Deatail review paper about the current therapeutic scenario of immune checkpoint inhibitors (in particular nivolumab, ipilimumab, atezolizumab, pembrolizumab, durvalumab) for the treatment of hepatocellular carcinoma. The first part of this review is dedicated to the concluded and ongoing clinical trials. The second part deals with the hot topics in the field of immunotherapy borrowing concepts from other cancers and adapting them to the specific scenario of hepatocellular carcinoma.
Immune checkpoint inhibitors (ICIs) are beginning to show promise in the clinical management of hepatocellular carcinoma (HCC). Most recently, the anti-programmed death protein-1 (PD-1) agent atezolizumab combined with bevacizumab demonstrated superiority to sorafenib in a Phase 3 randomised clinical trial in the frontline setting. Other ongoing trials of immunotherapy for HCC are exploring different drug combinations, such as a double checkpoint blockade with PD-1 and anti-Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) agents or with tyrosine kinase inhibitors. Moreover, ICIs are being tested in the adjuvant and neoadjuvant settings trying to resolve long-time unmet needs in HCC. The results of the ongoing trials will be critical to understanding the extent of the therapeutic role of ICIs in the complex and multifaceted clinical scenario of HCC. Still, there are some critical points which need further attention to clarify the best use of ICIs in HCC patients. For instance, the actual eligibility rate of patients in the real-life scenario, the prompt identification and correct management of immune-mediated adverse events, the identification of biomarkers predicting response or resistance, and strategies to prevent the tumour escape from ICI effect.
Deatail review paper about the current therapeutic scenario of immune checkpoint inhibitors (in particular nivolumab, ipilimumab, atezolizumab, pembrolizumab, durvalumab) for the treatment of hepatocellular carcinoma. The first part of this review is dedicated to the concluded and ongoing clinical trials. The second part deals with the hot topics in the field of immunotherapy borrowing concepts from other cancers and adapting them to the specific scenario of hepatocellular carcinoma.
- hepatocellular carcinoma
- liver cirrhosis
- checkpoint inhibitors
- immunotherapy
- immune-oncology drugs
- ipilimumab
- nivolumab
- atezolizumab
- bevacizumab
- pembrolizumab
- durvalumab
- programmed death-1
- Cytotoxic T-lymphocyte-associated protein 4
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
Immune checkpoint inhibitors (ICIs) are beginning to show promise in the clinical management of hepatocellular carcinoma (HCC). Most recently, the anti-programmed death protein-1 (PD-1) agent atezolizumab combined with bevacizumab demonstrated superiority to sorafenib in a Phase 3 randomised clinical trial in the frontline setting. Other ongoing trials of immunotherapy for HCC are exploring different drug combinations, such as a double checkpoint blockade with PD-1 and anti-Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) agents or with tyrosine kinase inhibitors. Moreover, ICIs are being tested in the adjuvant and neoadjuvant settings trying to resolve long-time unmet needs in HCC. The results of the ongoing trials will be critical to understanding the extent of the therapeutic role of ICIs in the complex and multifaceted clinical scenario of HCC. Still, there are some critical points which need further attention to clarify the best use of ICIs in HCC patients. For instance, the actual eligibility rate of patients in the real-life scenario, the prompt identification and correct management of immune-mediated adverse events, the identification of biomarkers predicting response or resistance, and strategies to prevent the tumour escape from ICI effect.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related morbidity worldwide, and the majority of HCC cases occur in a background of chronic liver inflammation [1]. Patients with advanced HCC had no effective therapies until 2008, when sorafenib, a multitarget tyrosine kinase inhibitor (TKI), demonstrated a benefit compared with placebo in terms of both overall survival (OS) and time to progression (TTP) [2]. Even if different frontline and second-line treatments with similar mechanisms of action (lenvatinib, regorafenib, cabozantinib, ramucirumab) have been identified as effective since 2016 [3], the research of drugs inhibiting other tumour pathways remains a top priority [4].
