Minimally invasive surgery allows for fewer complications as microdevices operate through small incisions or natural orifices. Thermomagnetic-responsive microgrippers are microscopic multi-fingered devices that respond to temperature changes due to the presence of thermal-responsive polymers. Polymeric devices, made of poly(N-isopropylacrylamide-co-acrylic acid) (pNIPAM-AAc) and polypropylene fumarate (PPF), self-fold due to swelling and contracting of the hydrogel layer. In comparison, soft metallic devices feature a pre-stressed metal bilayer and polymer hinges that soften with increased temperature. Both types of microdevices can self-actuate when exposed to the elevated temperature of a tumor microenvironment, allowing for direct targeting for biopsies. Microgrippers can also be doped to become magnetically responsive, allowing for direction without tethers and the retrieval of microdevices containing excised tissue. The smaller size of stimuli-responsive microgrippers allows for their movement through hard-to-reach areas within the body and the successful extraction of intact cells, RNA and DNA.
- smart materials
- microgrippers
- stimuli responsive
- soft robotics
1. Introduction
2. Mechanisms of Thermomagnetic Self-Actuating Microgrippers
Most of the advantages of using this technology stem from the ability of microgrippers to self-fold in response to temperature changes. In fully polymeric microgrippers, the thermal response comes from the pNIPAM-AAc hydrogel layer (Figure 2). Specifically, pNIPAM is a thermal responsive polymer with a lower critical solution temperature (LCST) in the range of 30 to 35 °C [11][5]. pNIPAM is copolymerized with acrylic acid (AAc), a hydrophilic monomer, to modify the LCST to 36 °C, which is closer to body temperature [9][6].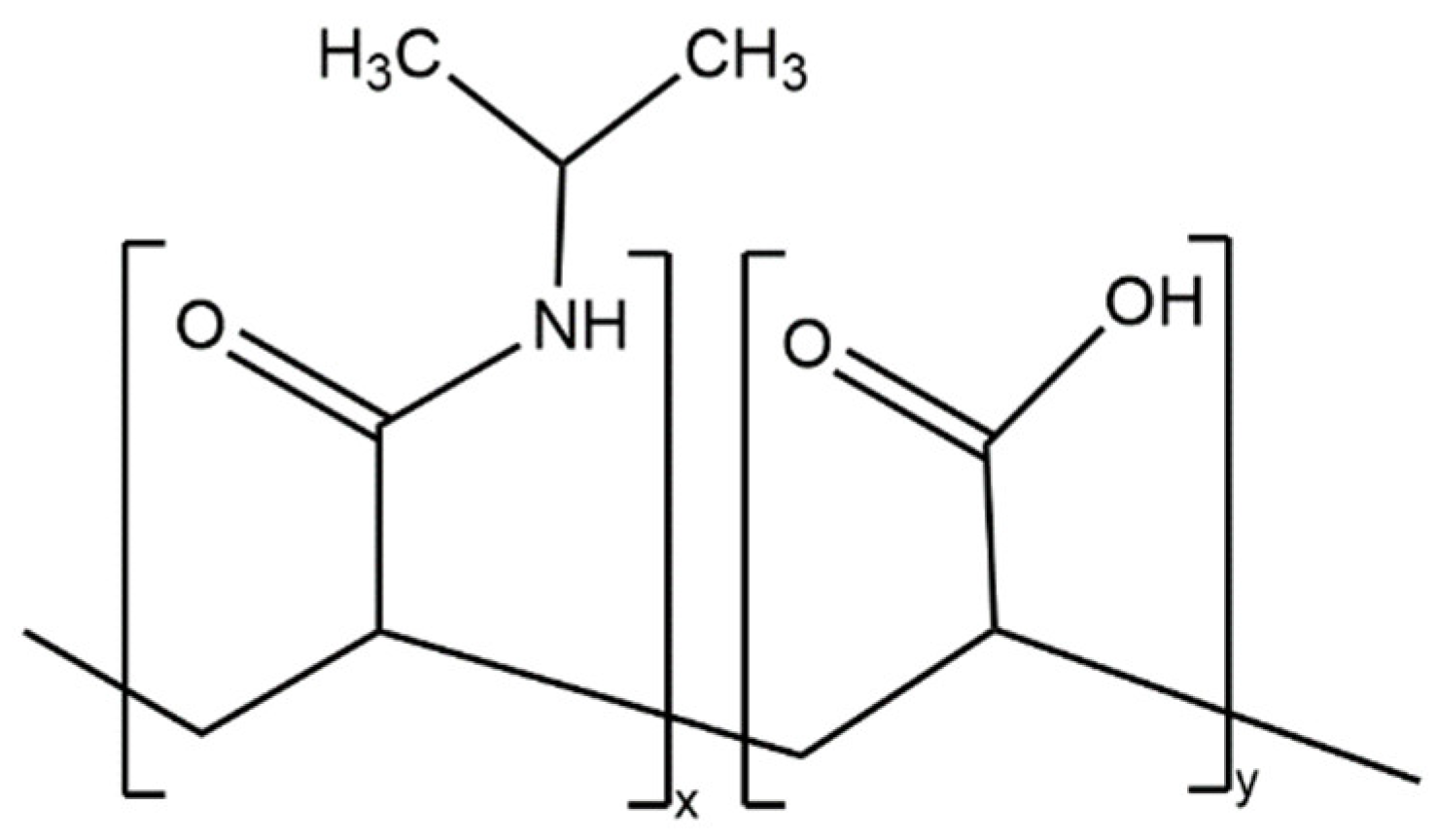
3. Fabrication and Design of Thermomagnetic-Responsive Microgrippers
Both types of microgrippers, polymeric and soft metallic, are commonly modeled after a hand, with several flexible fingers that form a star-like shape [5][3]. The multi-fingered design of thermomagnetic-microgrippers allows the devices to grip onto tissue and other small molecules. Typically, these devices feature four to six fingerlike projections to ensure that the devices can hold their grasp when being directed under high magnetic fields [18][9]. Furthermore, for the devices to be capable of excising cells for biopsies, the microgrippers must be large enough to encapsulate cells within the device. Cells range in size from 10 to 100 µm, making the ideal size for microgrippers to be between 10 µm to 1 mm. Due to the fabrication methods, microgrippers range in size from 300 μm to 1.5 mm, making them large enough to capture cells within the fingers of the device but small enough to be easily delivered [5][3]. Polymeric microdevices are designed to have thermal-responsive hydrogel hinges paired with stiff polymer segments. The hydrogel layer is typically made up of pNIPAM-AAc due to its thermal responsiveness, swelling ability and low shear modulus of 162 kPa [9][6]. Polypropylene fumarate is often used to make up the stiff polymer segments due to its nonswelling behavior and high shear modulus of 16 MPa [9][6]. SU-8 is an alternative polymer that can make up the stiff segments of microgrippers [19][10]. SU-8 is a photopatternable epoxy resin with a high elastic modulus of 3.6 GPa and excellent chemical and