Monkeypox, once a rare zoonotic disease, has been endemic to some African countries since its original identification among humans in 1970. Since then, cases in non-endemic regions have been linked to returning travelers or those who had contact with transported animals. The causative agent, Monkeypox virus, belongs to Orthopoxviruses, the same family as Variola—the causative organism for smallpox. Although most monkeypox outbreaks until recently were linked to zoonotic transmission, secondary human–human transmission in smallpox-unvaccinated individuals was observed in a small proportion of overall cases. Smallpox was declared to be eradicated in 1980, and since its eradication, Monkeypox virus has been the most significant poxvirus to cause human disease. The 2022 monkeypox outbreak marks a significant paradigm shift in the human and poxvirus association, with new modes of transmission and concerns of viral evolution and entrenchment as a sexually transmitted disease. Monkeypox clinically resembles smallpox but is far milder. At this time, there are no approved therapies for monkeypox, and antiviral agents effective against smallpox are being utilized. Additionally, preventive strategies being utilized include smallpox vaccinations such as JYNNEOS and ACAM2000. In this narrative review, we discuss the virology, epidemiology, transmission, clinical manifestations, diagnosis, management, and prevention strategies associated with monkeypox.
- public health emergency
- sexual health
- monkeypox
- smallpox
- JYNNEOS
- ACAM2000
- tecovirimat
- brincidofovir
1. History and Introduction
2. Virology and Pathogenesis
MPV, a double-stranded DNA (dsDNA) virus, is similar in structure to other Orthopoxviruses, i.e., Variola, Vaccinia, and Cowpox virus. MPV, like other poxviruses, has a brick-like, oval structure measuring 200–250 nm. The viruses are characterized by a dumbbell-shaped nucleus housing the linear double-stranded deoxyribonucleic acid (dsDNA) of ~197 kb, surrounded by lateral bodies [21]. The virions are enclosed by a lipoprotein outer membrane with surface tubules. Palindromic hairpins covalently combine the ends of the DNA strands, while inverted terminal repeats, which hold the origins of replication for DNA viruses, comprise a hairpin loop, tandem repeats, and some open reading frames (ORFs). DNA synthesis is initiated at one end of the inverted terminal repeats and continues to the other end [22]. Similar to other Orthopoxviruses, the MPV genome consists of 190 largely non-overlapping ORFs of ≥60 amino acid residues, as well as structural features [21]. Additionally, the central part of the MPV genome consists of highly conserved genes, seen across Orthopoxviruses. Uniquely to MPV, significant lengths of its right side genome contain duplications of the four left terminal ORFs as a part of terminal inverted repeat. These terminal regions exhibit considerable variations as a result of ORF truncations and deletions. There is an approximate 84.5–84.6% overlap of genome nucleotide sequences between MPV and the Variola virus [21]. The lifecycle of MPV in the host-cell cytoplasm is largely similar to other Orthopoxviruses [22][23][24][25][22,23,24,25]. The entry of the virus into the host cell is mediated by the fusion of viral proteins (glycoproteins B5 and A34) to host-cell membrane glycosaminoglycans [26]. This triggers the virion to release its contents into the host-cell cytoplasm. In an immediate next phase, as early as 30 min post-infection, viral RNA-polymerase transcribes the early expression of viral proteins and causes the uncoating of its entire genome [23][27][23,27]. The next intermediate phase, taking place approximately 100 min post-infection, involves the expression of a series of genes orchestrating the recruitment of viral DNA-polymerase for replication. In the subsequent late phase, 2–48 h post infection, structural proteins are produced through the transcription of late genes, the recruitment of the host-cell endoplasmic reticulum and Golgi apparatus, and the assembly of spherical proto-virions in host cytoplasmic viral factories [23][27][28][23,27,28]. Mature virions can be released from the host cell via lysis as a non-enveloped intracellular mature virion (IMV) or bud out as an external enveloped virion (EEV) having acquired a double membrane from the Golgi apparatus. The EEV further sets up a cellular microtubule transport that fuses to the host-cell membrane lipoprotein before being released outside [22][23][27][22,23,27]. Interestingly, these two forms of mature virions are believed to exhibit variable antigenic properties and host-cell attachment sites. These variations