This priority is determined by multiple unmet needs. First, the disease control rate obtained with TKIs rarely exceeds 50–60%, with an objective response rate constantly <10%. Indeed, a relatively high number of patients show a progression at the first imaging evaluation with the practical failure of obtaining tumour downstage or neoadjuvant/adjuvant strategies. Indeed, a high number of patients progress after the first imaging evaluation with the practical failure of obtaining tumour downstage or neoadjuvant/adjuvant strategies. Second, even patients achieving a disease control will experience a relatively short progression-free survival, limiting the possibility of achieving long-term survivals in a sizeable number of patients. Third, most patients will experience TKI-related adverse events (AEs) impairing their quality of life and adherence to the treatment strategy. Moreover, as the safety profile of TKIs widely overlaps between different molecules, patients who are intolerant to sorafenib are theoretically prone to experience similar AEs if treated with other TKIs. As an example, the registrative trial of regorafenib explicitly excluded patients who did not tolerate sorafenib at a reduced dose of 400 mg/day. Consequently, most regulatory agencies approved the use of regorafenib only for patients who progressed under sorafenib, but not for those intolerant to the drug.
The presence of tumour-infiltrating lymphocytes expressing programmed death protein-1 (PD-1) in HCC mass and their correlation with outcome suggested that immunotherapeutic approaches could play a protective role against the progression of this cancer [5[5][6],6], as reported for other tumours [7,8][7][8]. Immune checkpoints are a normal part of the immune system. Their role is to prevent hyperimmune responses leading to tissue damage. The most known immune checkpoint are PD-1 and Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). In the oncological setting, pathological activation of PD-1 by its ligands, in particular ligand 1 (PD-L1), can result in the immune escape of the cancer cells. Thus, preventing the activation of the PD-1 receptor can restore the ability of immune cells to recognise and kill tumour cells [9,10][9][10]. On the other hand, CTLA-4 is mainly expressed on T cells and regulates the proliferation of activated lymphocytes. In physiological conditions, CTLA-4 regulates the end of the T-cell activity and prevents an excess in T-cell responses. Instead, in pathological conditions, it inhibits the activation, proliferation and production of tumour antigen-activated T cells in the tumour microenvironment (TME) [9,10][9][10]. Over time, many different PD-1, PD-L1, and CTLA-4 inhibitor agents have been developed that, collectively, are known as “"immune checkpoint inhibitors”" (ICIs) (Figure 1).
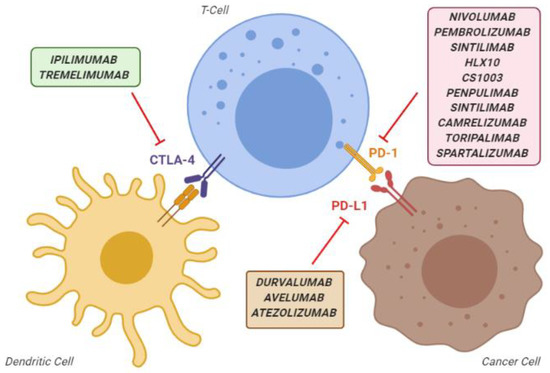
Overview of the main immune checkpoint inhibitors, classified according to their mechanism of action. CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed death-1 protein; PD-L1, programmed death-ligand 1.
Despite early promising results in HCC, the first significant Phase 3 trial testing the PD-1 inhibitor nivolumab vs. sorafenib in 2019 failed, as this ICI failed to demonstrate superiority to the TKI [11]. However, only a few months later, a combination of the PD-ligand 1 (PD-L1) atezolizumab with the monoclonal anti-VEGF (vascular endothelial growth factor) antibody bevacizumab significantly increased OS in comparison with sorafenib, ending a 12-year history of failures in searching therapies able to outperform sorafenib [12]. Indeed, previously only another TKI (lenvatinib) had succeeded in substantially challenging sorafenib in a noninferiority trial, but without reaching the threshold of a statistically significant superiority [13].
2. Open Problems
2.1. Eligibility in the Real-World Clinical Practice
The target population of the concluded ICI studies on HCC includes patients with an advanced-stage (BCLC-C) cancer or an intermediate-stage (BCLC-B) neoplasm not amenable to surgery or locoregional procedures. However, in the real-world clinical practice, not all of these patients are eligible to receive ICIs as monotherapy or combination, as they may have specific contraindications.