thermal stability [20][11]. Soft robotic microgrippers are composed of pre-stressed metallic bilayer hinges that are flattened with a thermosensitive polymer layer [10][12]. Paraffin wax is most often used for the thermosensitive polymer trigger layer. This material was chosen due to its biocompatibility and chemical and biological inertness [16][13]. Furthermore, the phase-transition temperature of paraffin wax is near body temperature [16][13]. The thickness of the paraffin wax layer plays a large role in the bending ability of the microgripper. Polymeric thermomagnetic-responsive microgrippers are fabricated through serial photolithography [9][6]. The fabrication process of polymeric pNIPAM-AAc. First, a layer of PPF is deposited onto a silicon wafer through spin coating and cross-linked by applying UV light through a dark field mask. Next, pNIPAM-AAc mixed with 5% weight concentration iron oxide is poured on top of the PPF layer. This layer is then also cross-linked by exposing the polymer layer to UV light through a dark field mask. Uncrosslinked polymers are then removed by submerging the silicon wafer in alcohol to reveal the star-shaped polymeric microgrippers [9][6]. For soft metallic microgrippers, photolithography with electrodeposition is utilized to fabricate the metallic bilayer, then the thermo-sensitive polymer is spin-coated to the surface [6][4]. First, the stressed metallic bilayer and the embedded magnetic material are created through UV photolithography [6][4]. The metal layers are then electrodeposited onto a mold where molten paraffin wax is spin coated onto the metal components. Once cooled, the microgripper is encapsulated within a rigid paraffin wax shell [17][14].4. Applications of Thermomagnetic-Responsive Microgrippers in Minimally Invasive Surgery
Thermomagnetic-responsive microgrippers have to capability to be used to improve minimally invasive biopsies. When exposed to an elevated temperature in the presence of a tumor, the fingers of the microgrippers can self-fold to grasp onto the surrounding tissue and excise cells for a minimally invasive biopsy. Furthermore, the magnetic responsiveness of the microgrippers allows surgeons to easily retrieve the deployed microgrippers and the tissue captured by the devices to perform DNA disease-diagnostic analysis. In vitro testing of polymeric microgrippers composed of PPF and pNIPAM-AAc verified the thermal responsiveness of the devices. Microgrippers submerged in solution were able to fold in both directions in response to temperature changes. Above 36 °C, the gripper is closed with the PPF segments on the external surface. The hydrogel layer appears opaque, indicating that the polymer network is collapsed and that the pNIPAM-AAc is hydrophobic [9][6]. It was also verified that, below 36 °C, the microgripper closes in the opposite direction with the PPF segments on the inside surface of the gripper. The hydrogel layer appears clear at this temperature, indicating that the polymer network is swollen with water and hydrophilic. This self-folding and unfolding behavior was observed for over 50 cycles between 28 °C and 43 °C confirming that the self-actuation mechanism is reversible. These results confirmed that pNIPAM-AAc microgrippers self-fold at temperatures near-physiological conditions and that they are capable of self-actuating multiple times, which could be useful for storage prior to surgery. The