modulate the host immune response and infectivity of progeny virions [27]. Additionally, one study showed that the EEV plays a key role in viral dissemination while the IMV plays a predominant role in host–host transmission [29]. These factors of virion replication and egress need to be looked at carefully and may hold insights into the new transmission modes associated with the 2022 global monkeypox outbreak. Recent studies have shown evidence of microevolution within the MPV genome sequence that might be indicative of the changes observed in the transmission chain [30][31][30,31]. The infected cell hosts most of the viral DNA replication, transcription, assembly, and release, as well as all housekeeping genes present on the conserved central region of the genome [22]. Significantly, there is an 83.5–93.6% commonality between the Variola and MPV putative virulence and immunomodulatory amino acid sequences. Alongside these significant similarities, Vaccinia virus vaccination can elicit cross-reactive immunity against similar targets on MPV for neutralizing antibody and T-cell activity [32]. Furthermore, previous studies have shown that certain mutations in genes for two interferon (IFN) resistance-encoding intracellular proteins (causing IFN sensitivity) could have been key in making the human–human transmission of MPV less efficient in earlier outbreaks, as these genes were intact in other Orthopoxviruses such as Variola [21]. Other differences in complement-binding proteins compared to Variola and the presence of an L-1β-binding protein in MPV may contribute to a less pronounced clinical disease, while the absence of the latter in Variola has been associated with significant pathogenicity and fever [21]. The phylogenetic analysis of Orthopoxviruses have shown that MPV is slightly distant from the Variola and Vaccinia viruses while the Cowpox virus may be a progenitor [21][33][21,33]. These and other discrete differences in the MPV genome, compared with other Orthopoxviruses, require attention and may hold answers regarding its unique virulence, pathogenesis and transmission, particularly as the 2022 monkeypox global outbreak exhibits differences in these aspects from previous outbreaks. Phylogenetically, MPV has been grouped into two genetic clades based on geography, disease severity, and sequence homology (the Central African/Congo Basin (CB) and West African (WA) clades), and reports suggest that the latter has been associated with a milder form of disease [34][35][34,35]. The human–human transmissibility, disease-associated morbidity, mortality, and viraemia are more severe with the CB clade [34]. The most significant difference between the two clades is associated with the DNA sequence diversity at the terminal region for genes that encode the host-response modulation proteins [34][35][36][34,35,36]. Prior epidemiological surveys assessing monkeypox showed that infection with either clade has a similar serological response in smallpox-unvaccinated individuals but is associated with higher mortality rates in the CB-clade-infected patients [36][37][36,37].3. Clinical Manifestations and Diagnosis
Early diagnosis and robust management are key to curtailing the extent of the ongoing monkeypox outbreak as transmission continues. The diagnosis of monkeypox is based on patient history, clinical presentation, and diagnostic testing. Gathering a detailed travel and sexual history are of utmost importance given evolving transmission patterns and disease spread beyond its endemic distribution. The clinical features of monkeypox traditionally include prodromal symptoms such as fever, malaise, chills, lymphadenopathy, myalgia, or headache, along with a pleomorphic, often umbilicated skin rash. The lesions have been described as firm, deep-seated, well-circumscribed, and potentially affecting palms and soles [38][53]. Macules, papules, vesicles and pustules progress to scabs and eventually desquamate. An individual is considered infectious until all skin lesions have completely re-epithelialized. Pitted scars or skin with variable pigmentation may result at the sites of prior skin lesions. Monkeypox is generally a self-limited disease that resolves over 2–4 weeks. Severe cases can occasionally occur and are more common among children, and worse outcomes remain a possibility among immunocompromised hosts. The features of severe disease include encephalitis, sepsis, hemorrhage, confluent skin lesions, and other complications resulting in hospitalization. The potential complications of monkeypox include encephalitis, corneal ulceration and scarring resulting in a loss of vision, the secondary bacterial infection