2.2. Safety
The inhibition of physiological immune checkpoints may be associated with immune-related AEs (irAEs) targeting the skin, gut, thyroid, adrenal glands, lung and liver [15][14]. For monotherapies with PD-1 /PD-L1 inhibitors, the risk of irAEs is dose-independent, with an incidence of 27% for all Grades, and 6% for Grade ≥3 [37][15]. Instead, with CTLA-4 inhibitors, the overall incidence of irAEs is dose-dependent and remarkably higher, reaching 72% for all Grades and 24% for Grade ≥3 [38][16]. Generally, these events are easily manageable, delaying the subsequent scheduled dose and using corticosteroids in severe or unresponsive cases. A recent meta-analysis reports 42 (0.6%) cases of fatal irAEs among 6528 patients treated with ICIs, with ipilimumab-induced colitis being the leading cause of death [39][17]. Furthermore, a minimal number of fatal outcomes due to immune-related pneumonitis [40][18] and myocarditis [41][19] have been reported.
Despite this acceptable safety profile of ICIs, a justifiable concern on the expected risk/benefit ratio accompanies their use in cirrhotic patients, for different reasons. First, immune-related hepatitis can precipitate an acute-on-chronic liver failure with a high risk of severe liver decompensation and death. Second, the use of corticosteroids to treat severe irAEs is particularly problematic in cirrhosis, especially in terms of increased risk of infections and ascitic decompensation. Third, cirrhosis is known to disrupt the liver’s homeostatic immune function, provoking per se a condition, including both systemic inflammation and immunodeficiency [42][20]. Indeed, a study enrolling patients treated with ICIs for different cancers seemed to suggest that hepatic AES were related to a worse prognosis [43][21].
2.3. Unpredictable Efficacy, the Need for Biomarkers
All trials of anti-PD-1/PD-L1 for HCC consistently identified a subgroup of 15–20% of patients obtaining an objective response (with an increase of this proportion up to 36% using combination regimes) [27][22]. These patients also obtained the most important benefit in terms of OS. Therefore, the identification of predictors of response would have a crucial role in optimising the cost-effectiveness of therapy with ICIs. At the same time, predictors of futility might channel patients to other treatments (TKIs, for instance), avoiding the cost and risk of pointless irAES.
Historically, immunostaining of tumour specimen with anti-PD-L1 antibodies was the first approach used to predict the response to ICIs. However, in most studies, PD-L1 expression was not predictive of response. When it was claimed as predictive, different thresholds of PD-L1 were identified in different tumours (from 1% to 50%) [46][23]. Moreover, the determination of PD-L1 expression suffers from the intrinsic variability of immunohistochemistry [47][24]. Moreover, the biological characteristics of malignancy, including intratumoral heterogeneity and tumour microenvironment, play an essential role in reducing the reliability of this technique [48][25]. Notably, Bensch et al. [49][26] performed the first-in-human study assessing PD-L1 expression by radionuclide imaging (89 Zr-atezolizumab), finding a good correlation between increased tumour uptake and response to anti-PD1 therapy. However, the reliability of this intriguing non-invasive way to detect PD-L1 expression that avoids sample biases needs further validation.
The role of PD-L1 expression has been evaluated in a patient subgroup of the CheckMate-459 study testing nivolumab vs sorafenib. In the nivolumab arm, the OS did not differ between patients with low and high PD-L1 expression. At the same time, surprisingly, the OS was different in the sorafenib arm, being about 14 months in patients with low PD-L1 expression and eight months in those overexpressing PD-L1 [11]. Altogether, these results suggest that PD-L1 expression has a negative prognostic effect in HCC patients, and a PD1 blockade (but not sorafenib) can reverse this negative effect on survival. Nevertheless, the benefit of nivolumab cannot be predicted by PD-L1 expression alone, as it did not affect the OS of patients undergoing this treatment.