gripping strength of polymeric microdevices was also verified in vitro to be strong enough to extract cells from tissue. pNIPAM-AAc and PPF microgrippers were able to extract fibroblast cells from a cell clump when pipetted from a cold storage solution to a 37 °C environment [9][6]. These results validated the ability of the microgrippers to excise cells from tissue without the use of tethers, indicating potential usefulness for cell collection during minimally invasive biopsies. In vitro experiments also confirmed that the position of polymeric microgrippers doped with iron oxide can be manipulated through the application of a magnetic field. The position of the device was able to be controlled with a magnetic probe, indicating the potential for the microgrippers to be controlled via magnetic fields in clinical settings [9][6]. In vitro testing verified that microgrippers controlled via a closed-loop system were also capable of manuevering around obstacles while performing pick-and-place procedures. The ability of thermomagnetic-responsive microgrippers to autonomously recognize and sort materials was also verified in vitro. Closed-loop controlled microgrippers were able to recognize colored polyester beads and move each one to the area marked with the corresponding color. The spherical beads were 0.5 mm in diameter and weighed around 0.6 mg. The average error of the object drop-off was 0.85 ± 0.41 mm, which fell into the range of the drop-off location boundaries. This demonstrated that microgrippers can also autonomously recognize and manipulate micro-scale objects with high precision [19][10].5. Summary
In conclusion, thermomagnetic-responsive microgrippers have the potential to revolutionize minimally invasive surgical techniques. With the ability to self-fold in response to temperature changes and the ability to be guided via a magnetic field, thermomagnetic-responsive microdevices eliminate the need for external control tethers, which greatly increases the accessibility and maneuverability of the devices. Microgrippers are 10- to 100- times smaller than the current biopsy forceps or robotic grippers used for minimally invasive biopsies. This small size allows the devices to access extremely hard-to-reach areas of the body and greatly reduces the pain and discomfort associated with surgery, leading to faster recovery times and reduced surgery costs. Polymeric microgrippers doped with iron oxide nanoparticles self-actuate in response to temperature changes due to changes in the solubility of the polymer network. On the other hand, soft robotic microdevices self-fold due to a change in the Young’s modulus of the polymer trigger layer. The sharp tips of the star-shaped design allow these devices to dig into surrounding tissue and excise cells. The thermal responsiveness of both fully polymeric and soft robotic microgrippers enables the devices to self-fold when exposed to high-temperature tumor regions. The magnetic responsiveness allows surgeons to easily control the position of the microgrippers and retrieve tissue samples containing DNA for minimally invasive biopsies. Future research around thermomagnetic-responsive microgrippers will focus on improving device tracking methods, reducing the number of devices left in the body and expanding this technology to other surgical techniques.References
- Mack, M.J. Minimally Invasive and Robotic Surgery. JAMA 2001, 285, 568–572.
