.
- Cook, S.R.; Maiti, P.K.; Chaves, A.V.; Benchaar, C.; Beauchemin, K.A.; McAllister, T.A. Avian (IgY) anti-methanogen antibodies for reducing ruminal methane production: In vitro assessment of their effects. Aust. J. Exp. Agric. 2008, 48, 260–264.
- Williams, Y.J.; Popovski, S.; Rea, S.M.; Skillman, L.C.; Toovey, A.F.; Northwood, K.S.; Wright, A.D.G. A vaccine against rumen methanogens can alter the composition of archaeal populations. Appl. Environ. Microbiol. 2009, 75, 1860–1866.
- Wedlock, D.N.; Pedersen, G.; Denis, M.; Buddle, B.M.; Dey, D.; Janssen, P.H. Development of a vaccine to mitigate greenhouse gas emissions in agriculture: Vaccination of sheep with methanogen fractions induces antibodies that block methane production in vitro. N. Z. Vet. J. 2010, 58, 29–36.
- Leahy, S.C.; Kelly, W.J.; Altermann, E.; Ronimus, R.S.; Yeoman, C.J.; Pacheco, D.M.; Li, D.; Kong, Z.; McTavish, S.; Sang, C.; et al. The genome sequence of the rumen methanogen Methanobrevibacter ruminantium reveals new possibilities for controlling ruminant methane emissions. PLoS ONE 2010, 5, e8926.
- Wedlock, D.N.; Janssen, P.H.; Leahy, S.C.; Shu, D.; Buddle, B.M. Progress in the development of vaccines against rumen methanogens. Animal 2013, 7 (Suppl. 2), 244–252.
- Subharat, S.; Shu, D.; Zheng, T.; Buddle, B.M.; Janssen, P.H.; Luo, D.; Wedlock, D.N. Vaccination of cattle with a methanogen protein produces specific antibodies in the saliva which are stable in the rumen. Vet. Immunol. Immunopathol. 2015, 164, 201–207.
- Zhang, L.; Huang, X.; Xue, B.; Peng, Q.; Wang, Z.; Yan, T.; Wang, L. Immunization against rumen methanogenesis by vaccination with a new recombinant protein. PLoS ONE 2015, 10, e0140086.
- Subharat, S.; Shu, D.; Zheng, T.; Buddle, B.M.; Kaneko, K.; Hook, S.; Janssen, P.H.; Wedlock, D.N. Vaccination of sheep with a methanogen protein provides insight into levels of antibody in saliva needed to target ruminal methanogens. PLoS ONE 2016, 11, e0159861.
- Patil, P.K.; Bayry, J.; Ramakrishna, C.; Hugar, B.; Misra, L.D.; Prabhudas, K.; Natarajan, C. Immune responses of sheep to quadrivalent double emulsion Foot-and-Mouth Disease vaccines: Rate of development of immunity and variations among other ruminants. J. Clin. Microbiol. 2002, 40, 4367–4371.
- Lobato, F.C.F.; Lima, C.G.R.D.; Assis, R.A.; Pires, P.S.; Silva, R.O.S.; Salvarani, F.M.; Carmo, A.O.; Contigli, C.; Kalapothakis, E. Potency against enterotoxemia of a recombinant Clostridium perfringens type D epsilon toxoid in ruminants. Vaccine 2010, 28, 6125–6127.
- Moreira, G.M.S.G.; Salvarani, F.M.; Da Cunha, C.E.P.; Mendonça, M.; Moreira, Â.N.; Gonçalves, L.A.; Pires, P.S.; Lobato, F.C.F.; Conceição, F.R. Immunogenicity of a trivalent recombinant vaccine against Clostridium perfringens alpha, beta, and epsilon toxins in farm ruminants. Sci. Rep. 2016, 6, 22816.
- Iqbal, M.W.; Zhang, Q.; Yang, Y.; Li, L.; Zou, C.; Huang, C.; Lin, B.; Wasim Iqbal, M. Comparative study of rumen fermentation and microbial community differences between water buffalo and Jersey cows under similar feeding conditions. J. Appl. Anim. Res. 2018, 46, 740–748.
- Watson, D.L.; Colditz, I.G.; Andrew, M.; Gill, H.S.; Altmann, K.G. Age-dependent immune response in Merino sheep. Res. Vet. Sci. 1994, 57, 152–158.
- Nguyen, T.C. The immune response in sheep: Analysis of age, sex and genetic effects on the quantitative antibody response to chicken red blood cells. Vet. Immunol. Immunopathol. 1984, 5, 237–245.
- Shu, Q.; Bird, S.; Gill, H.; Duan, E.; Xu, Y.; Hillard, M.; Rowe, J. Antibody response in sheep following immunization with Streptococcus bovis in different adjuvants. Vet. Res. Commun. 2001, 25, 43–54.
- Gill, H.S.; Shu, Q.; Leng, R.A. Immunization with Streptococcus bovis protects against lactic acidosis in sheep. Vaccine 2000, 18, 2541–2548.
- Shu, Q.; Hillard, M.A.; Bindon, B.M.; Duan, E.; Xu, Y.; Bird, S.H.; Rowe, J.B.; Oddy, V.H.; Gill, H.S. Effects of various adjuvants on efficacy of a vaccine against Streptococcus bovis and Lactobacillus spp. in cattle. Am. J. Vet. Res. 2000, 61, 839–843.
- Yáñez-Ruiz, D.R.; Macías, B.; Pinloche, E.; Newbold, C.J. The persistence of bacterial and methanogenic archaeal communities residing in the rumen of young lambs. FEMS Microbiol. Ecol. 2010, 72, 272–278.
- De Barbieri, I.; Hegarty, R.S.; Silveira, C.; Gulino, L.M.; Oddy, V.H.; Gilbert, R.A.; Klieve, A.V.; Ouwerkerk, D. Programming rumen bacterial communities in newborn Merino lambs. Small Rumin. Res. 2015, 129, 48–59.
- Lawan, A.; Jesse, F.F.A.; Idris, U.H.; Odhah, M.N.; Arsalan, M.; Muhammad, N.A.; Bhutto, K.R.; Peter, I.D.; Abraham, G.A.; Wahid, A.H.; et al. Mucosal and systemic responses of immunogenic vaccines candidates against enteric Escherichia coli infections in ruminants: A review. Microb. Pathog. 2018, 117, 175–183.
More