Meanwhile, other putative biomarkers are under investigation. Several modern pieces of research are exploring the relationship between DNA damage/mutations and tumour immunogenicity. Tumour Mutational Burden (TMB) is a quantitative measure of the total number of nonsynonymous mutations per coding area of the tumour genome and is considered a surrogate marker of tumour immunogenicity reflecting neoantigen load. TMB is usually calculated using next-generation sequencing (NGS) techniques on tumour samples. Moreover, new blood tests (bTMB), exploring a limited number of genes, are under investigation in an attempt to obtain liquid biopsies in patients with tumours inaccessible to biopsies [50][27]. TMB determination, however, suffers from the same limitations of PD-L1 staining, namely, the lack of standardised thresholds and variability in quantification methods [51][28].
Mutations in the mismatch repair (MMR) system and microsatellite instability (MSI) are other DNA alterations potentially associated with increased tumour immunogenicity. In particular, tumours harbouring an erroneous MMR system will accumulate DNA mutations, which can lead to the presence of high levels of mutation-associated neoantigens [52[29][30],53], so that anti-PD1 agents are now prescribed to patients with colorectal cancers showing MSI [54][31]. The role of these DNA alterations in guiding the HCC treatment remains to be established.
2.4. Resistance to Immune Checkpoint Inhibitors
Despite the overall encouraging results of immunotherapy, most HCC patients under anti-PD1/PD-L1 and anti-CTLA4 agents eventually experience a disease progression. Resistance to ICIs can be primary or acquired.
The primary resistance to ICIs is due to a paucity (or even lack) of intratumoral immune infiltrate, which suggest a defective immune cell trafficking. Interestingly, this profile of “"immune exclusion”" is often associated with an activated Wnt/ß-catenin pathway signalling in HCC [55][32], giving support to the role of Wnt/ß-catenin activation as biomarker predictive of resistance to ICIs.
Primary resistance to ICIs may also derive from a more complex alteration of the immune system, with other immune pathways lying outside of the classical PD-1/PD-L1 and CTLA-4 checkpoints. For instance, lymphocyte activation gene-3 (LAG-3) is involved in the inhibition of CD8+ T cell and NK cell functions. Its expression is associated with a poor prognosis in HCC patients [56][33]. Moreover, T-cell immunoglobulin and mucin-containing protein-3 (TIM-3) and its ligand galectin-9 can activate a complex cascade that ultimately leads to T-cell exhaustion [56][33].
It can be argued that LAG-3, TIM-3 and PD-1 act synergistically, facilitating the HCC immune evasion, and could mediate the resistance to the classical PD-1/PD-L1 blockade [57,58][34][35]. Some trials are currently investigating the association effects of ICIs combined with TIM-3 (NCT03099109) and LAG-3 (NCT01968109) inhibitors in solid tumours.
Tumour microenvironment (TME) is another possible player in the development of primary resistance to ICIs. TME includes not only immune cells, but also blood vessels, fibroblasts, signalling molecules and the extracellular matrix surrounding the tumour [59][36]. Indoleamine-pyrrole 2,3-dioxygenase 1 (IDO-1) is a heme-containing enzyme physiologically expressed in many tissues and cells, which is activated during tumour development, helping malignant cells escape eradication by the immune system (56). Tumours with high IDO1 deplete the essential amino acid tryptophan from TME, resulting in T-cell anergy and immune suppression [59,60][36][37]. EPACADOSTAT and INCAGN01949 are two drugs targeting IDO and the T-cell costimulatory molecule CD134, which are being tested in combination with ICIs for HCC (NCT02178722, NCT03241173).