- Ghosh, A.; Yoon, C.; Ongaro, F.; Scheggi, S.; Selaru, F.M.; Misra, S.; Gracias, D.H. Stimuli-responsive soft untethered grippers for drug delivery and robotic surgery. Front. Mech. Eng. 2017, 3, 7.
- Gultepe, E.; Randhawa, J.S.; Kadam, S.; Yamanaka, S.; Selaru, F.M.; Shin, E.J.; Kalloo, A.N.; Gracias, D.H. Biopsy with Thermally-Responsive Untethered Microtools. Adv. Mater. 2013, 25, 514–519.
- Yin, C.; Wei, F.; Zhan, Z.; Zheng, J.; Yao, L.; Yang, W.; Li, M. Untethered microgripper-the dexterous hand at microscale. Biomed. Microdevices 2019, 21, 1–18.
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory, and application. Prog. Polym. Sci. 1992, 17, 163–249.
- Breger, J.C.; Yoon, C.; Xiao, R.; Kwag, H.R.; Wang, M.O.; Fisher, J.P.; Nguyen, T.D.; Gracias, D.H. Self-Folding Thermo-Magnetically Responsive Soft Microgrippers. ACS Appl. Mater. Interfaces 2015, 7, 3398–3405.
- Sanz, B.; von Bilderling, C.; Tuninetti, J.S.; Pietrasanta, L.; Mijangos, C.; Longo, G.S.; Azzaroni, O.; Giussi, J.M. Thermally-induced softening of PNIPAm-based nanopillar arrays. Soft Matter 2017, 13, 2453–2464.
- Cho, H.; Jeon, S.; Yang, J.; Baek, S.Y.; Kim, D. Hydrogel Nanoparticle as a Functional Coating Layer in Biosensing, Tissue Engineering, and Drug Delivery. Coatings 2020, 10, 663.
- Ongaro, F.; Scheggi, S.; Ghosh, A.; Denasi, A.; Gracias, D.H.; Misra, S. Design, characterization, and control of thermally responsive and magnetically-actuated micro-grippers at the air-water interface. PLoS ONE 2017, 12, e0187441.
- Ongaro, F.; Scheggi, S.; Yoon, C.; van den Brink, F.; Oh, S.H.; Gracias, D.H.; Misra, S. Autonomous planning, and control of soft untethered grippers in unstructured environments. J. Micro-Bio. Robot. 2017, 12, 45–52.
- Xu, T.; Yoo, J.H.; Babu, S.; Roy, S.; Lee, J.-B.; Lu, H. Characterization of the mechanical behavior of SU-8 at microscale by viscoelastic analysis. J. Micromech. Microeng. 2016, 26, 105001–105012.
- Yim, S.; Gultepe, E.; Gracias, D.H.; Sitti, M. Biopsy using a Magnetic Capsule Endoscope Carrying, Releasing, and Retrieving Untethered Microgrippers. IEEE Trans. Biomed. Eng. 2014, 61, 513–521.
- Jin, Q.; Yang, Y.; Jackson, J.A.; Yoon, C.; Gracias, D.H. Untethered Single Cell Grippers for Active Biopsy. Nano Lett. 2020, 20, 5383–5390.
- Ghosh, A.; Liu, Y.; Artemov, D.; Gracias, D.H. Magnetic Resonance Guided Navigation of Untethered Microgrippers. Adv. Healthc. Mater. 2021, 10, 2000869.