of skin lesions, and respiratory tract infections such as bronchopneumonia and sepsis [18]. Disease prognosis depends on several factors including age, prior smallpox vaccination status, medical comorbidities, use of immunosuppressive drugs, and the severity of the illness. Although the true extent of asymptomatic monkeypox remains unknown, fresh reports of this phenomenon have emerged this year. Three Belgian male attendees of a sexual health clinic were identified to be MPV PCR-positive on samples collected from the anorectal region, with eventual spontaneous viral clearance [39][54]. The salient clinical features of the 2022 monkeypox cases include dermal lesions over the external genitalia, anal region, and oral mucosa, which are most likely portals of exposure and viral entry. Thornhill et al. described similar clinical presentations among those with and without HIV [40][48]. Sore throat, penile edema, rectal pain have also been observed among patients from London, UK with PCR-confirmed monkeypox [41][55]. Patel et al. also reported a variable timeline of systemic and mucocutaneous symptoms among cases and described several cases where patients solely presented with dermatological symptoms without systemic features of the disease. Novel clinical findings from the 2022 cases also include mucocutaneous ulcers and fewer skin lesions, less disseminated disease, and proctitis causing anorectal pain [40][48].4. Management
4.1. Tecovirimat
Tecovirimat (TPOXX) was approved by the FDA in 2018 for the treatment of smallpox in adults and children. It inhibits VP37, a viral envelope wrapping protein, and disrupts viral replication and release. It is currently available for use in the US under an expanded access investigational new drug protocol at no cost (EA-IND) [42][63]. Tecovirimat is available in oral and intravenous formulations. While efficacy data on the use of TPOXX against monkeypox are lacking, a favorable safety profile with common adverse effects such as headache, nausea, vomiting and abdominal pain has been reported. Neutropenia was also reported for use with one trial participant [43][64]. Intravenous formulation use may result in infusion site erythema, pain and swelling [42][63]. Thornhill et al. reported on the treatment of recent monkeypox cases with TPOXX [40][48]. Adler et al. described the management of a human monkeypox case with TPOXX with a favorable outcome [44][65]. Monkeypox in a returning traveler from Nigeria to the US, treated with TPOXX, was also recently described [45][66].4.2. Brincidofovir
Brincidofovir was approved by the FDA for use against smallpox in adults and pediatric patients in June 2021. It is a prodrug of cidofovir and contains a lipid conjugate. Intracellularly, it is converted to cidofovir and eventually its active metabolite, cidofovir diphosphate (CDP), which incorporates into viral DNA and inhibits viral DNA polymerase, thereby inhibiting viral replication. Large-scale human data on the use of brincidofovir against MPV are lacking, but an animal model showed trends of protection against lethal monkeypox, with 29–57% survival rates among infected prairie dogs depending on the time of treatment initiation [46][67]. Adler et al. also described three human cases of monkeypox treated with brincidofovir. Treatment cessation occurred due to the elevation of liver enzymes [44][65]. Brincidofovir is available as an oral formulation (tablet and oral suspension) and has a better renal safety profile than cidofovir [44][65].4.3. Cidofovir
Cidofovir has the same mechanism of action as its prodrug, brincidofovir. Large-scale human data on efficacy of cidofovir against monkeypox are lacking. However, animal data on its use against Orthopoxviruses including cowpox, vaccinia, ectromelia and rabbitpox exist [47][68]. Thornhill et al. reported cases amid the 2022 monkeypox outbreak that were treated with cidofovir. It is only available as an intravenous formulation and can have significant renal toxicity [40][48].4.4. Vaccinia Immune Globulin Intravenous (VIGIV)
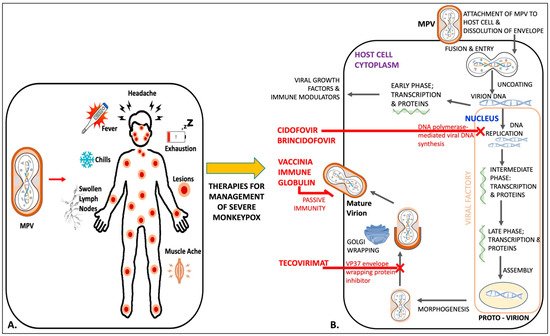
VIGIV is FDA-licensed for the treatment of complications after Vaccinia vaccination, including vaccinia (progressive or severe generalized), eczema vaccinatum, and aberrant infections due to Vaccinia virus. It can also be used for vaccinia infections in those with certain skin conditions [48][69]. Data for its use against monkeypox are lacking, but it is available in the US as a response measure in the event of Orthopoxvirus outbreaks under an EA-IND. Figure 1 is an illustration of mechanisms of action of available therapies against monkeypox, along with its clinical symptoms.