The role of TME is strictly related to the phenomenon of the epithelial to mesenchymal transition (EMT), a cellular process that enables epithelial cells to gain mesenchymal features leading to an aggressive and motile phenotype [61][38]. Several animal models and in vivo patient studies have shown that the activation of EMT in HCC promotes tumour progression and metastasis [62][39]. Moreover, EMT can promote an immunosuppressive TME by recruitment of tumour-associated macrophages, regulation of immune checkpoint molecules and immune resistance to NK cell-mediated lysis [63,64][40][41]. The association between EMT and immunosuppression has been reported in different cancer types, including HCC [65][42]. The role of tumour growth factor beta (TGF beta) is also of particular interest. This multifunctional cytokine plays multiple key activities, because of its role in immune and stem cell regulation and differentiation [66,67][43][44]. In many cancer cells, the TGF-β signalling is disrupted [68][45], and therefore, TGF-β is no longer able to downregulate the cell cycle, causing a simultaneous proliferation of both cancer and surrounding stromal cells in the setting of an immunosuppressive and pro-angiogenic microenvironment [69][46]. Additionally, TGF-β can convert effector T-cells into regulatory T-cells [70][47] and exerts inhibitory effects on B-cells [71][48], turning off the inflammatory reaction and favouring tumour immune escape. Investigation on the combination of nivolumab and the TGF-beta inhibitors galunisertib and ascrinvacumab (NCT02423343, NCT03893695) is currently in progress and will provide valuable information about the ability of these combinations in overcoming resistance to ICIs.
The VEGF signalling pathway can also provoke immune resistance as it induces Fas ligand, leading to cell death in tumour-infiltrating CD8+ T cells [72][49]. The results of the ImBrave-150 trial testing atezolizumab-bevacizumab would support the hypothesis of a consistent role of this pathway in HCC progression. However, the design of this trial does not clarify whether the efficacy of atezolizumab-bevacizumab derived from a synergistic or additional effect [73][50].
Currently, there are no data suggesting that the cirrhotic microenvironment actually affects the efficacy of ICIs. Indeed, when the first trials of immunotherapy for HCC were designed, this hypothesis (deriving from pre-clinical experiences) [42][20] was considered, but subsequent clinical data showed a similar efficacy both in viral and nonviral patients. Even more relevant, no differences between cirrhotic and noncirrhotic patients have been so far demonstrated.
Acquired resistance to ICIs is an even more complex phenomenon and is rapidly becoming a hot topic as its occurrence hampers long-term results in patients responding to immunotherapy. Differently from classical chemotherapies and TKIs, ICIs have not a direct an antitumour effect as they act by enhancing the cytotoxicity of the immune system. The acquired resistance to ICIs probably relies on different events, for which, however, dynamic mutations in tumour cells still play a pivotal role. In particular, mutations in genes codifying for target antigens of the HLA system (resulting in a loss of expression of HLA genes on tumour cells) or in genes involved in the interferon signalling may be involved in this phenomenon [74][51]. While some strategies to overcome these events can be hypothesised (i.e., enhancing the natural killer T-cell response in case of HLA loss), mechanistic and clinical studies are needed to highlight these phenomena further. Interestingly, on ongoing trial for the HCC treatment, combining nivolumab and ABX196 relies on the possibility of activating the natural killer T-cells, potentially overcoming the acquired resistance derived from the HLA loss (NCT03419481).
2.5. The Radiological Evaluation of Response
Historically, the RECIST 1.1 [75][52] have been used as the preferred radiological criteria to assess the response to the systemic drugs for most malignancies, including HCC. However, in the case of HCC, the modified RECIST criteria (mRECIST) assess the response to locoregional treatments and have also been endorsed for the evaluation of systemic therapies [76][53]. Whether the information provided by the mRECIST in the systemic setting is superior is still a matter of debate [77[54][55],78], but the leading regulatory agencies still require a RECIST 1.1-based evaluation.
However, the advent of immunotherapy poses some unique challenges that cannot be addressed by both RECIST1.1 and mRECIST. In early trials of ipilimumab for melanoma, the investigators described an initial disease behaviour meeting the RECIST criteria for progressive disease, followed by marked and durable responses [79][56]. This pattern was called “"pseudoprogression”" and was attributed to a delayed response to ICIs and prompted the RECIST working group to propose new immune-related response criteria (iRECIST) [80][57]. According to these criteria, an increase of the tumour burden or even the appearance of new lesions should be classified as unconfirmed progression (iUPD) [80][57], and if the patients are clinically stable, ICIs should not be discontinued, and a new imaging assessment should be scheduled in the next 4.-8 weeks. In case of further increase of the tumour burden, radiological progression is confirmed (iCPD), and the treatment should be discontinued. If the tumour remains stable or shrinks, the imaging showing iUPD is regarded as a novel “"baseline imaging”" for the subsequent evaluations [80][57].
The combinations of ICIs with either TKIs or anti-VEGF agents could prevent pseudoprogression, and consequently, the applicability of iRECIST for combination therapies is debatable and should be investigated.
3. Conclusions
From an expert perspective, accumulating data on ICIs would indicate that these agents can provide answers to some of the current issues in the treatment of HCC.
First, ICIs and their combination provide an objective response rate which is considerably higher than TKIs in monotherapy. These data suggest that pharmacological downstaging strategies for HCC can now be possible. The pertinent implications are manifold. First, patients with intermediate-stage HCC, which are not ideal candidates for transarterial procedures (for instance patients with nodules larger than 6 cm or more than six nodules), due to the low probability of achieving a complete response and for the relatively high risk of hepatic decompensation, could receive upfront systemic treatment, followed by locoregional procedures in case of successful downstaging. Clearly, the feasibility of this strategy and the best cut-offs for tumour size and number have to be defined by future studies. Moreover, the pharmacological downstaging offers can be useful in patients who are a borderline candidate for surgery, and this specific aspect is already being investigated in dedicated trials. Second, a clinically meaningful number of patients treated with ICIs can achieve a durable response, in stark contrast with what occurs with TKIs. At the state-of-art, patients showing an objective response are the most obvious candidates to achieve long-term survival. Thus, identifying combination strategies which augment the biological effects of ICIs will probably be the primary target of future studies. Third, there are no crossed toxicities between TKIs and ICIs; thus, the availability of two different classes of biological agents represents an upmost benefit for patients intolerant to one class.
Nevertheless, there also some open problems which must be necessary to consider, and possibly resolved, to further improve the therapeutic scenario of HCC. Firstly, biomarkers predicting treatment efficacy are still needed. The current lack of such biomarkers designs a scenario affected by a “"dilution bias”" with a too high percentage of non-responding patients and exposed to the treatment risks. Moreover, this bias adversely affects the cost-effectiveness of ICI treatment. Secondly, despite the increased possibility of achieving long-term responses, disease progression still occurs in most patients, stressing the need to identify agents able to overcome the primary resistance to ICIs and preventing the secondary resistance. Lastly, a word of caution about toxicity: Therapeutic combinations, including ICIs, aimed at increasing the treatment efficacy can also amplify the toxicity. Moreover, since the immune-related AEs are known to occur even after the treatment stop, more long-term data on safety are needed.
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462, doi:10.1056/NEJMra1713263.
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390, doi:10.1056/NEJMoa0708857.
- Tovoli, F.; Negrini, G.; Benevento, F.; Faggiano, C.; Goio, E.; Granito, A. Systemic treatments for hepatocellular carcinoma: Challenges and future perspectives. Hepatic Oncol. 2018, 5, HEP01, doi:10.2217/hep-2017-0020.
- Nishida, N.; Arizumi, T.; Hagiwara, S.; Ida, H.; Sakurai, T.; Kudo, M. MicroRNAs for the Prediction of Early Response to Sorafenib Treatment in Human Hepatocellular Carcinoma. Liver Cancer 2017, 6, 113–125, doi:10.1159/000449475.
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700, doi:10.1038/nrgastro.2015.173.
- Shi, F.; Shi, M.; Zeng, Z.; Qi, R.-Z.; Liu, Z.-W.; Zhang, J.-Y.; Yang, Y.-P.; Tien, P.; Wang, F.-S. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer 2011, 128, 887–896, doi:10.1002/ijc.25397.
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461, doi:10.1016/j.ccell.2015.03.001.
- Whiteside, T.L.; Demaria, S.; Rodriguez-Ruiz, M.E.; Zarour, H.M.; Melero, I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1845–1855, doi:10.1158/1078-0432.CCR-16-0049.
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1974–1982, doi:10.1200/JCO.2014.59.4358.
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232, doi:10.1038/s41590-018-0044-z.
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, v874–v875, doi:10.1093/annonc/mdz394.029.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905, doi:10.1056/NEJMoa1915745.
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet Lond. Engl. 2018, 391, 1163–1173, doi:10.1016/S0140-6736(18)30207-1.
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet Lond. Engl. 2017, 389, 2492–2502, doi:10.1016/S0140-6736(17)31046-2.
- Wang, P.-F.; Chen, Y.; Song, S.-Y.; Wang, T.-J.; Ji, W.-J.; Li, S.-W.; Liu, N.; Yan, C.-X. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front. Pharm. 2017, 8, 730, doi:10.3389/fphar.2017.00730.
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.-E.; Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. Bmc Med. 2015, 13, 211, doi:10.1186/s12916-015-0455-8.
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318, doi:10.1158/2326-6066.CIR-16-0237.
- Abdel-Rahman, O.; Fouad, M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Adv. Respir. Dis. 2016, 10, 183–193, doi:10.1177/1753465816636557.
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755, doi:10.1056/NEJMoa1609214.
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396, doi:10.1016/j.jhep.2014.08.010.
- Chan, S.L.; Yip, T.C.-F.; Wong, V.W.-S.; Tse, Y.-K.; Yuen, B.W.-Y.; Luk, H.W.-S.; Lui, R.N.-S.; Chan, H.L.-Y.; Mok, T.S.-K.; Wong, G.L.-H. Pattern and impact of hepatic adverse events encountered during immune checkpoint inhibitors – A territory-wide cohort study. Cancer Med. doi:10.1002/cam4.3378 [In Press].
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, JCO.20.00808, doi:10.1200/JCO.20.00808.
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278, doi:10.1186/s40425-019-0768-9.
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 208–222, doi:10.1016/j.jtho.2016.11.2228.
- Rasmussen, J.H.; Lelkaitis, G.; Håkansson, K.; Vogelius, I.R.; Johannesen, H.H.; Fischer, B.M.; Bentzen, S.M.; Specht, L.; Kristensen, C.A.; von Buchwald, C.; et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 1003–1006, doi:10.1038/s41416-019-0449-y.
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858, doi:10.1038/s41591-018-0255-8.
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448, doi:10.1038/s41591-018-0134-3.
- Immuno-Oncology | OncologyPRO. Available online: https://oncologypro.esmo.org/education-library/handbooks/immuno-oncology (accessed on 21 July2020).
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413, doi:10.1126/science.aan6733.
- Sinicrope, F.A.; Sargent, D.J. Molecular pathways: Microsatellite instability in colorectal cancer: Prognostic, predictive, and therapeutic implications. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1506–1512, doi:10.1158/1078-0432.CCR-11-1469.
- Sarshekeh, A.M.; Overman, M.J.; Kopetz, S. Nivolumab in the treatment of microsatellite instability high metastatic colorectal cancer. Future Oncol. 2018, 14, 1869–1874, doi:10.2217/fon-2017-0696.
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro de Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826, doi:10.1053/j.gastro.2017.06.007.
- Matsuda, Y.; Yamagiwa, Y.; Fukushima, K.; Ueno, Y.; Shimosegawa, T. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2008, 38, 1098–1111, doi:10.1111/j.1872-034X.2008.00387.x.
- Yan, W.; Liu, X.; Ma, H.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut 2015, 64, 1593–1604, doi:10.1136/gutjnl-2014-307671.
- Yarchoan, M.; Xing, D.; Luan, L.; Xu, H.; Sharma, R.B.; Popovic, A.; Pawlik, T.M.; Kim, A.K.; Zhu, Q.; Jaffee, E.M.; et al. Characterization of the Immune Microenvironment in Hepatocellular Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 7333–7339, doi:10.1158/1078-0432.CCR-17-0950.
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80, doi:10.1126/science.aaa6204.
- Audrito, V.; Managò, A.; Gaudino, F.; Sorci, L.; Messana, V.G.; Raffaelli, N.; Deaglio, S. NAD-Biosynthetic and Consuming Enzymes as Central Players of Metabolic Regulation of Innate and Adaptive Immune Responses in Cancer. Front. Immunol. 2019, 10, 1720, doi:10.3389/fimmu.2019.01720.
- 1428, doi:10.1172/JCI39104.
- Immune Checkpoint Blockade Therapies for HCC: Current Status and Future Implications. Available online: https://hrjournal.net/article/view/3207 (accessed on 15 August2020).
- Soundararajan, R.; Fradette, J.J.; Konen, J.M.; Moulder, S.; Zhang, X.; Gibbons, D.L.; Varadarajan, N.; Wistuba, I.I.; Tripathy, D.; Bernatchez, C.; et al. Targeting the Interplay between Epithelial-to-Mesenchymal-Transition and the Immune System for Effective Immunotherapy. Cancers 2019, 11, doi:10.3390/cancers11050714.
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.-P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846, doi:10.1002/1878-0261.12093.
- Ye, L.-Y.; Chen, W.; Bai, X.-L.; Xu, X.-Y.; Zhang, Q.; Xia, X.-F.; Sun, X.; Li, G.-G.; Hu, Q.-D.; Fu, Q.-H.; et al. Hypoxia-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma Induces an Immunosuppressive Tumor Microenvironment to Promote Metastasis. Cancer Res. 2016, 76, 818–830, doi:10.1158/0008-5472.CAN-15-0977.
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630, doi:10.1038/nrm3434.
- Nakao, A.; Afrakhte, M.; Morn, A.; Nakayama, T.; Christian, J.L.; Heuchel, R.; Itoh, S.; Kawabata, M.; Heldin, N.-E.; Heldin, C.-H.; et al. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 1997, 389, 631–635, doi:10.1038/39369.
- The Hallmarks of Cancer: Cell. Available online: https://www.cell.com/cell/fulltext/S0092-8674(00)81683-9?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867400816839%3Fshowall%3Dtrue (accessed on 21 September2020).
- Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. Role of Transforming Growth Factor β in Human Disease. Available online: https://www.nejm.org/doi/10.1056/NEJM200005043421807 (accessed on 21 September 2020).
- Eisenstein, E.M.; Williams, C.B. The T reg /Th17 Cell Balance: A New Paradigm for Autoimmunity. Pediatr. Res. 2009, 65, 26–31, doi:10.1203/PDR.0b013e31819e76c7.
- Roes, J.; Choi, B.K.; Cazac, B.B. Redirection of B cell responsiveness by transforming growth factor β receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 7241–7246, doi:10.1073/pnas.0731875100.
- Kim, J.M.; Chen, D.S. Immune escape to PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1492–1504, doi:10.1093/annonc/mdw217.
- Kelley, R.K. Atezolizumab plus Bevacizumab—A Landmark in Liver Cancer. N. Engl. J. Med. 2020, 382, 1953–1955, doi:10.1056/NEJMe2004851.
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723, doi:10.1016/j.cell.2017.01.017.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer Oxf. Engl. 1990 2009, 45, 228–247, doi:10.1016/j.ejca.2008.10.026.
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60, doi:10.1055/s-0030-1247132.
- Lencioni, R.; Montal, R.; Torres, F.; Park, J.-W.; Decaens, T.; Raoul, J.-L.; Kudo, M.; Chang, C.; Ríos, J.; Boige, V.; et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J. Hepatol. 2017, 66, 1166–1172, doi:10.1016/j.jhep.2017.01.012.
- Bruix, J.; Reig, M.; Sangro, B. Assessment of treatment efficacy in hepatocellular carcinoma: Response rate, delay in progression or none of them. J. Hepatol. 2017, 66, 1114–1117, doi:10.1016/j.jhep.2017.02.032.
- Wolchok, J.D.; Rollin, L.; Larkin, J. Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 2503–2504, doi:10.1056/NEJMc1714339.
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152, doi:10.1016/S1470-2045(17)30074-8.
